Abstract
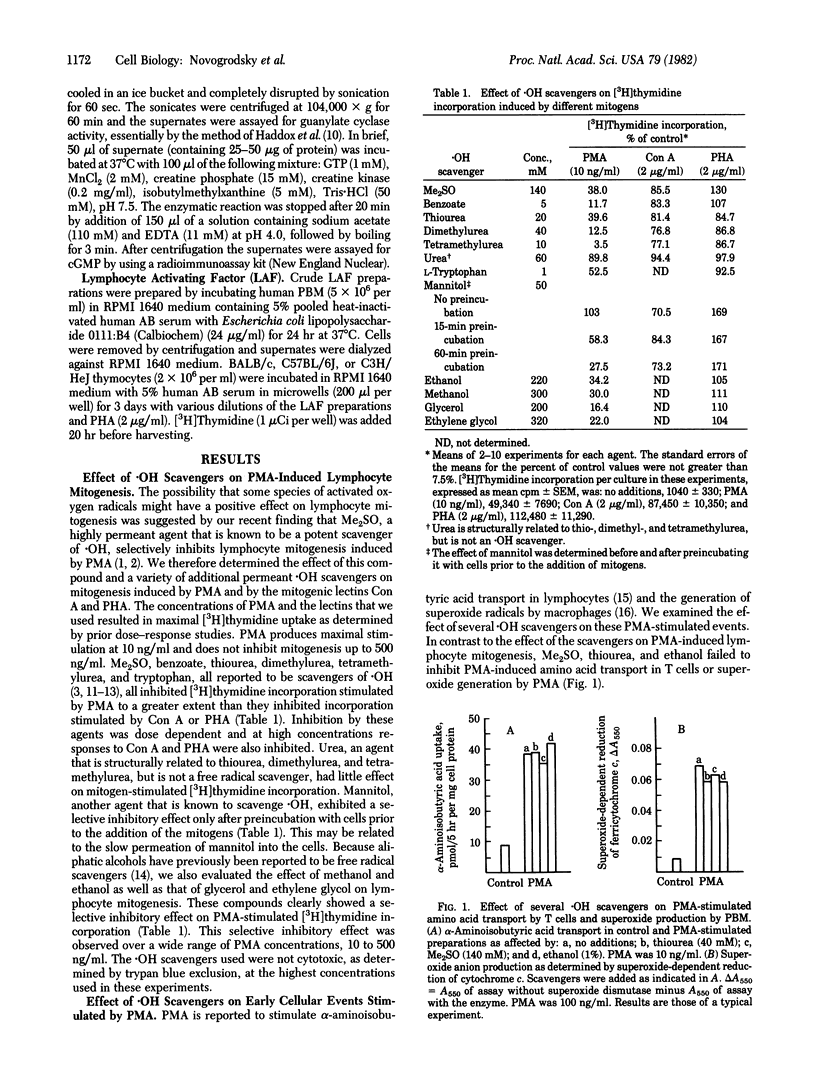
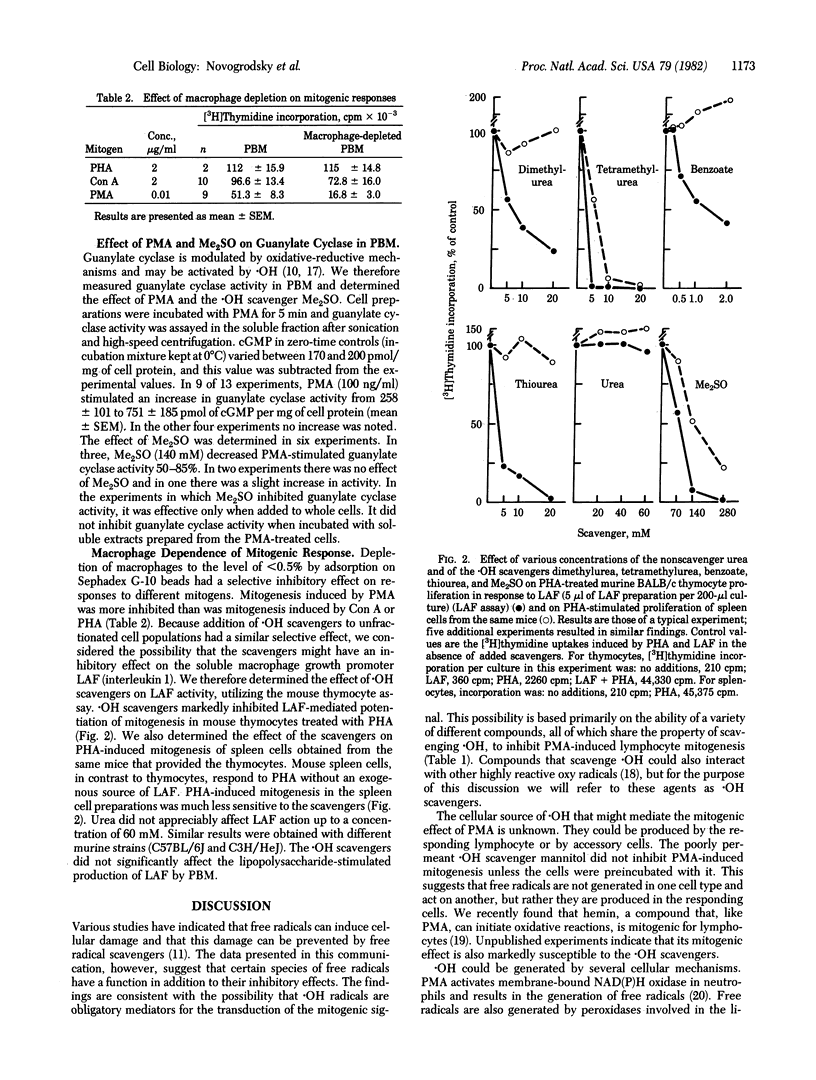
Agents that are known to be scavengers of hydroxyl radicals inhibit lymphocyte mitogenesis induced by phorbol myristate acetate (PMA) to a greater extent than they inhibit mitogenesis induced by concanavalin A or phytohemagglutinin. These agents include dimethyl sulfoxide, benzoate, thiourea, dimethylurea, tetramethylurea, L-tryptophan, mannitol, and several other alcohols. Their inhibitory effect is not associated with cytotoxicity. The hydroxyl radical scavengers do not inhibit PMA-dependent amino acid transport in T cells or PMA-induced superoxide production by monocytes. Thus, they do not inhibit the primary interaction of PMA with responding cells. Treatment of peripheral blood mononuclear cells with PMA increased cellular guanylate cyclase in most experiments, and dimethyl sulfoxide tended to inhibit this increase. In addition to inhibition of PMA-induced mitogenesis, hydroxyl radical scavengers markedly inhibited the activity of lymphocyte activating factor (interleukin 1). The differential inhibition of lymphocyte mitogenesis induced by different mitogens appears to be related to the differential macrophage requirements of the mitogens. The data suggest that hydroxyl radicals may be involved in mediating the triggering signal for lymphocyte activation. Some of the hydroxyl radical scavengers are inducers of cellular differentiation,. nd it is possible that their differentiating activity is related to their ability to scavenge free radicals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffey R. G., Hadden E. M., Hadden J. W. Phytohemagglutinin stimulation of guanylate cyclase in human lymphocytes. J Biol Chem. 1981 May 10;256(9):4418–4424. [PubMed] [Google Scholar]
- Coffey R. G., Hadden J. W. Phorbol myristate acetate stimulation of lymphocyte guanylate cyclase. Biochem Biophys Res Commun. 1981 Jul 30;101(2):584–590. doi: 10.1016/0006-291x(81)91299-7. [DOI] [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974 Apr 25;249(8):2447–2452. [PubMed] [Google Scholar]
- Cohen G. The generation of hydroxyl radicals in biologic systems: toxicological aspects. Photochem Photobiol. 1978 Oct-Nov;28(4-5):669–675. doi: 10.1111/j.1751-1097.1978.tb06993.x. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Kuehl F. A., Jr Reduction of hydroperoxides in the prostaglandin biosynthetic pathway by a microsomal peroxidase. J Biol Chem. 1979 May 10;254(9):3295–3302. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Opposing effects of mitogenic and nonmitogenic lectins on lymphocyte activation. Evidence that wheat germ agglutinin produces a negative signal. J Biol Chem. 1976 Jul 10;251(13):4017–4025. [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox M. K., Stephenson J. H., Moser M. E., Goldberg N. D. Oxidative-reductive modulation of guinea pig splenic cell guanylate cyclase activity. J Biol Chem. 1978 May 10;253(9):3143–3152. [PubMed] [Google Scholar]
- Heikkila R. E., Winston B., Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976 May 1;25(9):1085–1092. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. P., Kierszenbaum F., Waksman B. H. Mechanisms of action of "lymphocyte-activating factor" (LAF). III. Evidence that LAF acts on stimulated lymphocytes by raising cyclic GMP in G1. J Immunol. 1978 Dec;121(6):2386–2391. [PubMed] [Google Scholar]
- Kelly J. P., Johnson M. C., Parker C. W. Effect of inhibitors of arachidonic acid metabolism on mitogenesis in human lymphocytes: possible role of thromboxanes and products of the lipoxygenase pathway. J Immunol. 1979 Apr;122(4):1563–1571. [PubMed] [Google Scholar]
- Kensler T. W., Wertz P. W., Mueller G. C. Inhibition of phorbol ester-accelerated amino acid transport in bovine lymphocytes. Biochim Biophys Acta. 1979 Jun 1;585(1):43–52. doi: 10.1016/0304-4165(79)90323-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3',5'-monophosphate formation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Quittner S., Rubin A. L., Stenzel K. H. Transglutaminase activity in human lymphocytes: early activation by phytomitogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1157–1161. doi: 10.1073/pnas.75.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Rubin A. L., Stenzel K. H. A new class of inhibitors of lymphocyte mitogenesis: agents that induce erythroid differentiation in Friend leukemia cells. J Immunol. 1980 Apr;124(4):1892–1897. [PubMed] [Google Scholar]
- Parker C. W., Kelly J. P., Falkenhein S. F., Huber M. G. Release of arachidonic acid from human lymphocytes in response to mitogenic lectins. J Exp Med. 1979 Jun 1;149(6):1487–1503. doi: 10.1084/jem.149.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler H. D., Christoff G., Taylor E. Cryoprotective agents as inducers of erythroleukemic cell differentiation in vitro. Blood. 1976 Mar;47(3):363–368. [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. Signal requirements for T lymphocyte activation. I. Replacement of macrophage function with phorbol myristic acetate. J Immunol. 1979 Oct;123(4):1749–1754. [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Stenzel K. H., Rubin A. L., Novogrodsky A. Mitogenic and co-mitogenic properties of hemin. J Immunol. 1981 Dec;127(6):2469–2473. [PubMed] [Google Scholar]
- Stenzel K. H., Schwartz R., Rubin A. L., Novogrodsky A. Chemical inducers of differentiation in Friend leukaemia cells inhibit lymphocyte mitogenesis. Nature. 1980 May 8;285(5760):106–108. doi: 10.1038/285106a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Lehrer R. I. NAD(P)H oxidase activity in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1980 Dec;66(6):1409–1418. doi: 10.1172/JCI109994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibaldi J., Benjamin J., Cabbat F. S., Heikkila R. E. Protection against alloxan-induced diabetes by various urea derivatives: relationship between protective effects and reactivity with the hydroxyl radical. J Pharmacol Exp Ther. 1979 Nov;211(2):415–418. [PubMed] [Google Scholar]


