Abstract
An increasing number of patients older than 65 years are referred for and have access to organ transplantation, and an increasing number of older adults are donating organs. Although short-term outcomes are similar in older versus younger transplant recipients, older donor or recipient age is associated with inferior long-term outcomes. However, age is often a proxy for other factors that might predict poor outcomes more strongly and better identify patients at risk for adverse events. Approaches to transplantation in older adults vary across programs, but despite recent gains in access and the increased use of marginal organs, older patients remain less likely than other groups to receive a transplant, and those who do are highly selected. Moreover, few studies have addressed geriatric issues in transplant patient selection or management, or the implications on health span and disability when patients age to late life with a transplanted organ. This paper summarizes a recent trans-disciplinary workshop held by ASP, in collaboration with NHLBI, NIA, NIAID, NIDDK, and AGS, to address issues related to kidney, liver, lung, or heart transplantation in older adults and to propose a research agenda in these areas.
Keywords: transplantation, liver, kidney, heart, lung, elderly, aging
Introduction
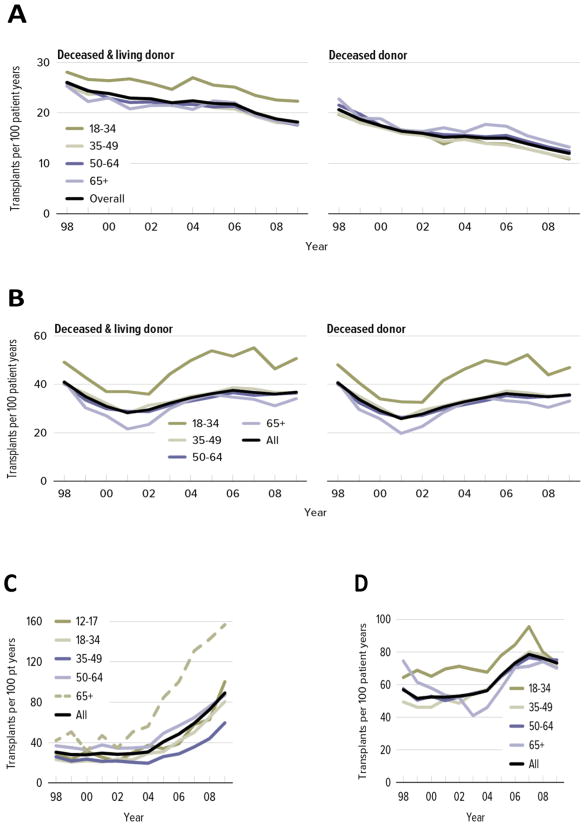
The patient population on organ transplant waiting lists is growing older, particularly as the number of patients aged 65 years and older increases (1)(Figure 1). Older adults have gained more access to transplantation over time (2), but transplantation rates among these patients still vary across organs (Figure 2). For kidney, the proportion of older patients placed on the waiting list is lower than that of other age groups, even though older patients represent about half of all patients with end-stage renal disease (ESRD). However, of the older patients listed for kidney transplant, the proportion who receive transplants is similar to that in younger age groups. For lung, transplantation rates increase with age because of the high rate of transplantation for idiopathic pulmonary fibrosis, a disorder primarily affecting older adults (3).
Figure 1.
Proportion of patients on transplant waiting lists, by age (1), for (A) kidney, (B) liver, (C) lung, and (D) heart.
Figure 2.
Transplant rates for adults on the waiting list, by age (1), for (A) kidney, (B) liver, (C) lung, and (D) heart.
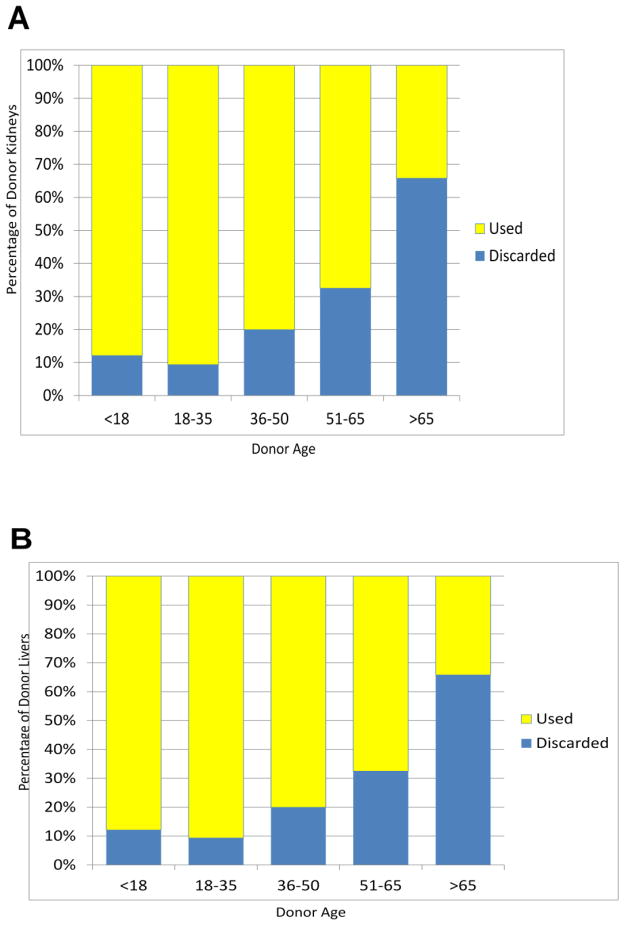
The average age of donor organs is also increasing, but the impact of older donor age on transplantation varies by organ. On the basis of data from old studies, the clinical decision to use “older” hearts for transplantation is rare. The number of heart donations remains flat overall, and the number of older donors remains small. In contrast, the number of lung and kidney donations is increasing (4), even as the proportion of donors older than 65 years remains flat. Lung transplant data suggest that donor organ age has minimal impact on short-term survival and that a combination of age and longer ischemic time (5), not donor age alone, is associated with inferior outcomes. Similarly, the outcomes for patients receiving older kidneys or livers are only slightly worse than those for patients receiving younger organs. Yet many transplantation centers still exclude donors aged 55 years and older, and particularly for kidney, organs from older donors are more likely to be discarded (Figure 3) (1) when their inclusion could expand the donor pool.
Figure 3.
Proportion of donor organs discarded, by age. (A) kidney, (B) liver. (Based on data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR)).
The likelihood of receiving organs from expanded criteria donors (ECD) increases with recipient age (6, 7). In addition, older donor age increases the likelihood that an organ will be classified as ECD (8), but for heart and lung donations, there are no common accepted definitions for ECD organs. Thus classification of ECD organs often depends on expert opinion and, in the case of the lung, on criteria unrelated to risk factors that affect post-transplantation outcomes. Moreover, despite evidence that some patients will benefit from ECD organs (9), and even though patients’ willingness to receive these organs can be influenced by careful presentation of evidence (10), the proportion of those patients who are willing to receive such organs is low, even among centers with longer waiting times (11).
Donor Organ Quality
Large studies, the majority of which have focused on kidney transplantation, have shown that organ quality declines with older donor age. The quantity and quality of nephrons in the donor kidney, and particularly the initial number of healthy glomeruli, are more important determinants of allograft survival than donor age. Techniques to accurately quantify the number of functioning glomeruli have not yet been established, and surrogate measures, such as body surface area (12) or kidney weight, are not useful or practical in the existing allocation system (13). Estimates from autopsy studies in individuals without known kidney disease suggest that on average, glomerular filtration rate and the number of functioning glomeruli decline with age (14). Estimates based on renal physiology and morphometry show similar numbers and trends for functioning glomeruli in living donors (15) but a significantly lower number in older deceased donors (16, 17) (Figure 3). Consistent with these observations, allograft survival is considerably better for living donor kidneys, even those from older donors (18). However, the rate of allograft failure is still excessive for older donor kidneys, compared with younger ones.
Injury to renal tubular epithelial cells following procurement and implantation (19, 20) might also affect donor kidney quality. The risk for ESRD following postischemic acute renal failure is substantial among older adults, particularly those with pre-existing chronic kidney disease, compared with younger patients (21). The mechanism of this injury is unknown, but evidence suggests that repair of epithelial cells is limited by cellular senescence, and atubular glomeruli have been observed in kidneys from older adults and in renal allografts with chronic allograft nephropathy (22). Thus in deceased donor allografts, the reduction of the number of nephrons below a critical threshold could render the allograft more susceptible to accelerated decline, and subsequent injury from rejection or drug toxicity could hasten complete allograft failure. Further study on the mechanism and prevention of such injuries could guide interventions to increase the longevity of allografts from older donors.
Organ Allocation
Organ allocation systems vary by specific organ and by programmatic tendencies. The Lung Allocation Score, which includes age as a variable, grades disease severity and physiologic reserve. The Model for End-Stage Liver Disease (MELD) predicts waitlist mortality but predicts post-transplant outcomes only at scores above 35. The likelihood of patients dying while waiting for a liver depends on the donor service area in which they reside. A regional sharing and prioritization system for MELD scores of 15 or more has received widespread support, and recent evidence (23) supports a similar system for MELD scores higher than 35. An allocation system that accounts for several predictors of post-transplant mortality and considers post-transplant lifetime relative to lifetime on the waitlist has also been proposed. However, this system is seen as too complicated and thus has mixed support.
Kidney allocation policy is based primarily on waiting time and local organ distribution, and it only minimally addresses potential outcomes and immunologic matching. Thus the potential survival of the donor organ and that of the recipient are often mismatched, increasing the need for re-transplant and decreasing the number of potential life-years realized from a transplant. Moreover, current kidney allocation does not consider recipient age, except for pediatric patients. Efforts to improve kidney allocation by age-matching donors and recipients have been controversial because of perceptions of age discrimination, and there is no evidence that age-matching improves outcomes (24). A newer model incorporates longevity matching, which includes age and other metrics for prediction, and replaces the current ECD system with a Kidney Donor Profile Index score (25), which accounts for several clinical and demographic factors, including donor age. With this model, calculation of waiting time includes the amount of time a patient has lived with ESRD. Simulations suggest that the number of older patients receiving kidneys will decrease by approximately 5%, based on a difference in average age between donors and recipients. However, allocation systems based more heavily on recipient functional status and prognostic variables, rather than age alone, are more likely to be acceptable to the public.
Recipient Selection and Management
Illness severity is often better than age in predicting post-operative complications. However, despite recent gains in access for older patients on the waiting list and evidence that older patients benefit from transplantation if their waiting time is shorter (26–29), older patients still have less access to transplantation than other age groups because they are not placed on the waiting list (30). Moreover, older patients who are referred for transplant often are highly selected, and they are less likely to receive an organ from a living donor (31). Comprehensive risk assessments, based on stronger predictors than age and accounting for end points such as independence and quality of life, might be needed to evaluate risk versus benefit for older recipients.
Transplant recipients are selected based on the likelihood of successful outcomes, and age is often used as a determinant. However, age is a surrogate for many other health and functional issues, and there is no specific predictive rule that examines most age-related variables to determine which patients will do well after a transplant. Thus, transplant physicians judge candidate suitability based on their clinical experience and a subjective assessment of physical fitness, or the so-called “eyeball test.” More objective measures of fitness have focused on lean trunk muscle size for liver transplantation and a 6-minute walk test for lung transplantation, and both of these factors correlate with post-transplant survival (32, 33). Among older patients undergoing elective surgery, an American Society of Anesthesiologists (ASA) risk score combined with a frailty assessment is more predictive of outcomes than the ASA score alone (34). In kidney transplant recipients, frailty is strongly associated with early allograft dysfunction (35). Thus evaluations of physical performance, which might better represent a patient’s physiologic age, might prove a more objective approach to recipient selection.
While physical function can be affected by organ failure and transplantation (36–39), it might also play a role in a recipient’s post-transplantation recovery time, risk for disability, cardiovascular health, and health-related quality of life (HRQOL). Dialysis patients reporting a higher level of physical function are at lower risk for post-transplantation hospitalizations and death (40), and post-transplantation gains in exercise capacity and muscle strength are higher with exercise than with usual care (41). To date, no exercise intervention trials have examined the potential benefits of pre-transplant exercise on post-transplant outcomes in ESRD patients.
For most solid organs, organ failure has been associated with cognitive impairment (42–47), and several potential mechanisms are supported by modest data (42, 48–54). Chronic organ failure has been associated with encephalopathy and dementia, especially in renal disease, and acute insult to the organ can cause delirium that, when recurrent, can lead to chronic encephalopathy and dementia. In addition, the risk for cognitive impairment increases with age. Cognitive impairment leads to medical non-adherence and thus to higher rejection rates (55–57). However, the use of cognitive impairment as an exclusion criterion for transplant varies by organ (58–61), and assessments are usually done only if dementia is suspected, rather than by formal, standard evaluation.
Psychosocial well-being improves after transplantation in most patients, but not to normative levels. For example, rates of emotional distress and psychiatric disorders are higher, and the rate of gainful employment lower, among transplant recipients, compared with the general population (62–65). Psychosocial well-being is routinely assessed to aid in decisions to list patients for transplants and to guide interventions to ameliorate psychosocial contraindications to transplant (59, 60, 66). Post-transplant medical adherence, mental health outcomes, and quality of life for older recipients are similar to, and occasionally better than, those for younger recipients (67–71). Nevertheless, little evidence suggests that age is a major predictor of post-transplant psychosocial outcomes, and few analyses of psychosocial well-being in transplant recipients have been stratified by age. A more complete understanding of age effects on psychosocial outcomes could aid the development of interventions tailored to recipient age.
Comorbidities might also influence outcomes. For example, post-operative survival is shorter among cancer patients who have two or more comorbidities when undergoing tumor resection (72). However, few data address the impact of comorbidities on post-transplantation outcomes, particularly in older patients. Thus pre-operative assessments of comorbidities vary across centers, and patients older than 70 with multiple serious comorbidities are unlikely to receive transplants unless their functional status is exceptional. Risk assessments based on comorbidities may be confounded by the primary organ failure that can be reversed with transplantation, but few studies have addressed reversibility by age.
Immunosuppression in the Older Transplant Recipient
Aging broadly influences diverse affects of immunity including exaggerated inflammation and altered innate immunity important in host defense (73, 74), but cell-mediated immunity is most clearly affected. A precipitous decline in the production of naïve T cells and a resulting decline in T-cell diversity have been observed in the older immune system. In addition, clonally expanded T-cells, particularly in the CD8 T-cell compartment, lead to a narrower repertoire and accumulation of “exhausted” and “senescent” T cells (75–77). These changes can be exacerbated by persistent viral infections such as cytomegalovirus, which re-stimulates memory T cells over a lifetime (78–80). Senescent T cells are marked by absent CD28 expression, increased CD2 expression, altered dependence on co-stimulation versus adhesion ligand-receptor interactions, and overproduction of pro-inflammatory cytokines (81). As a result, mechanisms underlying organ rejection likely differ between younger and older transplant recipients.
Consistent with this hypothesis, mouse studies suggest that co-stimulation blockade is less effective in older individuals (82). Further, while immune senescence generally impairs immune responses, the impact of acute rejection is more profound in older recipients (83,84). In addition, the risk for delayed graft function associated with acute rejection (85–87) is higher for older donor organs. Thus, the impact of the immunobiology of aging on transplantation deserves investigation, and immunosuppression protocols for older transplant recipients must balance the risk for acute rejection with the risk for poor cardiovascular, infectious, and/or metabolic outcomes.
Immunosuppression protocols also must account for age-related physiological changes that alter the pharmacokinetics, pharmacodynamics, and potential toxicity of immunosuppressive drugs (88). Therapeutic blood target ranges associated with efficacy and toxicity might be different in older recipients. In addition, because older recipients are more likely to have comorbidities and thus take multiple medications, they are at higher risk for adverse drug interactions. Studies exploring immunosuppressant pharmacokinetic disposition among older kidney recipients have yielded mixed results (89–91), and their sample sizes are too small for interpretation. Few studies have assessed antibody therapy in the older recipient. Biologic assays that measure overall immunosuppression would be useful.
Despite possible age-related immunologic and pharmacologic changes, immunosuppression protocols are typically similar regardless of age (92). However, risk stratification data suggest there is a link between IL-2RA and risk for cardiovascular death and that the impact of acute rejection is most profound when both donor and recipient are classified as high risk (92). Tailored immunosuppression strategies such as calcineurin inhibitor avoidance (93, 94) and mycophenolic acid withdrawal (95) are designed to reduce morbidity and might improve patient and graft survival. There are also likely to be important effects of age on autonomic disturbances (e.g. postural hypotension) and neuropsychiatric issues that could influence drug choices. The benefit of steroid avoidance is less clear (96) but perhaps more important in seniors in whom age-related bone loss, glucose intolerance, and other metabolic effects complicate steroid therapy. Moreover, previous studies are largely retrospective, single-center studies without controls, and they have not collected data on older patients.
Long-Term Outcomes
Although short-term outcomes are acceptable for older transplant recipients across organs, long-term outcomes differ by age (6, 97). Older donor organs also have been associated with inferior long-term outcomes (98), for example increased risk for graft loss.
Older adults are more susceptible than young adults to infections that are often more severe and arise from a wider range of pathogens. Among waitlisted patients, the risk for infection-related death increases with age (99). It is not clear which changes in immunity contribute to these risks, but immunosuppressive drugs further impair host defenses. Risk for death from infection increases exponentially with age among kidney transplant recipients (99), and among kidney and lung recipients older than 60 years, infection is the leading cause of increased mortality seen in the first post-operative year (100, 101). Yet no guidance exists to help clinicians prevent or manage infections in older transplant patients. Multiple factors reduce host defense in senior transplant recipients; vaccine efficacy decreases markedly with age (102), antibiotic prophylaxis in older patients likely leads to complications such as C. difficile infection or antimicrobial resistance in specific pathogens, and less virulent pathogens (e.g. non-vaccine serotypes of S. pneumoniae) cause disease more frequently in old than young adults (103).
Cardiovascular disease (CVD) is an important predictor of mortality in transplant recipients (104–106). Among kidney recipients, even in those screened for CVD before transplantation, left ventricular dysfunction and a patient’s Framingham Risk Score can predict cardiac events (105, 106). In non-transplant patients, traditional risk factors such as hyperlipidemia or smoking can predict post-operative cardiac risk, but coronary artery disease revealed by coronary imaging further increases that risk, even if the disease is not hemodynamically significant (107). However, cardiac assessments of transplant candidates focus only on their ability to survive the surgery itself. How best to predict long-term cardiac risk, particularly among patients with coronary disease, is not clear.
Cancer incidence increases with age in the general population. Overall, transplant recipients are at twice the age-adjusted risk for cancers; the risk is elevated for non-Hodgkin lymphoma and cancers of the lung and kidney, but not for breast or prostate cancer (108). Because the spectrum of cancers and the underlying pathways to cancer differ between transplant recipients and the general population (109), it is unlikely that transplantation simply accelerates age-related processes leading to cancer. It is unclear whether immunosuppression regimens affect the biology of a cancer, although some evidence suggests that cancers behave more aggressively in immunosuppressed patients. Immunosuppression could reduce the ability to clear early cancer precursors (109).
Although data on quality of life following transplantation are few, the quality-of-life benefit does not appear to differ between older and younger recipients (110, 111). In some cases, reported life satisfaction among recipients older than 60 years is actually higher (112, 113). Across organs, the most important aspects of HRQOL differ between older and younger recipients (114, 112, 115). However, the effects of age on post-transplant HRQOL are complex, whether in older patients receiving a transplant or in patients aging after a transplant. One study suggests an immediate drop, then rebound, in quality of life for donors (116), but more data are needed.
Health Disparities in Transplantation
Across all ages, ESRD incidence of end-stage renal disease is four times greater among racial and ethnic minorities (117), but Hispanics are less likely to receive pre-emptive transplants, and African Americans are less likely to be waitlisted and transplanted in general (118, 119). Compromised health literacy (120), increased likelihood of higher disease severity requiring emergency treatment (121), variable or suboptimal discussions of transplantation and living organ donations (122, 123), and an apparent hesitation on the part of minorities to donate organs (124–127) all contribute to this disparity. Racial and ethnic disparities in access to transplantation have improved somewhat with the aid of home-based educational approaches (128), changes in donor kidney allocation policy (129, 130), amended Medicare coverage rules, the establishment of the National Living Donor Assistance Center, and changes in provider reimbursements for patient education.
Little is known about racial and ethnic differences in long-term post-transplant outcomes (131). Across organs, graft and patient survival rates are highest for Asian and Hispanic recipients and lowest for African American recipients, with Non-Hispanic Whites in the middle. Among kidney recipients, the benefits of pre-emptive transplantation are observed predominantly among White recipients. Hepatitis C infection, a risk factor for graft failure, is seen at higher rates among African American recipients, and disparities in outcomes are confounded by issues of access to transplantation. However, adjustment for these factors does not completely explain the worse outcomes in African Americans. More work is needed to identify factors underlying disparities in outcome, and no specific data address age or functional status issues in minority versus non-minority transplant recipients.
Health Care Utilization and Cost
The full cost implications of transplanting older recipients or using organs from older donors are poorly defined. The financial impact of elderly candidates includes the incremental costs of additional evaluation, age-specific costs incurred in the short term following transplant, and longer-term costs that are related but more difficult to attribute to the transplant procedure. The financial impact of older donor organs includes costs related to discarded organs, longer-term organ non-function and dysfunction, and shorter graft survival. Cost-effectiveness analyses often emphasize quality-adjusted life years saved (QALYS), but it is not clear whether the comparator in such analyses is the younger donor or recipient or a therapeutic alternative to transplantation. Moreover, cost variations between centers for otherwise comparable patient populations arise from variations in practice, which are more likely to reflect institutional culture than patient needs. Selection biases against transplantation in older adults might reflect providers’ risk aversion rather than empirical evidence, which might become more important as regulators and payers focus more on patient outcomes. Moreover, analyses of cost- and comparative-effectiveness must also address the moral and ethical appropriateness of therapeutic decisions, especially as patient-centeredness and shared decision-making become more prevalent in health care delivery.
Despite these difficulties in analyzing cost-effectiveness data, a decision analysis of kidney transplantation in older adults (113) suggests that the incremental cost of a QALYS is sensitive to older age, length of time on the waitlist, and potential for resource utilization associated with co-morbidities and post-transplant complications. However, resource utilization among older donors and recipients is difficult to predict, and the few studies that have addressed it (132–135) have produced conflicting results or failed to compare predictors in older versus younger patients. As suggested by data from other high-intensity care scenarios (136, 137), the inclusion of geriatric assessments (138) can prove useful in decisions about transplantation in older adults, but whether these assessments can predict resource utilization among older recipients is not yet clear.
Future Research Directions
Although advanced age has been associated with compromised post-transplant outcomes, it is likely a proxy for stronger predictors, such as functional status, physiologic organ reserve, or comorbidity. There is a critical need to address basic science issues of aging in transplantation with the potential to shape clinical investigations and protocols, but rodent models of aging may not closely mimic human aging (139). Further, few clinical studies have directly explored transplantation in older patients, but further research in this population could guide the education, selection, and management of appropriate candidates.
Critical areas of research need are comprehensively listed in Table 1, but can be summarized into several broad themes:
Table 1.
Questions and Potential Datasets or Cohorts for Future Biomedical, Health Outcomes, and Public Policy Research
| Area | Question or Cohort |
|---|---|
| Organ Allocation |
|
| Reducing Health Disparities |
|
| Recipient Selection |
|
| Recipient Counseling |
|
| Immunobiology and Immunosuppression |
|
| Pre-, Peri-, and Postoperative Management |
|
| Outcomes of Transplantation |
|
| Organ Specific Research Needs | |
| Heart |
|
| Kidney |
|
| Lung |
|
| Liver |
|
| Datasets and Cohorts | |
| Organ Transplantation |
|
| Geriatric |
|
| Genomics/Biomarkers | Genomics of Transplantation Cooperative Research Program |
| Quality of Life | Database on Health-Related Quality of Life (Singer) |
What critical immune mechanisms that change with age and life-long viral infections (e.g. CMV, hepatitis C) differentially affect transplant outcomes, and how should these influence patient management?
Age serves as a surrogate for many other factors, such as functional status, body composition, or co-morbidity. What is the contribution of each, and how can that better guide selection of donors and recipients, organ allocation systems, and clinical management of transplant recipients?
What is the impact of transplantation and chronic immune suppression on the aging process in various organ systems? Does that alter prevention and treatment strategies?
How can functional outcomes, co-morbidity, life-expectancy, and patient-centered results be better incorporated into transplant programs and quality metrics to assess “success” in older patients?
Several datasets and cohorts are available to explore general and organ-specific questions about the role of age in issues of organ allocation, donor and recipient selection, long-term outcomes, and health disparities (Table 1). However, efforts to collect and test the role of novel risk predictors in older adults, particularly those not in registries, are critical, as existing registries lack the granularity required to move this field forward. Future research will require multidisciplinary collaborations, harmonization of existing databases with primary data collection, and a uniform language of variables to include in new studies. This research will also need measures that are reliable, reproducible, easy to use, and focused on end points valued by older recipients. Some of these measures could aid in predicting post-operative outcomes and in stratifying candidates based on anticipated risk.
Figure 4.
Changes in glomerular filtration rate (GFR) and glomerulare number, by age. (A) Changes in GFR (17). (B) Changes in glomerular number, estimated from autopsy studies (14). (C) Changes in glomerular number from deceased donors, estimated by combining renal physiology with morphometry from kidney biopsies, compared with estimates from autopsy studies (16). (D) Changes in glomerular number from living donors, estimated by combining renal physiology with morphometry from kidney biopsies (15).
Acknowledgments
This workshop was supported by generous grants to ASP from the National Institute on Aging (1 U13 AG040938-01) and the John A. Hartford Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIA or the National Institutes of Health. In addition, the views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
We are grateful to Nancy Woolard for her assistance with organizing the workshop. To see the agenda, a list of workshop moderators and attendees, and workshop presentations, please visit http://www.im.org/AcademicAffairs/Aging/IGP/ExpandingResearchEfforts/Pages/SolidOrganTransplantation.aspx.
Abbreviations
- AGS
American Geriatrics Society
- ASA
American Society of Anesthesiologists
- ASP
Association of Specialty Professors
- CVD
cardiovascular disease
- ECD
expanded criteria donor
- ESRD
end-stage renal disease
- HRQOL
health-related quality of life
- IPF
idiopathic pulmonary fibrosis
- MELD
Model for End-stage Liver Disease
- NHLBI
National Heart, Lung, and Blood Institute
- NIA
National Institute on Aging
- NIAID
National Institute of Allergy and Infectious Diseases
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- QALYS
quality-adjusted life-year saved
Footnotes
Workshop speakers were:
Michael Abecassis, M.D., M.B.A., Northwestern University Feinberg School of Medicine
Cornelius J. Clancy, M.D., University of Pittsburgh
Mary Amanda Dew, Ph.D., University of Pittsburgh
Eric A. Engels, M.D., M.P.H., National Cancer Institute
Michael J. Englesbe, M.D., University of Michigan Medical School
John Friedewald, M.D., Northwestern University
Jagbir Gill, M.D., University of British Columbia
Erica L. Hartmann, M.D., Piedmont Transplant Center
Kevin P. High, Wake Forest School of Medicine
Jeffrey Hosenpud, M.D., Mayo Clinic
Pamela Jacobson, Pharm.D., University of Minnesota
Bertram Kasiske, M.D., University of Minnesota
John Lake, M.D., University of Minnesota
Preeti N. Malani, M.D., University of Michigan Medical School, VA Ann Arbor Healthcare System
Anne M. Murray, M.D., M.Sc., University of Minnesota
Minh-Hong Nguyen, M.D., University of Pittsburgh
Neil R. Powe, M.D., University of California, San Francisco
Peter P. Reese, M.D., M.S.C.E., University of Pennsylvania
Dorry L. Segev, M.D., Ph.D., Johns Hopkins University
Lianne G. Singer, M.D., University of Toronto, Toronto General Hospital
Jane C. Tan, M.D., Stanford University
Winfred W. Williams, M.D., Harvard University
David W. Zaas, M.D., Duke University School of Medicine
Disclosure
The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Annual Data Report. 2011. Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. OPTN/SRTR 2010. [Google Scholar]
- 2.Schaeffner ES, Rose C, Gill JS. Access to kidney transplantation among the elderly in the United States: a glass half full, not half empty. Clin J Am Soc Nephrol. 2010;5:2109–2114. doi: 10.2215/CJN.03490410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of United Network for Organ Sharing data. J Am Coll Surg. 2009;208:400–409. doi: 10.1016/j.jamcollsurg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, McBride MA, Montgomery RA. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Waddell TK, Shargall Y, Pierre AF, Fadel E, Uy K, Chaparro C, Hutcheon M, Singer LG, Keshavjee S. Impact of donors aged 60 years or more on outcome after lung transplantation: results of an 11-year single-center experience. J Thorac Cardiovasc Surg. 2007;133:525–531. doi: 10.1016/j.jtcvs.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Huang E, Poommipanit N, Sampaio MS, Kuo HT, Reddy P, Gritsch HA, Pham PT, Wilkinson A, Danovitch G, Bunnapradist S. Intermediate-term outcomes associated with kidney transplantation in recipients 80 years and older: an analysis of the OPTN/UNOS database. Transplantation. 2010;90:974–979. doi: 10.1097/TP.0b013e3181f5c3bf. [DOI] [PubMed] [Google Scholar]
- 7.Segev DL. Evaluating options for utility-based kidney allocation. Am J Transplant. 2009;9:1513–1518. doi: 10.1111/j.1600-6143.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 8.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 9.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Reese PP, Tehrani T, Lim MA, Asch DA, Blumberg EA, Simon MK, Bloom RD, Halpern SD. Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection. Clin J Am Soc Nephrol. 2010;5:917–923. doi: 10.2215/CJN.08251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grams ME, Womer KL, Ugarte RM, Desai NM, Montgomery RA, Segev DL. Listing for expanded criteria donor kidneys in older adults and those with predicted benefit. Am J Transplant. 2010;10:802–809. doi: 10.1111/j.1600-6143.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasiske BL, Snyder JJ, Gilbertson D. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol. 2002;13:2152–2159. doi: 10.1097/01.asn.0000024564.22119.3d. [DOI] [PubMed] [Google Scholar]
- 13.Giral M, Foucher Y, Karam G, Labrune Y, Kessler M, de Ligny BH, Buchler M, Bayle F, Meyer C, Trehet N, Daguin P, Renaudin K, Moreau A, Soulillou JP. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol. 2010;21:1022–1029. doi: 10.1681/ASN.2009121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 15.Tan JC, Busque S, Workeneh B, Ho B, Derby G, Blouch KL, Sommer FG, Edwards B, Myers BD. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78:686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan JC, Workeneh B, Busque S, Blouch K, Derby G, Myers BD. Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephrol. 2009;20:181–188. doi: 10.1681/ASN.2008030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, Lemley KV, Myers BD. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003;64:1417–1424. doi: 10.1046/j.1523-1755.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang P, Gill J, Dong J, Rose C, Yan H, Landsberg D, Cole EH, Gill JS. Living Donor Age and Kidney Allograft Half-Life: Implications for Living Donor Paired Exchange Programs. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.09990911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Dafoe D, Alfrey E, Myers BD. Backleak, tight junctions, and cell- cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon O, Corrigan G, Myers BD, Sibley R, Scandling JD, Dafoe D, Alfrey E, Nelson WJ. Sodium reabsorption and distribution of Na+/K+-ATPase during postischemic injury to the renal allograft. Kidney Int. 1999;55:963–975. doi: 10.1046/j.1523-1755.1999.055003963.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagtalunan ME, Oberbauer R, Haas M, Barlan M, Mayer G, Olson JL, Meyer TW. Atubular glomeruli in patients with chronic allograft rejection. Transplantation. 1996;61:1166–1171. doi: 10.1097/00007890-199604270-00008. [DOI] [PubMed] [Google Scholar]
- 23.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasiske BL, Snyder J. Matching older kidneys with older patients does not improve allograft survival. J Am Soc Nephrol. 2002;13:1067–1072. doi: 10.1681/ASN.V1341067. [DOI] [PubMed] [Google Scholar]
- 25.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 26.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 27.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945–951. doi: 10.1093/ndt/gfh022. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DW, Herzig K, Purdie D, Brown AM, Rigby RJ, Nicol DL, Hawley CM. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69:794–799. doi: 10.1097/00007890-200003150-00020. [DOI] [PubMed] [Google Scholar]
- 29.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol. 2006;1:532–538. doi: 10.2215/CJN.01130905. [DOI] [PubMed] [Google Scholar]
- 30.Grams ME, Kucirka LM, Hanrahan CF, Montgomery RA, Massie AB, Segev DL. Candidacy for kidney transplantation of older adults. J Am Geriatr Soc. 2012;60:1–7. doi: 10.1111/j.1532-5415.2011.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng FL, Reese PP, Mulgaonkar S, Patel AM. Barriers to living donor kidney transplantation among black or older transplant candidates. Clin J Am Soc Nephrol. 2010;5:2338–2347. doi: 10.2215/CJN.03040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, Vargas HE, Douglas DD. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 34.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, James NT, Kucirka LM, Hall EC, Berger JC, Montgomery RA, Desai NM, Dagher NN, Sonnenday CJ, Englesbe MJ, Makary MA, Walston JD, Segev DL. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 36.Bohannon RW, Hull D, Palmeri D. Muscle strength impairments and gait performance deficits in kidney transplantation candidates. Am J Kidney Dis. 1994;24:480–485. doi: 10.1016/s0272-6386(12)80905-x. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann EL, Kitzman D, Rocco M, Leng X, Klepin H, Gordon M, Rejeski J, Berry M, Kritchevsky S. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Painter P, Hanson P, Messer-Rehak D, Zimmerman SW, Glass NR. Exercise tolerance changes following renal transplantation. Am J Kidney Dis. 1987;10:452–456. doi: 10.1016/s0272-6386(87)80192-0. [DOI] [PubMed] [Google Scholar]
- 39.Painter PL, Luetkemeier MJ, Moore GE, Dibble SL, Green GA, Myll JO, Carlson LL. Health-related fitness and quality of life in organ transplant recipients. Transplantation. 1997;64:1795–1800. doi: 10.1097/00007890-199712270-00029. [DOI] [PubMed] [Google Scholar]
- 40.Kutner NG, Zhang R, Bowles T, Painter P. Pretransplant physical functioning and kidney patients’ risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin J Am Soc Nephrol. 2006;1:837–843. doi: 10.2215/CJN.01341005. [DOI] [PubMed] [Google Scholar]
- 41.Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, Tomlanovich SL, Ascher NL. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74:42–48. doi: 10.1097/00007890-200207150-00008. [DOI] [PubMed] [Google Scholar]
- 42.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Pegum N, Connor JP, Feeney GF, Young RM. Neuropsychological functioning in patients with alcohol-related liver disease before and after liver transplantation. Transplantation. 2011;92:1371–1377. doi: 10.1097/TP.0b013e3182375881. [DOI] [PubMed] [Google Scholar]
- 44.DiMartini A, Dew MA, Crone K. Organ transplant. In: Sadock V, Sadock B, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 45.O’Carroll RE, Turner F, Flatley K, McGregor LM, Hayes PC. Functional outcome following liver transplantation - a pilot study. Psychol Health Med. 2008;13:239–248. doi: 10.1080/13548500701403363. [DOI] [PubMed] [Google Scholar]
- 46.Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739–745. doi: 10.1002/hep.20095. [DOI] [PubMed] [Google Scholar]
- 47.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- 49.Bennett SJ, Sauve MJ. Cognitive deficits in patients with heart failure: a review of the literature. J Cardiovasc Nurs. 2003;18:219–242. doi: 10.1097/00005082-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray AM, Barzilay JI, Lovato JF, Williamson JD, Miller ME, Marcovina S, Launer LJ. Biomarkers of renal function and cognitive impairment in patients with diabetes. Diabetes Care. 2011;34:1827–1832. doi: 10.2337/dc11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurella TM. To predict dementia, should we be mindful of the kidneys? Clin J Am Soc Nephrol. 2011;6:1232–1234. doi: 10.2215/CJN.03390411. [DOI] [PubMed] [Google Scholar]
- 53.Kurella TM, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, Messe SR, Sehgal AR, Kusek J, DeSalvo KB, Cornish-Zirker D, Cohan J, Seliger SL, Chertow GM, Go AS. Vascular risk factors and cognitive impairment in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6:248–256. doi: 10.2215/CJN.02660310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurella TM, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22. doi: 10.1038/ki.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant. 1999;18:549–562. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 56.de Geest S, Borgermans L, Gemoets H, Abraham I, Vlaminck H, Evers G, Vanrenterghem Y. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation. 1995;59:340–347. [PubMed] [Google Scholar]
- 57.Paris W, Muchmore J, Pribil A, Zuhdi N, Cooper DK. Study of the relative incidences of psychosocial factors before and after heart transplantation and the influence of posttransplantation psychosocial factors on heart transplantation outcome. J Heart Lung Transplant. 1994;13:424–430. [PubMed] [Google Scholar]
- 58.Barbour KA, Blumenthal JA, Palmer SM. Psychosocial issues in the assessment and management of patients undergoing lung transplantation. Chest. 2006;129:1367–1374. doi: 10.1378/chest.129.5.1367. [DOI] [PubMed] [Google Scholar]
- 59.Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70:771–783. doi: 10.1037//0022-006x.70.3.771. [DOI] [PubMed] [Google Scholar]
- 60.Dew MA, Switzer GE, DiMartini AF, Matukaitis J, Fitzgerald MG, Kormos RL. Psychosocial assessments and outcomes in organ transplantation. Prog Transplant. 2000;10:239–259. doi: 10.1177/152692480001000408. [DOI] [PubMed] [Google Scholar]
- 61.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, Rush DN, Vazquez MA, Weir MR. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1 (Suppl 2):3–95. [PubMed] [Google Scholar]
- 62.Dew MA, DiMartini AF, vito Dabbs AJ, Fox KR, Myaskovsky L, Posluszny DM, Switzer GE, Zomak RA, Kormos RL, Toyoda Y. Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psychiatry. 2012;34:127–138. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DiMartini A, Sotelo J, Dew M. Organ transplantation. In: Levenson J, editor. The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. Washington, DC: American Psychiatric Publishing, Inc; 2010. [Google Scholar]
- 64.Paris W, White-Williams C. Social adaptation after cardiothoracic transplantation: a review of the literature. J Cardiovasc Nurs. 2005;20:S67–S73. doi: 10.1097/00005082-200509001-00008. [DOI] [PubMed] [Google Scholar]
- 65.van der Mei SF, Krol B, van Son WJ, de Jong PE, Groothoff JW, van den Heuvel WJ. Social participation and employment status after kidney transplantation: a systematic review. Qual Life Res. 2006;15:979–994. doi: 10.1007/s11136-006-0045-5. [DOI] [PubMed] [Google Scholar]
- 66.Dew MA, DiMartini AF. Transplantation. In: Friedman H, editor. Oxford Handbook of Health Psychology. New York: Oxford University Press; 2011. [Google Scholar]
- 67.Ortega T, Deulofeu R, Salamero P, Lauzurica R, Casanovas T, Cofan F, Nobel L, Jane L, Twose J, Ortega F. Perceived state of health is worse in kidney recipients younger than 60 years vs older than 60 years. Transplant Proc. 2009;41:2118–2121. doi: 10.1016/j.transproceed.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Dew MA, DiMartini AF, De Vito DA, Myaskovsky L, Steel J, Unruh M, Switzer GE, Zomak R, Kormos RL, Greenhouse JB. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 69.Dew MA, DiMartini AF, Steel J, De Vito DA, Myaskovsky L, Unruh M, Greenhouse J. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl. 2008;14:159–172. doi: 10.1002/lt.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dew MA, et al. Gen Hosp Psychiatry. in press. [Google Scholar]
- 71.Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8:263–270. doi: 10.1053/jlts.2002.31345. [DOI] [PubMed] [Google Scholar]
- 72.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 73.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6:446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 76.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith C, Miles JJ, Khanna R. Advances in direct T-cell alloreactivity: function, avidity, biophysics and structure. Am J Transplant. 2012;12:15–26. doi: 10.1111/j.1600-6143.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 78.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Nikolich-Zugich J. High specificity, not degeneracy, allows T cell alloresponses. Nat Immunol. 2007;8:335–337. doi: 10.1038/ni0407-335. [DOI] [PubMed] [Google Scholar]
- 80.Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–57. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, Kirk AD. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Du W, Shen H, Galan A, Goldstein DR. An age-specific CD8+ T cell pathway that impairs the effectiveness of strategies to prolong allograft survival. J Immunol. 2011;187:3631–3640. doi: 10.4049/jimmunol.1100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meier-Kriesche HU, Srinivas TR, Kaplan B. Interaction between acute rejection and recipient age on long-term renal allograft survival. Transplant Proc. 2001;33:3425–3426. doi: 10.1016/s0041-1345(01)02477-0. [DOI] [PubMed] [Google Scholar]
- 84.Heldal K, Hartmann A, Leivestad T, Svendsen MV, Foss A, Lien B, Midtvedt K. Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation. 2009;87:1045–1051. doi: 10.1097/TP.0b013e31819cdddd. [DOI] [PubMed] [Google Scholar]
- 85.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, Kim SJ. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pratschke J, Merk V, Reutzel-Selke A, Pascher A, Denecke C, Lun A, Said A, Schonemann C, Ulrich F, Reinke P, Frei U, Neuhaus P, Tullius SG. Potent early immune response after kidney transplantation in patients of the European senior transplant program. Transplantation. 2009;87:992–1000. doi: 10.1097/TP.0b013e31819ca0d7. [DOI] [PubMed] [Google Scholar]
- 87.de Fijter JW, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, Paul LC. Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol. 2001;12:1538–1546. doi: 10.1681/ASN.V1271538. [DOI] [PubMed] [Google Scholar]
- 88.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84:285–291. doi: 10.1097/01.tp.0000275423.69689.dc. [DOI] [PubMed] [Google Scholar]
- 89.Wang CX, Meng FH, Chen LZ, Ren B, Li SX, Fei JG, Qiu J, Deng SX, Li J, Chen SY. Population pharmacokinetics of mycophenolic acid in senile Chinese kidney transplant recipients. Transplant Proc. 2007;39:1392–1395. doi: 10.1016/j.transproceed.2007.02.082. [DOI] [PubMed] [Google Scholar]
- 90.Miura M, Satoh S, Kagaya H, Saito M, Inoue T, Tsuchiya N, Suzuki T, Habuchi T. No impact of age on dose-adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur J Clin Pharmacol. 2009;65:1047–1053. doi: 10.1007/s00228-009-0721-9. [DOI] [PubMed] [Google Scholar]
- 91.Staatz CE, Tett SE. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs Aging. 2005;22:541–557. doi: 10.2165/00002512-200522070-00001. [DOI] [PubMed] [Google Scholar]
- 92.Gill J, Sampaio M, Gill JS, Dong J, Kuo HT, Danovitch GM, Bunnapradist S. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6:1168–1178. doi: 10.2215/CJN.07540810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arbogast H, Huckelheim H, Schneeberger H, Illner WD, Tarabichi A, Fertmann J, Wimmer CD, Hillebrand GF, Mistry-Burchardi N, Thomae R, Acikgoz A, Land W. A calcineurin antagonist-free induction/maintenance strategy for immunosuppression in elderly recipients of renal allografts from elderly cadaver donors: long-term results from a prospective single centre trial. Clin Transplant. 2005;19:309–315. doi: 10.1111/j.1399-0012.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 94.Guba M, Rentsch M, Wimmer CD, Uemueksuez A, Illner WD, Schonermarck U, Land WG, Jauch KW, Arbogast H. Calcineurin-inhibitor avoidance in elderly renal allograft recipients using ATG and basiliximab combined with mycophenolate mofetil. Transpl Int. 2008;21:637–645. doi: 10.1111/j.1432-2277.2008.00658.x. [DOI] [PubMed] [Google Scholar]
- 95.Meier M, Bode W, Nitschke M, Wong W, Ison M, Kramer J, Jabs W, Burk C, Lehnert H, Fricke L. High rejection rates with low dose immunosuppression in old for old kidney transplantation. Transplantationsmedizin. 2011;23:118–126. [Google Scholar]
- 96.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van VP. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–577. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 97.Berger JC, Muzaale AD, James N, Hoque M, Wang JM, Montgomery RA, Massie AB, Hall EC, Segev DL. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin J Am Soc Nephrol. 2011;6:2887–2893. doi: 10.2215/CJN.04160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Demers P, Moffatt S, Oyer PE, Hunt SA, Reitz BA, Robbins RC. Long-term results of heart transplantation in patients older than 60 years. J Thorac Cardiovasc Surg. 2003;126:224–231. doi: 10.1016/s0022-5223(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 99.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 100.Gutierrez C, Al-Faifi S, Chaparro C, Waddell T, Hadjiliadis D, Singer L, Keshavjee S, Hutcheon M. The effect of recipient’s age on lung transplant outcome. Am J Transplant. 2007;7:1271–1277. doi: 10.1111/j.1600-6143.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 101.Cameron JS. Renal transplantation in the elderly. Int Urol Nephrol. 2000;32:193–201. doi: 10.1023/a:1007122322571. [DOI] [PubMed] [Google Scholar]
- 102.High KP, D’Aquila RT, Fuldner RA, Gerding DN, Halter JB, Haynes L, Hazzard WR, Jackson LA, Janoff E, Levin MJ, Nayfield SG, Nichol KL, Prabhudas M, Talbot HK, Clayton CP, Henderson R, Scott CM, Tarver ED, Woolard NF, Schmader KE. Workshop on immunizations in older adults: identifying future research agendas. J Am Geriatr Soc. 2010;58:765–776. doi: 10.1111/j.1532-5415.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 103.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 104.Gelson W, Hoare M, Dawwas MF, Vowler S, Gibbs P, Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240–1244. doi: 10.1097/TP.0b013e31821841ba. [DOI] [PubMed] [Google Scholar]
- 105.Fabrizii V, Winkelmayer WC, Klauser R, Kletzmayr J, Saemann MD, Steininger R, Kramar R, Horl WH, Kovarik J. Patient and graft survival in older kidney transplant recipients: does age matter? J Am Soc Nephrol. 2004;15:1052–1060. doi: 10.1097/01.asn.0000120370.35927.40. [DOI] [PubMed] [Google Scholar]
- 106.Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004;66:441–447. doi: 10.1111/j.1523-1755.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 107.Russo V, Zavalloni A, Bacchi Reggiani ML, Buttazzi K, Gostoli V, Bartolini S, Fattori R. Incremental prognostic value of coronary CT angiography in patients with suspected coronary artery disease. Circ Cardiovasc Imaging. 2010;3:351–359. doi: 10.1161/CIRCIMAGING.109.880625. [DOI] [PubMed] [Google Scholar]
- 108.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 110.Rebollo P, Ortega F, Baltar JM, varez-Ude F, Alvarez NR, varez-Grande J. Is the loss of health-related quality of life during renal replacement therapy lower in elderly patients than in younger patients? Nephrol Dial Transplant. 2001;16:1675–1680. doi: 10.1093/ndt/16.8.1675. [DOI] [PubMed] [Google Scholar]
- 111.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 112.Grady KL, Naftel DC, White-Williams C, Bellg AJ, Young JB, Pelegrin D, Patton-Schroeder K, Kobashigawa J, Chait J, Kirklin JK, Piccione W, Jr, McLeod M, Heroux A. Predictors of quality of life at 5 to 6 years after heart transplantation. J Heart Lung Transplant. 2005;24:1431–1439. doi: 10.1016/j.healun.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 113.Siegal B, Halbert RJ, McGuire MJ. Life satisfaction among kidney transplant recipients: demographic and biological factors. Prog Transplant. 2002;12:293–298. doi: 10.1177/152692480201200410. [DOI] [PubMed] [Google Scholar]
- 114.Rosenberger J, van Dijk JP, Nagyova I, Roland R, Geckova AM, van den Heuvel WJ, Groothoff JW. Do dialysis- and transplantation-related medical factors affect perceived health status? Nephrol Dial Transplant. 2005;20:2153–2158. doi: 10.1093/ndt/gfh965. [DOI] [PubMed] [Google Scholar]
- 115.Kugler C, Fischer S, Gottlieb J, Tegtbur U, Welte T, Goerler H, Simon A, Haverich A, Strueber M. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transplant. 2007;21:590–596. doi: 10.1111/j.1399-0012.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 116.Minnee RC, Bemelman WA, Polle SW, van Koperen PJ, Ter MS, Donselaar-van der Pant KA, Bemelman FJ, Idu MM. Older living kidney donors: surgical outcome and quality of life. Transplantation. 2008;86:251–256. doi: 10.1097/TP.0b013e31817789dd. [DOI] [PubMed] [Google Scholar]
- 117.U.S. Renal Data System. USRDS 2009 annual data report: Atlas of chronic kidney disease and end-stage renal disease. 2009. [Google Scholar]
- 118.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–44. 2. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu CY. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4:195–200. doi: 10.2215/CJN.03290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sheu J, Ephraim P, Powe N, Rabb H, Senga M, Evans K, Jaar B, Crews D, Greer R, Boulware L. Qual Health Res. doi: 10.1177/1049732312443427. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 123.Boulware LE, Meoni LA, Fink NE, Parekh RS, Kao WH, Klag MJ, Powe NR. Preferences, knowledge, communication and patient-physician discussion of living kidney transplantation in African American families. Am J Transplant. 2005;5:1503–1512. doi: 10.1111/j.1600-6143.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 124.U.S. Renal Data System. USRDS 2007 annual data report: Atlas of chronic kidney disease and end-stage renal diseaes in the United States. 2007. [Google Scholar]
- 125.Boulware LE, Ratner LE, Cooper LA, Sosa JA, LaVeist TA, Powe NR. Understanding disparities in donor behavior: race and gender differences in willingness to donate blood and cadaveric organs. Med Care. 2002;40:85–95. doi: 10.1097/00005650-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 126.Boulware LE, Ratner LE, Sosa JA, Cooper LA, LaVeist TA, Powe NR. Determinants of willingness to donate living related and cadaveric organs: identifying opportunities for intervention. Transplantation. 2002;73:1683–1691. doi: 10.1097/00007890-200205270-00029. [DOI] [PubMed] [Google Scholar]
- 127.Edwards TM, Essman C, Thornton JD. Assessing racial and ethnic differences in medical student knowledge, attitudes and behaviors regarding organ donation. J Natl Med Assoc. 2007;99:131–137. [PMC free article] [PubMed] [Google Scholar]
- 128.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. A randomized trial of a home-based educational approach to increase live donor kidney transplantation: effects in blacks and whites. Am J Kidney Dis. 2008;51:663–670. doi: 10.1053/j.ajkd.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 129.Hall EC, Massie AB, James NT, Garonzik Wang JM, Montgomery RA, Berger JC, Segev DL. Effect of eliminating priority points for HLA-B matching on racial disparities in kidney transplant rates. Am J Kidney Dis. 2011;58:813–816. doi: 10.1053/j.ajkd.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 130.Ashby VB, Port FK, Wolfe RA, Wynn JJ, Williams WW, Roberts JP, Leichtman AB. Transplanting kidneys without points for HLA-B matching: consequences of the policy change. Am J Transplant. 2011;11:1712–1718. doi: 10.1111/j.1600-6143.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 131.Fan P-Y, Ashby V, Fuller D, Boulware LE, Kao A, Norman S, Randall H, Young C, Kalbfleisch J, Leichtman A. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10 (Part 2):1090–1107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Showstack J, Katz PP, Lake JR, Brown RS, Jr, Dudley RA, Belle S, Wiesner RH, Zetterman RK, Everhart J. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. NIDDK Liver Transplantation Database Group. JAMA. 1999;281:1381–1386. doi: 10.1001/jama.281.15.1381. [DOI] [PubMed] [Google Scholar]
- 133.Shankar N, AlBasheer M, Marotta P, Wall W, McAlister V, Chandok N. Do older patients utilize excess health care resources after liver transplantation? Ann Hepatol. 2011;10:477–481. [PubMed] [Google Scholar]
- 134.Aloia TA, Knight R, Gaber AO, Ghobrial RM, Goss JA. Analysis of liver transplant outcomes for United Network for Organ Sharing recipients 60 years old or older identifies multiple model for end-stage liver disease-independent prognostic factors. Liver Transpl. 2010;16:950–959. doi: 10.1002/lt.22098. [DOI] [PubMed] [Google Scholar]
- 135.Morrissey PE, Yango AF. Renal transplantation: older recipients and donors. Clin Geriatr Med. 2006;22:687–707. doi: 10.1016/j.cger.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 136.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, Parodi S, Dal LD, Gioia F, Monfardini S, Aapro MS, Serraino D, Zagonel V. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 138.Kniepeiss D, Wagner D, Pienaar S, Thaler HW, Porubsky C, Tscheliessnigg KH, Roller RE. Solid organ transplantation: technical progress meets human dignity a review of the literature considering elderly patients’ health related quality of life following transplantation. Ageing Res Rev. 2012;11:181–187. doi: 10.1016/j.arr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 139.High KP, Akbar AN, Nikolich-Zugich J. Translational research in immune senescence: Assessing the relevance of current models. Semin Immunol. 2012 doi: 10.1016/j.smim.2012.04.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]