Abstract
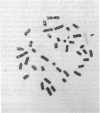
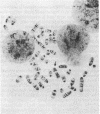
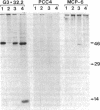
We have developed an approach to human developmental biology which exploits somatic cell genetics. With this system we have examined the production of the HLA-A,B,C antigens, A human-mouse somatic cell hybrid was constructed which contained a human X-7 chromosome translocation carrying the HLA region; this hybrid was used as a donor of the X-6 translocation in the technique of microcell transfer. The X-6 chromosome recipient was the mouse embryonal carcinoma cell line PCC4. The microcell hybrid MCP-6 retained the embryonal carcinoma phenotype as judged by shape and absence of H-2 expression. Nonetheless, the expression of the HLA-A,B,C genes was not extinguished. HLA-A,B,C antigen production of the cell surface, however, was not detected because this hybrid apparently could not make beta 2-microglobulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Bronson D. L., Benham F., Strickland S., Knowles B. B. A comparative study of eight cell lines derived from human testicular teratocarcinoma. Int J Cancer. 1980 Sep 15;26(3):269–280. doi: 10.1002/ijc.2910260304. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Bronson D. L., Wiles M. V., Goodfellow P. N. The expression of MHC antigens by human teratocarcinoma derived cell lines. Tissue Antigens. 1981 May;17(5):493–500. doi: 10.1111/j.1399-0039.1981.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Goodfellow P. N. Antigen expression by somatic cell hybrids of a murine embryonal carcinoma cell with thymocytes and L cells. Somatic Cell Genet. 1980 Mar;6(2):271–284. doi: 10.1007/BF01538801. [DOI] [PubMed] [Google Scholar]
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Artzt K., Jacob F. Letter: Absence of serologically detectable H-2 on primitive teratocarcinoma cells in culture. Transplantation. 1974 Jun;17(6):632–634. doi: 10.1097/00007890-197406000-00015. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Cross J. Differential staining of human and mouse chromosomes in interspecific cell hybrids. Nature. 1974 Sep 6;251(5470):77–79. doi: 10.1038/251077a0. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. HLA structure and function: a contemporary view. Tissue Antigens. 1981 Jan;17(1):9–20. doi: 10.1111/j.1399-0039.1981.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Lomakka G., Zech L. The 24 fluorescence patterns of the human metaphase chromosomes - distinguishing characters and variability. Hereditas. 1972;67(1):89–102. doi: 10.1111/j.1601-5223.1971.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Correani A., Croce C. M. Expression of the teratocarcinoma phenotype in hybrids between totipotent mouse teratocarcinoma and myeloma cells. J Cell Physiol. 1980 Oct;105(1):73–79. doi: 10.1002/jcp.1041050110. [DOI] [PubMed] [Google Scholar]
- Ege T., Ringertz N. R. Preparation of microcells by enucleation of micronucleate cells. Exp Cell Res. 1974 Aug;87(2):378–382. doi: 10.1016/0014-4827(74)90494-7. [DOI] [PubMed] [Google Scholar]
- Fellous M., Günther E., Kemler R., Wiels J., Berger R., Guenet J. L., Jakob H., Jacob F. Association of the H-Y male antigen with beta2-microglobulin on human lymphoid and differentiated mouse teratocarcinoma cell lines. J Exp Med. 1978 Jul 1;148(1):58–70. doi: 10.1084/jem.148.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Weitkamp L. R. Report of the committee on the genetic constitution of chromosome 6. Cytogenet Cell Genet. 1979;25(1-4):32–38. doi: 10.1159/000131397. [DOI] [PubMed] [Google Scholar]
- Gmür R., Solter D., Knowles B. B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980 Jun 1;151(6):1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Levy R., Povey S., McMichael A. A human X-linked antigen defined by a monoclonal antibody. Somatic Cell Genet. 1980 Nov;6(6):777–787. doi: 10.1007/BF01538976. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Hoppe P. C., Croce C. M. Chimeric mice derived from human-mouse hybrid cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1914–1918. doi: 10.1073/pnas.75.4.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immunology and the fetus. Lancet. 1978 Feb 11;1(8059):326–326. [PubMed] [Google Scholar]
- Jonasson J., Povey S., Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977 Apr;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- Jongsma A., van Someren H., Westerveld A., Hagemeijer A., Pearson P. Localization of genes on human chromosomes by studies of human-Chinese hamster somatic cell hybrids. Assignment of PGM3 to chromosome C6 and regional mapping of the PGD, PGM1 and pep-C genes on chromosome A1. Humangenetik. 1973 Dec 10;20(3):195–202. doi: 10.1007/BF00385730. [DOI] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lamm L. U., Svejgaard A., Kissmeyer-Nielsen F. PGM3: HL-A is another linkage in man. Nat New Biol. 1971 May 26;231(21):109–110. doi: 10.1038/newbio231109a0. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Ruddle F. H. Properties of teratocarcinoma-thymus somatic cell hybrids. Somatic Cell Genet. 1977 May;3(3):247–261. doi: 10.1007/BF01538744. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Ruddle F. H. Teratocarcinoma X friend erythroleukemia cell hybrids resemble their pluripotent embryonal carcinoma parent. Dev Biol. 1977 Mar;56(1):157–173. doi: 10.1016/0012-1606(77)90159-2. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Miggiano V., Bodmer W. Genetic analysis with human--mouse somatic cell hybrids. Nature. 1969 Jul 26;223(5204):358–363. doi: 10.1038/223358a0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Evrin P. E., Welsh K. I. Production of beta 2-microglobulin by normal and malignant human cell lines and peripheral lymphocytes. Transplant Rev. 1974;21(0):53–84. doi: 10.1111/j.1600-065x.1974.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Parham P., Bodmer W. F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978 Nov 23;276(5686):397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Povey S., Gardiner S. E., Watson B., Mowbray S., Harris H., Arthur E., Steel C. M., Blenkinsop C., Evans H. J. Genetic studies on human lymphoblastoid lines: isozyme analysis on cell lines from forty-one different individuals and on mutants produced following exposure to a chemical mutagen. Ann Hum Genet. 1973 Jan;36(3):247–266. doi: 10.1111/j.1469-1809.1973.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Povey S., Jeremiah S., Arthur E., Ber R., Fialkow P. J., Gardiner E., Goodfellow P. N., Karande A., Klein G., Quintero M. Deficiency of malic enzyme: a possible marker for malignancy in lymphoid cells. Ann Hum Genet. 1981 Jul;45(Pt 3):237–252. doi: 10.1111/j.1469-1809.1981.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Rousset J. P., Jami J., Dubois P., Avilès D., Ritz E. Developmental potentialities and surface antigens of mouse teratocarcinoma x lymphoid cell hybrids. Somatic Cell Genet. 1980 May;6(3):419–433. doi: 10.1007/BF01542793. [DOI] [PubMed] [Google Scholar]
- Solomon E., Bobrow M., Goodfellow P. N., Bodmer W. F., Swallow D. M., Povey S., Noël B. Human gene mapping using an X/autosome translocation. Somatic Cell Genet. 1976 Mar;2(2):125–140. doi: 10.1007/BF01542626. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki N., Nakamuro K., Natori T., Kreiter V. P., Pressman D. Common antigenic structures of HL-A antigens. V. An antigenic determinant characteristic of a 33,000-dalton fragment of HL-A molecules. Transplantation. 1974 Jul;18(1):74–78. doi: 10.1097/00007890-197407000-00012. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Travers P., Bodmer W. F., Patillo R. A. Expression of HLA-A, -B, and -C and beta 2-microglobulin antigens in human choriocarcinoma cell lines. J Exp Med. 1980 Aug 1;152(2 Pt 2):11s–17s. [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]