Abstract
Increasingly, US-sponsored research is carried out in developing countries, but how US Institutional Review Boards (IRBs) approach the challenges they then face is unclear.
METHODS
I conducted in-depth interviews of 2 hours each, with 46 IRB chairs, directors, administrators and members. I contacted the leadership of 60 IRBs in the United States (US) (every fourth one in the list of the top 240 institutions by National Institutes of Health (NIH) funding), and interviewed IRB leaders from 34 (55%).
RESULTS
US IRBs face ethical and logistical challenges in interpreting and applying principles and regulations in developing countries, given economic and health disparities, and limited contextual knowledge. These IRBs perceive wide variations in developing world IRBs/RECs’ quality, resources and training; and health systems in some countries may have long-standing practices of corruption. These IRBs often know little of local contexts, regulations and standards of care, and struggle with understandings of other cultures’ differing views of autonomy, and risks and benefits of daily life. US IRBs thus face difficult decisions, including how to interpret principles, how much to pay subjects and how much sustainability to require from researchers. IRB responses and solutions include trying to maintain higher standards for developing world research, obtain cultural expertise, build IRB infrastructure abroad, communicate with foreign IRBs, ‘negotiate’ for maximum benefits for participants and fearing ‘worst-case scenarios’.
CONCLUSIONS
US IRBs confront a series of tensions and dilemmas in reviewing developing world research. These data have important implications for increased education of IRBs/RECs and researchers in the US and abroad, and for research and practice.
Keywords: bioethics, empirical ethics, informed consent, research ethics, investigators, research protocols, developing world bioethics
INTRODUCTION
Increasingly, US-sponsored research is carried out abroad, raising numerous challenges for Institutional Review Boards (IRBs) and Research Ethics Committees (RECs) in both sponsor and host countries. Clinical trials are far cheaper to conduct in the developing world, and epidemics of HIV/AIDS, malaria and TB occur predominantly overseas, and are receiving more attention from wealthier countries. Yet US IRBs thus confront critical ethical and logistical questions, and how they do so is unclear.
In the developing world, IRBs and RECs (note: the term ‘IRB’ is used below to refer to both IRBs and RECs) are growing in numbers, and are now located in 113 countries,1 but often lack resources and training.2 Of African nations, one-third do not have a national IRB.3 Questions arise about the background and education of members. In Latin America,4 Tanzania,5 and South Africa,6 most IRB members are male. With informed consent in the developing world, problems emerge related to participants’ diminished autonomy, coercion, linguistic barriers, comprehension and communitarian values.7 Different cultures may hold varying conceptions of ‘selfhood’, ‘autonomy’ and ‘cures’.8 Of IRB members, those in the US are more likely than those in South African RECs to think that subjects in the developing world understand informed consent forms, risks and benefits and placebos.9
Research has begun to explore issues raised by research that is funded by the West but conducted in the developing world, but many questions remain. Particularly, it is not wholly clear whether US IRBs are aware of these issues, and how they view and respond to them. A few studies have examined logistical issues IRBs confront,10 but what problems US IRBs confront in reviewing research conducted in the developing world, and how they view and address these, remains uncharted.
I recently conducted an in-depth semi-structured interview study of views and approaches among IRB chairs, administrators and members toward research integrity (RI) and related issues concerning research conducted in the US and elsewhere. The study aimed to understand how US IRBs viewed and approached RI, which these participants defined very broadly, and why. Interviewees revealed how they defined, viewed and addressed integrity in research.11 They also revealed how they responded to violations of, and threats to, RI in a wide variety of ways, related to how they saw and approached the nature, roles and responsibilities of IRBs; interpreted and applied regulations and principles; and viewed and interacted with researchers and others. The interviews shed important light on many other, related issues concerning IRBs’ interpretations of regulations,12 conflicts of interest,13 use of so-called “community members,”14 and views of centralized IRBs.15 Interviewees also varied in how they responded to issues that arose with principal investigators (PIs) and IRBs/RECs in both the US and the developing world, and tried to improve these relationships. Since the study used qualitative methods, it allowed for further detailed explorations of these domains, shedding light on these issues.
METHODS
As described elsewhere,16 I conducted in-depth interviews of 2 hours each with 46 IRB chairs, directors, administrators and members. I contacted the leadership of 60 US IRBs around the country, representing every fourth one in the list of the top 240 institutions by NIH funding, that the NIH has made publicly available,17 and interviewed IRB leaders from 34 of these institutions (response rate = 55%). In some cases, I interviewed both a chair/director and an administrator from an institution (e.g. if the chair thought that the administrator might be better able to answer certain questions). From these 34 institutions, I thus interviewed a total of 39 chairs/directors and administrators. The institutions varied in location, size and public/private status. Inclusion of IRBs from a wide range of institutions allows for illumination of the roles of different social and institutional contexts on these issues. I also asked half of these leaders (every other one on the list by amount of NIH funding) to distribute information about the study to members of their IRBs, in order to recruit one member of each of these IRBs to be interviewed for the study as well. Thus, in addition to the 39 chairs/directors and administrators, I interviewed seven other members (six regular members and one community member) as well.
As summarized in Table I, these 46 individuals include 28 chairs/co-chairs; 10 administrators (including two directors of compliance offices); and seven members. In all, 27 were male and 19 were female. One was Asian/Pacific Islander, while the remaining 43 were Caucasian. Twenty one came from institutions in the Northeast, six from the Midwest, 13 from the West, and six from the South. From institutions ranked 1-50, 51-100, 101-150, 151-200, 201-250 in NIH funding, the number of interviewees were 13, 13, 7, 1, and, 12, respectively.
Table I.
Characteristics of the Sample
| Total | % (N=46) | |
|---|---|---|
| Type of IRB Staff | ||
| Chairs/Co-Chairs | 28 | 60.87% |
| Directors | 1 | 2.17% |
| Administrators | 10 | 21.74% |
| Members | 7 | 15.22% |
| Gender | ||
| Male | 27 | 58.70% |
| Female | 19 | 41.30% |
| Institution Rank | ||
| 1-50 | 13 | 28.26% |
| 51-100 | 13 | 28.26% |
| 101-150 | 7 | 15.22% |
| 151-200 | 1 | 2.17% |
| 201-250 | 12 | 26.09% |
| State vs. Private | ||
| State | 19 | 41.30% |
| Private | 27 | 58.70% |
| Region | ||
| Northeast | 21 | 45.65% |
| Midwest | 6 | 13.04% |
| West | 13 | 28.26% |
| South | 6 | 13.04% |
| Total # of Institutions Represented | 34 | |
The interviews focused on participants’ views of integrity of research, broadly defined, and on IRB responses to problems in these realms. The interviews shed important light on many other, related issues as well, and allowed for exploration of these domains in further depth. Relevant sections of the interview guide are attached (see Appendix A), through which we sought to obtain detailed descriptions of the above issues. From a theoretical standpoint, Geertz18 has advocated studying aspects of individuals’ lives, decisions and social situations not by imposing theoretical structures, but by trying to understand the individuals’ own experiences, drawing on their own words and perspectives to obtain a ‘thick description’.
In the methods, I adapted elements from grounded theory (Strauss & Corbin, 1990),19 as I have used and described in several prior studies.20 This approach was thus informed by techniques of ‘constant comparison’, in which data from different contexts are compared for similarities and differences to see if they suggest hypotheses.
I drafted the questionnaire, drawing on prior research we conducted and published literature, and then piloted it, analyzing the results of the interviews, and revising the interview guide further as we proceeded. Transcriptions and initial analyses of interviews occurred during the period in which the interviews were being conducted, enhancing validity, and these analyses helped shape subsequent interviews. Interviews were all conducted by me, by telephone. The Columbia University Department of Psychiatry Institutional Review Board approved the study, and all participants gave informed consent.
Once the full set of interviews were completed, subsequent analyses were conducted in two phases, primarily by a trained research assistant (RA) and me. In phase I, the RA and I independently examined a subset of interviews to assess factors that shaped participants’ experiences, identifying categories of recurrent themes and issues, which were subsequently given codes. We read each interview, systematically coding blocks of text to assign ‘core’ codes or categories (for example, instances when IRBs discussed research being conducted in the developing world). While reading the interviews, a topic name (or code) was inserted beside each excerpt of the interview to indicate the themes being discussed. We then worked together to reconcile these independently developed coding schemes into a single scheme. We then prepared a coding manual, defining each code and examining areas of disagreement until reaching consensus between them. New themes that did not fit into the original coding framework were discussed, and modifications were made in the manual when deemed appropriate.
In phase II of the analysis, an RA and I then independently performed a content analysis of the data to identify the principal subcategories, and ranges of variation within each of the core codes. The sub-themes identified by each coder were reconciled into a single set of ‘secondary’ codes and an elaborated set of core codes. These codes assess subcategories and other situational and social factors. Such subcategories include specific types of differences in interpretations (for example, specific differences IRBs had in reviewing research conducted in the developing world, such as questions about determining appropriate amounts of compensation or post-trial treatment sustainability).
Codes and sub-codes were then used in analysis of all of the interviews. To ensure coding reliability, two coders analyzed all interviews. Where necessary, multiple codes were used. The coders assessed similarities and differences between participants, examining categories that emerged, ranges of variation within categories and variables that may be involved.
We examined areas of disagreement through closer analysis until consensus was reached through discussion. We checked regularly for consistency and accuracy in ratings by comparing earlier and later coded excerpts. To ensure that the coding schemes established for the core codes and secondary codes are both valid (well grounded in the data and supportable) and reliable (consistent in meaning), they were systematically developed and well-documented.
RESULTS
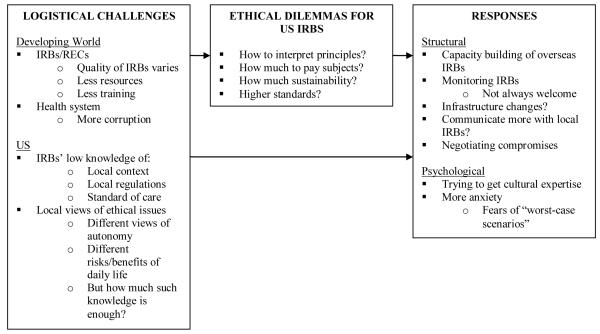
Overall, as indicated on Figure I, and described more fully below, three main sets of themes emerged, concerning logistical challenges, ethical dilemmas and IRB responses to these. These themes are closely interrelated.
Figure 1.
Issues Regarding Relationships with IRBs in the Developing World
Interviewees revealed that US IRBs vary from having reviewed research conducted in the developing world relatively frequently to relatively rarely, if at all. In general, those that have done so more frequently appear more comfortable in some ways, but are often more aware of the potential pitfalls and challenge which can serve to increase anxiety.
These challenges arose in reviewing research in both the US and the developing world, but in the latter case were often heightened due to cultural, social, legal and economic differences and geographic distance.
Logistical challenges
Lack of ‘local knowledge’
Geographic distance creates uncertainties as US IRBs try to affect events – interactions between research staff and subjects – in the developing world, far removed from themselves geographically, temporally, socially, economically and culturally. These IRBs cannot readily reflect the values of the community of participants, and have difficulty knowing what can ‘go wrong’. These IRBs may thus become either over or under-concerned about such risks. These IRBs hence worry about ‘worst-case scenarios’ that may be very unlikely or unrealistic. These boards may consequently seek extra precautions, not fully or directly knowing what is appropriate for a country, and may end up being overly paternalistic:
We worry a lot about third world research where our faculty are the PIs, and we are therefore the IRB – just knowing what’s right and reasonable for the country, or what could go wrong. For studies of sexually-transmitted diseases and HIV in India, women were very much at risk if their husbands found out about their participation, about results of testing. In each IRB, we have members who have done research in third world countries, but it’s a scary area. We take extra precautions. IRB17
Research conducted on other cultures may thus pose different risks that IRBs then need to consider.
Lack of ‘local knowledge’ about the logistical details that exist or may arise poses added uncertainties and challenges. Developing world IRBs can have unique local knowledge, but US IRBs generally have difficulty obtaining such input. For instance, an IRB in the local host country, but not in the US may be aware that a facility is now closed, or that the population targeted for recruitment may involve prisoners:
There’s local knowledge that we just wouldn’t have known. Like, questioning a certain institution’s ability to handle the research. Or the facility is under construction or renovation, and can’t be used. Or the study involves prisoners or people who become imprisoned during the study. IRB18
Local knowledge can thus involve logistical and procedural concerns as well as ethical principles. PIs should possess these details as well, but may not do so, or sufficiently convey it.
US IRBs often try to get additional cultural expertise:
Sometimes our IRB has to go the extra mile, and get someone familiar with the culture. For instance, elders of tribes may make the informed consent decisions for everyone in their group. We do our due diligence. IRB9
To know in advance the exact circumstances of research in the developing world may also be hard, if not impossible. For instance, a PI can arrive abroad, and realize that the situation is much different than he or she anticipated. IRBs may vary in anticipating and considering these exigencies.
Researchers end up in a country, and realize that things aren’t as thought, and they then have to make changes. We’ve worked closely with investigators at that point to try to accept and approve modifications, so they don’t have to jump on a plane, come home, and cancel the research until then. IRB28
Regardless of the country, researchers in the field encounter unanticipated obstacles, but given the increased distance, the magnitude, the unforseeability and the difficulties entailed in responding to these barriers can be higher in the developing world.
US IRBs may also be unsure about relevant local laws and regulations. ‘You can have the best intentions to follow the local rules, but can’t get a copy of them. The IRBs in a country may not even have a copy’ IRB18. Yet interviewees felt that obtaining a copy of a country’s laws and regulations concerning research can at times be difficult. This interviewee is referring not to international documents such as CIOMS and the Declaration of Helsinki, but to a country’s own relevant domestic laws or regulations.
Interviewees reported that compendiums of all of these relevant laws and regulations from all countries in the world are being developed, but are still often incomplete, or not up-to-date. Moreover, problems arise because regulations, once obtained, may be interpreted in different ways within or between countries – whether in the US or elsewhere.
Questions emerge, too, as to when dual IRB approval is needed. US regulations are also somewhat ambiguous in certain situations – for example, if a researcher is ‘merely a consultant’ or a co-investigator. Some PIs have argued that since they are doing research overseas, they do not need IRB approval from their home institution. IRBs may require approval from both institutions if the researcher plans to be an author in a published paper resulting from the study. But papers may have 20 to 30 authors, some of whom may have relatively small roles. Co-investigators range widely in their involvement, creating uncertainty and needs for judgment.
For research in the developing world, US IRBs feel they need to trust PIs, but may face subjective decisions in doing so. PIs:
…may be following all the regulations. But, we’re not there, and don’t know. We’re hoping for the best. We have to hope for that our investigator has research integrity, and is only doing research appropriately. IRB13
Knowledge of local logistical circumstances can thus affect the ethical concerns that can arise, and the ways principles get interpreted.
Problems, when arising, may be discovered not through the PI, but through a third party:
If the company is conducting studies in a developing country – China and India are very popular – it takes a long time to figure out if there were any human rights violations. Sometimes that information comes to us in a Newsweek article. IRB13
In the absence of data and knowledge, many IRB members thus have to ‘hop[e] for the best’, and given the increased uncertainty, may feel somewhat uneasy. Here and elsewhere, communication between IRBs in the US and developing countries was also often poor or non-existent.
Standard of care
The standard of care in a developing world country can be hard to ascertain as well. For instance, one IRB had to decide whether in another country, ultrasounds were part of standard prenatal care. The IRB was concerned about female foetuses being selectively aborted. If there were abortions, the IRB wanted to see these in adverse event (AE) reports, but arranging that proved hard:
The question came up: are ultrasounds really part of prenatal standard care in a country – or just part of the study – and if so, are they given once or twice, and when? The protocol wasn’t clear. In a society where elective abortions often happen because of gender selection, we didn’t want to be unintentionally contributing to girls getting aborted! We went back and forth with the PI to find out. We wanted to makesure that elective abortions were in the AE reports, so we could track them, and see if the ultrasound was contributing to gender elective abortions. IRB18
Yet trusting PIs in the developing world poses challenges – as it also does in the US as well. When this PI said that standard of care included one ultrasound, the IRB had to judge whether to accept his word, and how many resources to put into substantiating his claim. IRBs thus have to decide at what point to accept uncertainty. When the study site is abroad, obtaining additional certainty takes further energy and effort:
The PI answered that, ‘One early-stage ultrasound is standard’. Now we were in an ethical dilemma: do we take his word for it? And if not, how much of our resources do we put into independently finding more information? Because the PI’s answer was fairly convenient, since in early ultrasound, you’re not likely to know the gender. We took his word, but insisted that he include elective abortions in his AE reporting. That’s a kind of balanced, compromised solution, where we may not do all the due diligence that we should, but didn’t totally close our eyes. IRB18
Reviewing research in the developing world presents challenges, too, since many details lie beyond the control of US IRBs, which may then strive simply to ‘compromise’, and make the best plan they can.
Different views of autonomy
IRBs face challenges in understanding the cross-cultural contexts and meanings of ethical issues, and deciding whether and how these should shape reviews. For instance, differing notions of autonomy can affect decisions about who should consent for a participant, and how. Concepts of identity and relationships of the individual to the group, can vary widely as well, posing problems:
The identity of an individual is very different across societies. In the US, it’s very ‘one person, one identity’ – detachable from others. But in lots of societies, individual identity includes the kinship group, or having a husband, father or chief condone a decision, or tell the individual what to do. The researcher has to go through these gatekeepers first – a tribal chief, then maybe a head of household – to get permission to talk to the individual. These gatekeepers don’t necessarily speak for the individual, but the researcher has to get to the individual. We really cannot waive this process unless the subject is a child, and it’s a high benefit/low risk therapeutic intervention, where it’s reasonable to waive the child’s consent. You respect the authority of the different gatekeepers, but also go to the individual. You try to make it private and let them know they can say no. You have to get their individual consent. IRB18
In these situations, coercion may be possible: ‘In different societies, men, women and children do not always have the same abilities to say yes or no. Women can’t vote. Their ethics are based on their social structure’ IRB13.
IRBs’ concerns about the possibility of coercion may vary depending on the level of risk of the study:
We tend to have low risk studies, so I don’t worry too much about women’s freedom to say no, or, if the husband or the other men say, ‘Do this!’, that the woman feels coerced. I don’t worry about people being pressured into brain surgery, or something like that. IRB18
The fact that these particular studies are low risk alters the IRBs’ risk/benefit calculus.
Reviews can be complicated, too, since certain practices are accepted in other cultures, but not here. For instance, other cultures may implicitly condone domestic abuse, and women, if their HIV infection becomes known, can be beaten or divorced.
Different risks and benefits of daily life
Reviews of research in the developing world generate difficulties, too, because health and other behaviours can carry far different meanings, and thus pose differing harms and benefits than in the US. For instance, in studies examining the risks of children getting HIV from breastfeeding, bottle-feeding itself may be stigmatized since it may suggest that the mother is HIV-infected. Research may compare whether rates of HIV transmission through breast milk are less than that of dying from diarrhoea due to bottle-feeding that involves bad water. But this prejudice – which does not exist in the US, and about which IRBs here may be unaware – adds potential dangers. Breastfeeding may be better than bottle-feeding, but:
…goes so much against Western medical advice…If you’re urging people to bottle-feed, they need clean water and bottles, and have to know the proper mixtures. Otherwise the babies can die of diarrhoea. A lot of AE reports come in of babies dying of diarrhoea. We would ask the PI: what’s the background rate of deaths from diarrhoea? Babies’ chances of getting HIV through breast milk are less than dying of diarrhoea from bad formula. How do you balance that and honour the social norms around breastfeeding? Is it fair to put people at higher risk of stigmatization by asking them to bottle-feed? IRB18
To address such questions, IRBs, in deciding what changes to request, may ask PIs to address logistical issues to bolster support for vulnerable groups such as women:
We usually resolve these issues by emphasizing voluntary counselling testing and education during pregnancies, and building up support systems for women and couples to deal with disclosure, and counselling about the benefits of not breastfeeding, and how to prepare formulas. That’s hard: they don’t have clean water. You just can’t control it. If ARV treatment is available, we make sure there are referrals. IRB18
The quality of IRBs/RECs in the developing world and their reviews can also vary enormously – as in the US – but can be harder to ascertain, given differences in location and distance. As indicated earlier, IRBs in the developing world generally are under-resourced, lacking full-time staff and hobbled by poor infrastructure. Consequently, institutional entities such as grant and contract administration offices to ensure accountability may be absent or less robust.
These IRBs may agree or disagree with US reviews. Some developing world IRBs may worry that they are just ‘rubber-stamping’ Western IRB decisions, and subsequently either alter or continue that tendency. Others come up with points that Western IRBs had missed:
A lot in-country IRBs are just not very confident. The classic concern is that they are rubber-stamping the Western IRB decision. They don’t routinely stand up much to challenge our decisions. There are a couple of exceptions. One country’s Ministry of Health IRB is very good. Their letters to investigators detail concerns, and educate us. IRB18
IRB members abroad may also interpret regulations in ways that US IRBs or PIs disagree with.
Corruption
When conducting research in some developing countries, given different political contexts, and at times, lesser governmental oversight and transparency, corruption can pose obstacles as well: ‘There’s also a lot of corruption. Money goes into the pockets of institutional officials. So, there are a lot of challenges…’ IRB18.
Interviewees felt that in certain developing countries, corruption may be ‘a way of life’, forcing US IRBs to then struggle with how to proceed as a result – whether to accept it, and if so, how much, and how to decide. For instance, ideally, more funds from US grants could go to capacity building, but would need accountability, given corruption.
Financial deceit may include, for instance, kickbacks from drug manufacturers to pharmacists:
Corruption is usually off the record, on the QT. A study pharmacist dispensing ARVs is dealing with a pretty big study, a lot of subjects, making a lot of big orders. He’s going to be offered kickbacks by the drug manufacturing companies to order from them. So, the price will be inflated because he’s going to get some of it. But that is grant money! Or, a subcontracted institution has hardly any infrastructure for grants or contracts management, so the money goes right into somebody’s personal bank account to buy a new car. IRB18
In some developing countries, kickbacks may be considered just part of doing business. A US IRB may aim not to eliminate, but merely minimize these practices:
Very respected people in development say it’s just a part of doing business – that 5 or 10% is an acceptable rate of paying extra for corruption. If it’s 15 or 20%, it’s a problem. Bribes just sort of pave your way. IRB18
But, of course, US Federal grants do not allow these costs. Key questions thus emerge of how much, if any, to accept.
Ethical dilemmas for US IRBs
Given these challenges, US IRBs face several specific sets of decisions – for example, how to interpret principles. These dilemmas manifest themselves in several ways: ‘The study may have gone through a local ethics review board, and may be appropriate according to their social customs. But by whose standards – theirs, or the US?’ IRB13.
US IRBs may think that developing world IRBs interpret regulations either too rigidly, or too casually. These foreign IRBs may not understand or follow key aspects – for instance, that they can do expedited reviews:
They lack policy procedures for operational function. Some don’t meet regularly, but just when they get protocols. It’s pretty hard to run an operation with that level of uncertainty. They don’t always understand that they have flexibility to do expedited reviews, so they’re calling meetings when they don’t have to. They don’t understand how to work with exempt or expedited, or non-human subjects, research. They are often reviewing a study that doesn’t need IRB review. IRB18
In part, they may not understand the regulations because they may not have had sufficient training. As indicated earlier, IRB members in both the US and abroad report needs for additional training.
Sustainability
IRBs face quandaries of how much post-trial ‘sustainability’ is sufficient when PIs may be ‘parachuting’ into a community abroad. For instance, for studies that provide treatment to subjects, IRBs encounter dilemmas of how much post-study access to the treatment researchers should provide:
Sustainability after a study leaves is a huge concern. We consider post-study access to a proven beneficial treatment. We are looking for an arrangement or contract for free or low cost supply. You get into questions of who should get it. A very common consideration is whether not just subjects, but the whole village, should get the free treatment. IRB18
These issues emerge with research in the US, as well, but do so even more in the developing world, given the more limited health resources that often exist.
Difficulties arise if researchers claim that they simply cannot pay for such ongoing care. Conflicts can then ensue:
Is it OK if a researcher says, ‘I just can’t give free treatment, we just don’t have the money. It’s just a three-year NIH grant’? We would then want to know, ‘What efforts have you made to push back on that and work for the best possible deal?’ The worst-case scenario is that the researchers don’t get to do the study. IRB18
Still, US IRBs are frequently unsure how long exactly to require a PI to continue to provide treatment in a community after a study – for example, whether 12, 24 or 36 months is sufficient.
Avoiding exploitation or coercion: problems of compensation
One of the most difficult conundrums IRBs face regarding research in the developing world is how to avoid coercion – specifically, how much to pay participants. IRBs seek to avoid the Scylla and Charybdis of exploitation and coercion. But for research in the developing world, to know how to steer clear of both can be hard. Many US IRBs simply try to defer these decisions to in-country IRBs. ‘We need to know from researchers what the locals recommend – what they think is appropriate. It may not be money at all, but something else’ IRB11.
But often, developing world IRBs are themselves unsure how to proceed, because of deep uncertainties and assumptions about subjects’ conscious and unconscious motivations. The notion in the US that local IRBs know may thus be illusory. Abroad, subjects may be from the same country as IRB members, but from dramatically different socio-economic and education levels, which may impede these IRBs’ full awareness of these subjects’ motives. In the US as well, IRBs may view the motives for research participation far differently than do subjects themselves.
Higher standards?
For research in the developing world, US IRBs face dilemmas of whether the standard should be that of the West, or higher or lower, and if different, why, and how much so.
IRBs may seek higher standards and be wary of researchers conducting research in poorer countries simply because doing so was easier or less expensive:
There’s a higher standard. What’s the rationale for going to another country? Are we taking advantage of a population? There has to be a good reason. The answer ‘because it’s easier to do research there’ is wrong, and you’re dead in the water. IRB11
But IRBs then face questions of how much higher a standard to follow, and in what ways – how much is reasonable, realistic or aspirational. IRBs may aim high, with the hope that realistically, at least some modicum of improvement, even if far less, will transpire.
Practical IRB responses
IRBs may encourage a PI, too, to aim for a higher standard – for instance, by trying as much as possible to build the capacity of researchers and local health systems. But IRBs are often unclear as to how much exactly to request, and in what ways:
We’ve aimed high, insisting that our researchers help build capacity, to provide the best standards of care – like Western standards. As long as we keep aiming for the highest standard, and insisting that studies build capacity, that means a better chance of sustainability later, without us. If medication isn’t available for herpes simplex, take it there! We understand: we are shooting for the stars, and settle for the moon. IRB18
This interviewee’s acknowledgement of the aspirational nature of her comments implicitly raises questions, however, of whether and to what degree such capacity building should be the role of grants funded simply to do research.
Strengthening foreign IRBs
In response to the above deficits, several US IRBs work to assist developing world IRBs, actively providing forms and policies. The NIH has funded Fogarty programs to enhance training in research ethics in the developing world, assisting foreign IRBs. But, these interviewees suggested, many needs remain.
US IRBs may also audit research and IRBs in the developing world, though doing so can cause added stress. In auditing researchers, IRBs argue that they are trying to benefit the PIs. But these audits generally instill fear, as individuals are scrutinized and judged. Trust may not yet be as established as within the US:
I’m auditing an IRB in the developing world. The chair spent five weeks here…So, I don’t think he’s stressed. He knows I’m an ally, just there to help. I’m not trying to get him into trouble. But for the researchers, it is a little stressful, even though I try to say, ‘We’re not looking for trouble, or going to get you into trouble.’ It’s definitely about building trust. But it’s not perfect. IRB18
Understandably, developing world IRBs may also not always welcome foreign input. IRBs struggle with how much to pressure developing world IRBs to conform to Western standards and procedures. Many US IRBs try to ‘be as collegial as possible with PIs, and not be like an IRS audit’ IRB18. But tensions can still arise.
US IRBs may want to do site visits of developing world IRBs, but not have the resources to do so. Such personal relationships may be critical to establish mutual trust, and to educate overseas IRBs as effectively as possible.
IRBs may also try to encourage PIs to negotiate for the best deal – especially when industry is involved. ‘We deal with a lot of worst-case scenarios, and end up trying to find out what’s the best possible outcome that we can structure – the best possible deal we can propose’ IRB18.
As suggested earlier, US IRBs also try to obtain more cultural expertise. But despite these efforts to affect PIs’ and their own interactions, in the end, IRBs may also be left feeling anxious, fearing ‘worst-case scenarios’.
Given the many challenges involved, especially initially, these IRBs may simply be left feeling anxious about such studies, but proceed nonetheless.
CONCLUSIONS
Increasing amounts of research by US PIs in the developing world present US IRBs with a spectrum of logistical and ethical challenges in interpreting and applying principles and regulations, fuelled by vast differences in culture, economics and health. These IRBs perceive that IRBs/RECs in the developing world – as in the US – vary in resources, quality and training; and that health systems in these countries may face challenges due to lower resources and at times, less government oversight and hence, related, long-standing practices of corruption. US IRBs often have little knowledge of the local contexts, regulations and standards of care. Other cultures may have different views of autonomy and risks and benefits in daily life. Thus, these IRBs face a variety of ethical dilemmas, including how to interpret principles, how much to pay subjects, and how much sustainability to require. These IRBs often adopt psychological and social responses, including trying to obtain cultural expertise in the review process, ‘negotiating’ to provide the most possible benefits for study participants, attempting to build IRB infrastructure abroad and communicating more with foreign IRBs, trying to maintain higher standards for research conducted in the developing world, and fearing ‘worst-case scenarios’.
While prior reports have shown that developing world IRBs often face challenges,21 this paper presents the first qualitative data to examine how US IRBs view and approach research conducted in the developing world, and shows how US IRBs, in reviewing research, confront several heightened challenges that require careful consideration. Many of these dilemmas clearly exist with research conducted in the US as well, but are exacerbated in the developing world due to differences in culture, laws, economics and geographic distance. Participants may speak different languages and have lower literacy levels. Logistical and ethical challenges thus emerge. Complex notions such as ‘autonomy’, ‘rights’, ‘identity’, ‘coercion’, ‘consent’, ‘experiment’, ‘treatment’, ‘cure’, ‘randomization’ and ‘placebo’ can all vary considerably between cultures, in ways that affect practice. Questions such as how much sustainability to seek or require raise, too, larger questions of justice – how to define and operationalize the concept when two very different contexts collide.
IRBs can face not only differences in cultural views of ethical and other concepts, which they often acknowledge, but economic disparities as well. These operate at all levels – from health systems (regarding sustainability and corruption) to individual subjects (concerning how much compensation to provide). IRBs may need to become more explicitly aware of these various differences, particularly economic ones, and how to approach these. One could argue that such considerations fall outside the purview of the IRB, yet the Belmont Report addresses questions of justice.
IRBs thus struggle with procedural/bureaucratic, as well as ethical issues, and these two sets of concerns are closely intertwined. IRBs here seek to ensure that both certain procedures and ethical principles are followed. These data suggest that in different cultures, principles may be applied and seen in very different manners that do not necessarily function in the same way, nor protect subjects as optimally as possible. Clearly, future research is needed to explore these issues in further detail, to ascertain more fully how often, in what ways, and to what degrees divergences may occur.
These US IRB members’ comments may reflect a very ‘American’ view, with insufficient knowledge of the developing world. Yet these interviewees regularly acknowledged and discussed their sense that they had suboptimal information regarding circumstances in the developing world, and generally wished to gain more knowledge and reduce these deficits (e.g., as noted, RECs ‘educate us’). Moreover, rather than feeling that IRBs work well in the US, these interviewees routinely revealed deficits and problems in IRB functioning in the US as well. Thus, these interviewees tended to think not that US IRBs were in any way ‘superior’ to those in the developing world, but instead that IRBs in both the US and abroad all faced difficulties, including ambiguities in interpreting regulations and principles, and challenges related to inherent uncertainties in conducting research (which, by definition, has unknown outcomes).
At the same time, IRBs have difficulty knowing when they sufficiently understand other cultures, and institutional, political, social and economic contexts. Interviewees face decisions of how much detail is adequate and contains all of the necessary, relevant information. It is not clear how much due diligence an IRB needs to do, or how to know. Further guidelines and input here might be beneficial.
These data highlight, too, the need for IRBs to recognize that they may interpret regulations differently in key ways, without one approach necessarily being uniquely ‘right’ or ‘wrong’. In domestic research, US IRBs may interpret regulations in certain ways that do not readily apply or make sense abroad. More communication between IRBs in the US and the developing world can also help here. IRBs communicate poorly in part because they do so via the intermediary of PIs. However, direct communication may help IRBs mutually educate each other about the issues they perceive.
As the amount of research conducted in certain developing countries with less established regulatory oversight continues to increase, questions about corruption arise as well. Bribery and kickbacks may be accepted practices in some countries, but pose enormous challenges to US IRBs trying to ensure that research is conducted with integrity (which can include scientific and financial soundness). These concerns need not necessarily prevent research from being conducted, but require close attention. The possibility of such corruption makes funders, researchers and PIs uncomfortable, but documenting when and how it occurs, and how IRBs, funders and PIs respond to it, is vital. Anecdotally, PIs may report observations of such corruption to the Food and Drug Administration and other US agencies, but are at times unclear what then happens, and whether future researchers are warned to avoid certain collaborations abroad.
Questions emerge as to whether heightened IRB scrutiny and caution regarding research in certain developing countries is unfair and how much wariness is warranted. These concerns vary by the specific country, locale, researcher, type of study (e.g., degree and type of risks), but deserve closer analysis. IRBs are trying to affect downstream processes—interactions between PIs, staff and subjects – and in the developing world, these challenges mount. IRBs may be compromising, encouraging PIs to ‘negotiate the best deal possible’, or remain anxious and possibly obsess over details.
US IRBs may feel responsible to assist these foreign IRBs as much as possible, but how much US IRBs and researchers can do, and at what financial and logistical costs, is unclear. Particularly given other budget constraints, dilemmas surface of how much of an NIH grant should be used to conduct the current study vs. build ongoing infrastructure to assist future research. These questions extend beyond any one research project and raise broader social and political issues involving questions of global and domestic justice in our increasingly global world. Effort by ethicists and others can help guide these analyses.
This study has several potential limitations. These data are based on in-depth interviews with individual IRB members and chairs and did not include direct observation of IRBs making decisions, or investigation of IRB written records. Future research can also observe IRBs and examine such records. However, such additional data may be difficult to obtain since, anecdotally, IRBs have at times required that researchers obtain consent from all IRB members, as well as from the PIs and funders of protocols. These interviews also probed respondents’ experiences and views at present and in the recent past, but not prospectively over time to assess whether they changed their views, and if so, why. Future research can explore these issues over time as well.
A critic may argue that the study reflects a self-centred and asymmetrical view toward the developing world, and does not include the views of IRB members in the developing world themselves. Clearly, enormous needs exist for research on the views of REC members outside the US. However, this paper is based on data consisting of interviews of US IRB members, as a first step in probing some of these domains. Future research can explore these issues among REC members in other countries as well. Nonetheless, the present data are highly valuable in and of themselves in shedding light on challenges that exist.
These data have important implications for future research. Future studies can examine more fully on larger samples of IRBs in both the US and abroad, how often exactly IRBs encounter each of these sets of issues and respond in each of the ways suggested here (i.e., adopting ‘higher,’ equal or lower standards), and how they make decisions about these dilemmas – e.g., what factors may play roles. Further research is needed as well to investigate further how IRBs define and approach definitions of ‘justice,’ levels of risk in daily life in the developing world and possibilities of corruption.
At the same time, paradoxes arise. Data suggest that subjects in other cultures frequently do not understand informed consent forms, while US IRBs often appear to believe otherwise. Studies have shown that over 80% of subjects in the developing world do not understand basic concepts such as placebo, randomization and the ability to withdraw from a study at any time without negative consequences.22 Questions arise as to why these possibilities do not influence IRB deliberations more, and whether IRBs consider or ignore this evidence. The fact that at a certain point, IRBs may be seeking to follow regulations more than protecting human subjects poses critical questions that require additional research. Future studies can examine these phenomena, and how and why US IRBs differ in their approaches.
In sum, these data suggest a range of challenges that US IRBs face in reviewing research conducted in the developing world. These findings have critical implications for the education and practice of IRBs and PIs in both the US and developing countries. Given the enormous amount of research in the developing world that is funded by, or involves researchers from the US, these questions are thus crucial in the increasingly global world.
Supplementary Material
ACKNOWLEDGEMENTS
The author would like to thank Meghan Sweeney, Jason Keehn, Renée Fox and Paul Appelbaum for their assistance with this manuscript.
BIOGRAPHY
Robert Klitzman is a Professor of Clinical Psychiatry at the Columbia University Department of Psychiatry. He is also the Director of the Ethics and Policy Core of the HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Department of Psychiatry and Director of the Masters of Bioethics Program at Columbia University.
References
- 1.Bartlett EE. International Analysis of Institutional Review Boards Registered with the U.S. Office for Human Research Protections. J Empir Res Hum Res Ethics. 2008;3:49–56. doi: 10.1525/jer.2008.3.4.49. [DOI] [PubMed] [Google Scholar]
- 2.Kass NE, et al. The Structure and Function of Research Ethics Committees in Africa: A Case Study. PLoS Med. 2007;4:26–31. doi: 10.1371/journal.pmed.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nyika A, et al. Composition, Training Needs and Independence of Ethics Review Committees across Africa: Are the Gate-keepers rising to the Emerging Challenges? J Med Ethics. 2009;35:189–193. doi: 10.1136/jme.2008.025189. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bhan A, et al. Process, Pitfalls and Probity: Sharing Experiences on Setting Up and Running Ethics Committees in India. Indian J Med Ethics. 2010;7:48–51. doi: 10.20529/IJME.2010.019. [DOI] [PubMed] [Google Scholar]; Rivera R, Ezcurra E. Composition and Operation of Selected Research Ethics Review Committees in Latin America. IRB. 2001;23:9–12. [PubMed] [Google Scholar]
- 3.Kirigia JM, Wambebe C, Baba-Moussa A. Status of National Research Bioethics Committees in the WHO African Region. BMC Med Ethics. 2005;6:10. doi: 10.1186/1472-6939-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera, Ezcurra op. cit. note 2.
- 5.Ikingura JKB, Kruger M, Zeleke W. Health Research Ethics Review and Needs of Institutional Ethics Committees in Tanzania. Tanzania Health Research Bulletin. 2007;9:154–158. doi: 10.4314/thrb.v9i3.14320. [DOI] [PubMed] [Google Scholar]
- 6.Klitzman R. Views of the Process and Content of Ethical Reviews of HIV Vaccine Trials among Members of US Institutional Review Boards and South African Research Ethics Committees. Dev World Bioeth. 2008;8(3):207–218. doi: 10.1111/j.1471-8847.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 7.Mystakidou K, et al. Ethical and Practical Challenges in Implementing Informed Consent in HIV/AIDS Clinical Trials in Developing or Resource-limited Countries. SAHARA J. 2009;6:45–57. doi: 10.1080/17290376.2009.9724930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klitzman R, Siragusa J. Contexts Anyone? The Need for Contextualization in the Debate about the Moral Status of Embryos. Am J Bioeth. 2005;5(6):56. doi: 10.1080/15265160500320445. [DOI] [PubMed] [Google Scholar]
- 9.Klitzman op. cit. note 6.
- 10.De Vries R, Anderson MS, Martinson BC. Normal Misbehavior: Scientists talk about the Ethics of Research. JERHRE. 2006;1:43–50. doi: 10.1525/jer.2006.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Greene SM, Geiger AM. A Review finds that Multicenter Studies face Substantial Challenges but Strategies exist to Achieve Institutional Review Board Approval. J Clin Epidemiol. 2006;59:784–790. doi: 10.1016/j.jclinepi.2005.11.018. [DOI] [PubMed] [Google Scholar]; Larson E, et al. A survey of IRB Process in 68 US Hospitals. J Nurs Scholarship. 2004;36:260–264. doi: 10.1111/j.1547-5069.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- 11.Klitzman R. Views and Experiences of IRBs Concerning Research Integrity. J Law Med Ethics. 2011;39:513–528. doi: 10.1111/j.1748-720X.2011.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klitzman R. The Myth of Community Differences as the Cause of Discrepancies between IRBs. Amer J Bioeth. 2011;2:24–33. doi: 10.1080/21507716.2011.601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klitzman R. “Members of the Same Club”: Challenges and Decisions Faced by US IRBs in Identifying and Managing Conflicts of Interest. PLoS ONE. 2011;6:e22796. doi: 10.1371/journal.pone.0022796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klitzman R. “Community” IRB Members: Who are they, What do they do, and do they Represent Anyone? Acad Med. 2011 (in press) [Google Scholar]
- 15.Klitzman R. How Local IRBs View Central IRBs in the US. BMC Med Ethics. 2011;12 doi: 10.1186/1472-6939-12-13. doi:10.1186/1472-6939-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klitzman op. cit. note 11.; Klitzman op. cit. note 12.; Klitzman op. cit. note 13.; Klitzman op. cit. note 14.; Klitzman op. cit. note 15.
- 17.US Department of Health. Human Services (DHHS) National Institutes of Health Research Portfolio Online Reporting Tools. DHHS; Bethseda, MD: [Accessed 20 Apr 2011]. 2011. Funded Organizations. Available at: http://report.nih.gov/funded_organizations/index.aspx. [Google Scholar]
- 18.Geertz C. The Interpretation of Cultures: Selected Essays. Basic Books; New York, NY: 1973. [Google Scholar]
- 19.Strauss A, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage Publications; Newbury Park, CA: 1990. [Google Scholar]
- 20.Klitzman R, Bayer R. Mortal Secrets: Truth and Lies in the Age of AIDS. Johns Hopkins University Press; Baltimore, MD: 2003. [Google Scholar]; Klitzman R. Views and Approaches toward Risks and Benefits among Doctors who become Patients. Patient Educ Couns. 2006;64:61–68. doi: 10.1016/j.pec.2005.11.013. [DOI] [PubMed] [Google Scholar]; Klitzman R, Chung W. The Process of Deciding about Prophylactic Surgery for Breast and Ovarian Cancer: Patient Questions, Uncertainties, and Communication. Am J Med Genet. 2007;152A:52–66. doi: 10.1002/ajmg.a.33068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kass, et al. op. cit. note 2.; Nyika, et al. op. cit. note 2.; Klitzman op. cit. note 6.
- 22.Krosin M, et al. Problems in Comprehension of Informed Consent in Rural and Peri-urban Mali, West Africa. Clin Trials J. 2006;3:306–313. doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]; Gazzinelli MF, et al. Health Education through Analogies: Preparation of a Community for Clinical Trials of a Vaccine against Hookworm in an Endemic area of Brazil. PLoS Neglected Tropical Diseases. 2010;4(7):1–12. doi: 10.1371/journal.pntd.0000749. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sarkar R, et al. Comparison of Group Counseling with Individual Counseling in the Comprehension of Informed Consent: A Randomized Controlled Trial. BMC Medical Ethics. 2010;11(8) doi: 10.1186/1472-6939-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.