Abstract
Nitric oxide-releasing aspirin (NO-aspirin) represents a novel class of promising chemopreventive agents. Unlike conventional nonsteroidal anti-inflammatory drugs, NO-aspirin seems to be free of adverse effects while retaining the beneficial activities of its parent compound. The effect of NO-aspirin on pancreatic carcinogenesis was investigated by assessing the development of precursor pancreatic lesions and adenocarcinomas in KrasG12D/+ transgenic mice that recapitulate human pancreatic cancer progression. Six-week-old male p48Cre/+-LSL-KrasG12D/+ transgenic mice (20 per group) were fed diets containing 0, 1000, or 2000 ppm NO-aspirin. The development of pancreatic tumors was monitored by positron emission tomography imaging. All mice were killed at the age of 41 weeks and assessed for pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC) and for molecular changes in the tumors. Our results reveal that NO-aspirin at 1000 and 2000 ppm significantly suppressed pancreatic tumor weights, PDAC incidence, and carcinoma in situ (PanIN-3 lesions). The degree of inhibition of PanIN-3 and carcinoma was more pronounced with NO-aspirin at 1000 ppm (58.8% and 48%, respectively) than with 2000 ppm (47% and 20%, respectively). NO-aspirin at 1000 ppm significantly inhibited the spread of carcinoma in the pancreas (∼97%; P < .0001). Decreased expression of cyclooxygenase (COX; with ∼42% inhibition of total COX activity), inducible nitric oxide synthase, proliferating cell nuclear antigen, Bcl-2, cyclin D1, and β-catenin was observed, with induction of p21, p38, and p53 in the pancreas of NO-aspirin-treated mice. These results suggest that low-dose NO-aspirin possesses inhibitory activity against pancreatic carcinogenesis by modulating multiple molecular targets.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a devastating and almost uniformly lethal disease despite tremendous scientific efforts for the past six decades. It has the worst prognosis and is the fourth leading cause of cancer-related deaths in the United States, with a five-year survival of <5% [1,2]. The high mortality rate is due, in part, to the difficulties in establishing an early and accurate diagnosis as well as to the lack of effective prevention treatments. The treatment strategies for pancreatic carcinoma have been hampered significantly by a number of unique challenges like the first definitive diagnosis only at an advanced stage [3–5]. Therefore, the stepwise progression of PDAC development has been inaccessible for study, and the precursor cell types still remain an area of active interest. One of the major goals of the pancreatic cancer biomarker field is to improve patient survival by developing effective chemoprevention and treatment strategies enabled by a better understanding of the underlying etiological and pathophysiological mechanisms. Oncogenic Kras mutation, mostly at codon 12, is observed in more than 95% of patients with precancerous lesions of the pancreas and PDAC [3–5].
Development of genetically engineered mouse models of pancreatic adenocarcinomas that mimic human disease progression has facilitated better understanding of the molecular pathobiology and is leading to the strategies for prevention and treatment [4,5]. To study the role of the mutant Kras gene in the initiation of pancreatic carcinogenesis, expression of the mutant allele specifically in the pancreatic epithelial cells is achieved by crossing LSL-KrasG12D mice with p48Cre mice that express Cre-recombinase from a pancreatic specific promoter. The p48Cre/+-LSL-KrasG12D/+ mice develop pancreatic intraepithelial neoplasia (PanIN) lesions (PanIN-1A, PanIN-1B and high-grade PanIN-2 and PanIN-3) followed by progression to PDAC as mice age [6–9].
Epidemiological studies have shown a decreased incidence of cancer with long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase (COX) enzymes in several organ sites [10,11]. Overproduction of aberrant arachidonic acid (AA) metabolites, cytokines, and growth factors, as well as the activation of their signaling pathways are known to contribute to the inflammation and tumorigenesis. Similar to many other malignant tissues, pancreatic lesions also overexpress COX-2 [12,13].
Epidemiologic evidence on the use of NSAIDs on the incidence of pancreatic cancer has been supported by observational studies [14–17]. The use of aspirin was inversely associated with incidence of pancreatic cancer (0.67) as per the Nutrition Examination Study 1 cohort. In another cohort study of patients with rheumatoid arthritis, the age-standardized incidence ratios for pancreatic cancer were 1.12 for men, 0.68 for women, and 0.83 for both sexes. A statistically nonsignificant inverse association between pancreatic cancer and self-reported use of NSAIDs (mostly aspirin) was reported in one case-control study. A meta-analysis with a total of 11 studies (3 case-control studies, 7 cohort studies, and 1 randomized trial) involving 6386 pancreatic cancer cases does not indicate that use of aspirin or NSAIDs affects the risk of pancreatic cancer [14–18]. Another recent clinic-based case-control study showed that there is no risk of pancreatic cancer on NSAID usage and that the use of aspirin for 1 day/month was greatly associated with reduced risk of pancreatic cancer [19]. The clinical usefulness of NSAIDs combined with their potentially life-threatening toxicity has prompted intense efforts to improve their safety profile. Nitric oxide (NO)-donating NSAIDs (NO-NSAID) represent such an approach [20]. Conventional aspirin prevents human cancers, but its toxicity precludes its application as a chemopreventive agent. NO-releasing aspirin (NO-aspirin) consists of traditional aspirin bearing -ONO2, which releases NO. NO-aspirin was shown to be superior in efficacy and safety profile [20,21]. NO-aspirin was found to be >1000-fold more potent and safer than aspirin in cultured human cancer cells [22]. NO-aspirin was more potent than aspirin for the prevention of several cancers [20,22–26]. Its safety has been documented, at least preliminarily, in humans [27,28].
On the basis of these observations, we evaluated the chemopreventive effect of NO-acetylsalicylic acid (ASA) against pancreatic cancer using p48Cre/+-LSL-KrasG12D/+ transgenic mice that recapitulate many morphologic and molecular features of the human disease. Here, we report that NO-aspirin has potent chemopreventive properties and modulates key markers of pancreatic lesion progression.
Materials and Methods
Animals, Diets, and Care
All animal experiments were done in accordance with the institutional guidelines of the American Council of Animal Care. Required numbers of activated KrasG12D/+ mice were generated as described below. Animals were housed in ventilated cages under standardized conditions (21°C, 60% humidity, 12-hour light/12-hour dark cycle, 20 air changes/hour) in the University of Oklahoma Health Sciences Center Rodent Barrier Facility. Semi-purified modified AIN-76A diet ingredients were purchased from Bio-Serv, Inc. (Frenchtown, NJ). NO-NSAID (NO-Aspirin) (Figure 1A) were procured from the Division of Cancer Prevention, National Cancer Institute chemoprevention drug repository. NO-aspirin (1000 and 2000 ppm) was premixed with small quantities of casein and then blended into the diet using a Hobart Mixer. Both control and experimental diets were prepared weekly and stored in the cold room. Agent content in the experimental diets was determined periodically in multiple samples taken from the top, middle, and bottom portions of individual diet preparations to verify uniform distribution. Mice were allowed ad libitum access to the respective diets and automated tap water purified by reverse osmosis.
Figure 1.
(A) Experimental design for preventive efficacy evaluation of NO-aspirin in male p48Cre/+-LSL-KrasG12D/+ mice. At 6 weeks of age, groups (20/group activated p48Cre/+-LSL-KrasG12D/+ or 12/group wild-type) of mice were fed AIN-76A diets containing 0, 1000, or 2000 ppm NO-aspirin continuously for 35 weeks, and each pancreas was evaluated histopathologically for marker expressions as described in the text. (B) Genotyping of p48Cre/+-LSL-KrasG12D/+ mice offspring by PCR (lanes 1 and 4 represent Cre, lanes 2, 5, 6, 8, and 10 represent wild type, lane 11 represents Kras, and lanes 3, 7, and 9 represent activated Kras). Pancreata of (C) wild-type, (D and E) untreated p48Cre/+-LSL-KrasG12D/+, and (F) NO-aspirin-treated p48Cre/+-LSL-KrasG12D/+ mice at 41 weeks of age. NO-aspirin treatment significantly decreased the size of the pancreas compared to that of untreated mice. (G–L) Typical histologic appearance of the wild-type pancreas (G), PanIN-1 lesions (H), PanIN-2 lesions (I), PanIN-3 lesions (J), carcinoma (K), and NO-aspirin-treated pancreas (L). No evidence of PanINs or PDAC was seen in the pancreas of wild-type mice. Significant increase in the normally appearing pancreas was seen with NO-aspirin treatment in p48Cre/+-LSL-KrasG12D/+ mice.
Breeding and Genotyping Analysis
LSL-KrasG12D/+ and p48Cre/+ mice were maintained in a C57BL/6 heterozygous genetic background. LSL-KrasG12D/+ and p48cre/+ mice were bred, and the male offspring of activated KrasG12D/+ (12%) were generated at required quantities. The genotype of each pup was confirmed by tail DNA extraction and polymerase chain reaction (PCR) as described elsewhere [8,9,29].
Bioassay: Efficacy of NO-Aspirin
Genotyped male p48Cre/+-LSL-KrasG12D/+ transgenic mice were used in the efficacy study. The experimental protocol is summarized in Figure 1B. Five-week-old mice were selected and randomized, so that average body weights in each group were equal (n = 20/group p48Cre/+-LSL-KrasG12D/+ mice and n = 12 C57BL/6 wild-type mice), and were fed AIN-76A diet for 1 week. At 6 weeks of age, mice were fed AIN-76A experimental diets containing 0, 1000, or 2000 ppm NO-aspirin (Figure 1A) until termination of the study. Mice were routinely checked for signs of weight loss or any signs of toxicity or any abnormalities. Food intake and body weight of each animal were measured once weekly for the first 6 weeks and then once a month until termination. After 35 weeks (∼9 months) on experimental diets, all mice were killed by CO2 asphyxiation and necropsied; pancreata were collected from all groups (Figure 1, C–F), weighed, and snap frozen in liquid nitrogen for further analysis. Pancreata (head to tail) required for histopathologic identification of PanIN lesions and PDAC and for evaluation of various molecular markers by immunohistochemistry (IHC; Figure 1, G–L) were fixed in 10% neutral buffered formalin as previously described.
Positron Emission Tomography-Computed Tomography Imaging
Positron emission tomography-computed tomography (PET-CT) imaging was performed on C57Bl/6 wild-type and treated and untreated p48Cre/+-LSL-KrasG12D/+ mice (n = 4) at the end of the experiment. Radiolabeled [18F]fluorodeoxyglucose (FDG) was intravenously injected in mice through tail vein. The probe was allowed to distribute for 2 hours before PET-CT imaging was performed. Mice were anesthetized with 2% isoflurane in an oxygen stream and positioned in the field of view of X-PET/X-O-CT machine (Gamma Medica-Ideas, Northridge, CA). A fly-mode CT was acquired (2 minutes) to establish anatomic landmarks before repositioning the animal for PET imaging. Approximately 20 minutes of list-models PET data were acquired. The acquired image data were reconstructed using a filtered backprojection algorithm. Both PET and CT images were fused together using Amira 3.1 software provided with the imaging system (Figure 2).
Figure 2.
PET and CT images of wild-type and p48Cre/+-LSL-KrasG12D/+ mice (n = 4). The p48Cre/+-LSL-KrasG12D/+ mice were fed control diet or control diet containing NO-aspirin for 35 weeks. The PET-CT imaging was performed at 41 weeks of age. About 2 hours before imaging, FDG was intravenously injected into the mice through tail vein. Images are from wild-type, treated, and untreated p48Cre/+-LSL-KrasG12D/+ mice. A significant accumulation of FDG was observed in the pancreas of untreated p48Cre/+-LSL-KrasG12D/+ mice, with a significant decrease in the pancreas of the treated mice. Moreover, the wild-type mouse pancreas did not show any significant accumulation of FDG.
Tissue Processing and Histologic Analysis of PanIN Lesions and PDAC
Formalin-fixed, paraffin-embedded tissues were sectioned (4 µm) and stained with hematoxylin and eosin. Twenty sections of each pancreas were histologically evaluated by a pathologist blinded to the experimental groups. PanIN lesions and carcinoma were classified according to histopathology criteria as recommended and previously published by our group [8,9,29]. Briefly, to quantify the progression of PanIN lesions, the total number of ductal lesions and their grade were determined (Figure 1, G–L). Pancreatic ducts of the entire fixed specimen (head, body, and tail of the pancreatic sections) were analyzed for each animal. The relative proportion of each PanIN lesion grade to the overall number of analyzed ducts was recorded for each animal. Similarly, pancreatic carcinoma and normal appearing pancreatic tissue were evaluated for all the animals.
Immunohistochemistry
The effects of NO-aspirin on expression of proliferating cell nuclear antigen (PCNA), COX-2, β-catenin, and inducible nitric oxide synthase (iNOS) were evaluated by IHC. Briefly, paraffin sections were deparaffinized in xylene, rehydrated through graded ethanol solutions, and washed in phosphate-buffered saline (PBS). Antigen retrieval was carried out by heating sections in 0.01 M citrate buffer (pH 6) for 30 minutes in a boiling water bath. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in PBS for 5 minutes. Nonspecific binding sites were blocked using protein block for 20 minutes. Sections were then incubated overnight at 4°C with 1:300 dilutions of monoclonal antibodies against PCNA, COX-2, β-catenin, and iNOS (Abcam, Cambridge, MA and Santa Cruz Biotechnology, Santa Cruz, CA). After several washes with PBS, the slides were incubated with appropriate secondary antibody for 2 hours and then washed and incubated with avidin-biotin complex reagent (Zymed Laboratories, Camarillo, CA). After rinsing with PBS, the slides were incubated with the chromogen 3, 3′-diaminobenzidine for 3 minutes, then rinsed and counterstained with hematoxylin. Non-immune rabbit immunoglobulins were substituted for primary antibodies as negative controls. Slides were observed under an Olympus microscope 1X701, and digital computer images were recorded with an Olympus DP70 camera.
Analysis of COX-1/2 Activity Using Radio-High-Performance Liquid Chromatography
Frozen pancreatic tumor tissues from male mice fed 1000 and 2000 ppm NO-aspirin and/or control diet were homogenized using an ice-cold homogenizing buffer. The combined COX-1 and COX-2 activity assays were performed according to our previous published methods [30]. Briefly, 150 µl of the reaction mixture containing 12 µM [14C]AA (420,000 dpm), 1 mM epinephrine, 1 mM glutathione in 50 mM phosphate buffer (pH 7.4), and 30 mg of protein for each assay was used. After incubation at 37°C for 20 minutes, the reaction was terminated by adding 40 µl of 0.2 M HCl. The COX-mediated metabolites of AA were extracted with ethyl acetate (3 x 0.5 ml). The combined extracts were evaporated to dryness under N2 and dissolved in 1 ml of acetonitrile, and 10-µl samples were injected into a reversed-phase high-performance liquid chromatography (HPLC) system (Shimadzu Scientific Instruments, Columbia, MD) equipped with a Phenomenix C18 column (300 x 3.90 mm; pore size, 10 µm). [14C]prostaglandins (PGs) and [14C]thromboxane B2 (TXB2) were eluted with a gradient solvent system containing solvent A [acetonitrile/water/acetic acid (35:65:0.1%)] and solvent B [acetonitrile/water/acetic acid (65:30:0.1%)]. The eluted metabolites were monitored and quantified with an IN/US Systems β-RAM radio-HPLC detector. COX activities are presented as pmol [14C]AA metabolized/mg protein per minute.
Western Blot Assay
Pancreata harvested from mice fed with or without NO-aspirin were homogenized and lysed in ice-cold lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP40, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, 1 mM DTT, and 1x protease inhibitor cocktail] (Sigma, St Louis, MO). After a brief vortexing, the protein lysates were collected by centrifugation at 12,000g for 15 minutes at 4°C, and protein concentrations were measured with the Bio-Rad Protein Assay Reagent (Hercules, CA). Proteins (50 µg protein/lane) were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blocking with 5% milk powder, membranes were probed for expression of PCNA, p21, iNOS, COX-2, β-catenin, Bcl-2, p53, and β-actin in hybridizing solution (1:500, in TBS-Tween 20 solution) using their respective primary antibodies (Cell Signaling Technology [Danvers, MA], AbCam, and Santa Cruz Biotechnology) and then probed with their respective horseradish peroxidase-conjugated secondary antibodies. Detection was performed using the SuperSignal West Pico Chemiluminescence procedure (Pierce, Rockford, IL). The bands were captured on Ewen Parker Blue sensitive X-ray films and quantified by densitometry as previously described [29].
RNA Extraction and Quantitative Reverse Transcription-PCR Assay for Cyclin D1, p21, p38, and COX-2 mRNA Expressions
For cyclin D1, denaturation at 94°C for 3 minutes was followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for the cyclin D1 gene were given as follows: 5′-ATGGAACACCAGCTCCTGTG-3′, sense; 5′-ACCTCCAGCATCCAGGTGGC-3′, anti-sense. For p21, denaturation at 94°C for 3 minutes was followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for p21 were given as follows: 5′-TCCTGGTGATGTCCGACCTG-3′, sense; 5′-TCCGTTTTCGGCCCTGAG-3′, anti-sense. For p38, denaturation at 94°C for 3 minutes was followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for p38 were given as follows: 5′-TATGTGCAGCCGCCCTCCCT-3′, sense; AGCAGTGTGCCCATGGCGTC, antisense. For COX-2, denaturation at 94°C for 2 minutes was followed by 35 cycles at 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 1 minute. Oligonucleotide primer sequences used for COX-2 were given as follows: 5′-CCTGTGCCTGATGATTGC-3′, sense; 5′- CGGTGAAACTCTGGCTAG-3′, anti-sense. PCR was done using the Taq polymerase, 10 mMdeoxynucleoside triphosphates, and buffers from Invitrogen (Carlsbad, CA). The PCR products were visualized and photographed under UV illumination and quantified by densitometry.
Statistical Analysis
The data are presented as means ± SE. Differences in body weights were analyzed by analysis of variance. Statistical differences between control and treated groups were evaluated using Fisher's exact test for PDAC incidence, and unpaired t test with Welch's correction was used for PanINs and PDAC lesions. Differences between groups are considered significant at P < .05.
Results
General Bioassay Observations
Both the p48Cre/+-LSL-KrasG12D/+ and wild-type C57/BL/6 mice fed AIN-76A or NO-aspirin had steady body weight gains. As shown in Figure 3A, there was no significant difference between body weights in the mice fed either AIN-76A or diets supplemented with either 1000 or 2000 ppm NO-aspirin. None of the animals fed the NO-aspirin diets exhibited any observable toxicity or any gross morphologic changes to liver, spleen, kidney, or lung despite notable differences in pancreas weights. There were also no differences in the amount of water or food consumption among the treatment and control mice.
Figure 3.
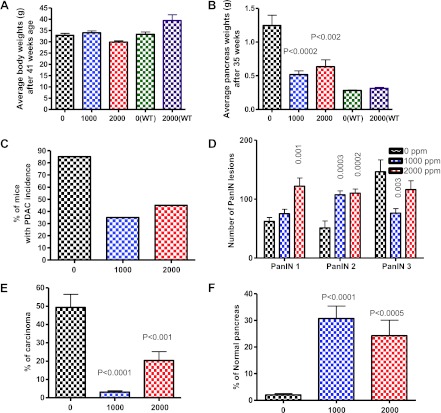
(A) Effect of NO-aspirin on body weight (means ± SE, N = 13 or 12) at the termination of the experiment. No statistically significant difference was observed between control and NO-aspirin-treated p48Cre/+-LSL-KrasG12D/+ or wild-type mice. (B) Effect of NO-aspirin on pancreas weights at the termination. Low-dose NO-aspirin significantly reduced the pancreatic tumor weights compared to high-dose NO-aspirin. (C) Effect of NO-aspirin on the incidence (percentage of mice with carcinomas) of PDAC. (D) Effect of NO-aspirin on the PanIN multiplicity (means ± SE). (E) Percentage of carcinoma spread per pancreas. (F) Effect of NO-aspirin on normal pancreas. The data in H to J were analyzed by unpaired t test with Welch's correction; values are considered statistically significant at P < .05.
PET-CT Noninvasive Imaging of Pancreatic Tumors in Mice
To monitor the tumor progression and confirm the inhibitory activity of NO-aspirin, we performed PET-CT imaging of the wild-type and transgenic mice (n = 4) with or withoutNO-aspirin treatment using FDG. Figure 2 shows the noninvasive PET-CT images of the wild-type, positive, and treated transgenic mice at end of the experiments. There was no tumor detected by this noninvasive imaging in the wild-type mouse pancreas, whereas untreated KrasG12D/+ transgenic mice accumulated high levels of FDG in the pancreas, confirming the presence of metabolically active pancreatic tumor. The FDG accumulation was significantly decreased in the pancreas of the treatment group. The three-dimensional PET-CT images indicated that the tumor growth rate was significantly inhibited in the treatment group when compared to the nontreatment group (Figure 2).
Effect of NO-Aspirin on Pancreas Weight and Progression of PanIN Lesions to PDAC in KrasG12D/+ Mice
Pancreatic weight is one of the important simple markers of PDAC development. In the C57BL/6 wild-type mice, the average pancreas weight was 0.28 g. However, a significant increase in pancreas weight was observed in p48Cre/+-LSL-KrasG12D/+ mice (1.24 g), almost five-fold more than the wild-type mouse pancreas (Figure 3B). A significant decrease in pancreas weights (58.8%, P < .0002 and 49.2%, P < .002) was observed in KrasG12D/+ mice fed 1000 and 2000 ppm NO-aspirin diets, respectively (Figures 1, C–F, and 3B). In addition, mice that were killed before termination (because of sickness) or found dead were ∼20% (4 of 20 mice) in the control group. Low-grade PanINs (1A and 1B) to high-grade PanINs (2 and 3) (Figure 1, G–L) were observed in all the p48Cre/+-LSL-KrasG12D/+ mice fed either AIN-76A diet alone or NO-aspirin diets. Extensive histopathologic analysis suggests that no pathologic alteration was observed in wild-type mice fed either AIN-76A or NO-aspirin-supplemented diets. The efficacy end points used in this study were inhibition of PanINs and PDAC. The criterion used for invasive disease is described in our earlier reports [29].
Figure 3C summarizes the efficacy of NO-aspirin on PDAC incidence in KrasG12D/+ mice. KrasG12D/+ induced an incidence of 85% (percentage of mice with PDAC, 17 of 20 mice developed PDAC), whereas 1000 ppm NO-aspirin-fed mice showed an incidence of 35% PDAC (7 of 20 mice with PDAC) and 2000 ppm NO-aspirin-fed mice had an incidence of 45% carcinoma (9 of 20 mice with PDAC). Control diet-fed KrasG12D/+ mice developed, on the average, about 62 PanIN-1, 51 PanIN-2, and 146 PanIN-3 lesions, whereas mice fed dietary NO-aspirin at 1000 ppm for 35 weeks developed about 75 PanIN-1, 107 PanIN-2, and 76 PanIN-3 lesions and those fed 2000 ppm developed about 121 PanIN-1, 110 PanIN-2, and 116 PanIN-3 lesions, respectively (Figure 3D). Overall, we observed a higher number of PanIN-1 and PanIN-2 lesions with the NO-aspirin treatment when compared to control diet. Most importantly, the carcinomas in situ, i.e., PanIN-3 lesions, were significantly reduced (48% and 20%) with the 1000 and 2000 ppm NO-aspirin treatments, respectively (Figure 3D), suggesting delay of progression. In addition, control diet-fed mice showed about 49% of the pancreas with invasive ductal carcinoma, whereas NO-aspirin-fed mice showed only an average of 3.14% and 20.37% invasive ductal carcinoma, with 1000 and 2000 ppm, respectively (Figure 3E). Furthermore, mice fed 1000 or 2000 ppm NO-aspirin exhibited 30% and 24%, respectively, normal appearing pancreatic tissue (free from the PanINs and PDAC) in comparison to control diet-fed pancreas showing ∼2% to be free from PanINs and PDAC (Figure 3F).
Inhibition by NO-Aspirin of PCNA-Labeled Cell Proliferation and COX-2 in PanINs and PDAC
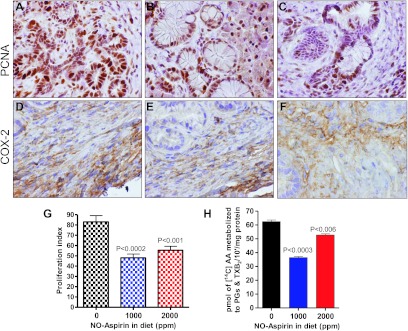
NSAIDs exert their effects in part by inhibiting the cell proliferation, COX-2, and inducing apoptosis. Tumor cell proliferation was measured by PCNA expression in pancreatic tumor tissues. As shown in Figure 4, A to C, PCNA-labeled cells were decreased in p48Cre/+-KrasG12D/+ mice fed NO-aspirin diets as compared with mice fed control diet alone. As shown in Figure 4G, the proliferative index (PI) was 83% in pancreatic tumor tissues of p48Cre/+-LSL-KrasG12D/+ mice on control diet. Mice fed 1000 or 2000 ppm NO-aspirin diet showed a decrease in PI by 41% and 33% (P < .001–.0002), respectively (Figure 4G). As shown in Figure 4, D to F, high expression of COX-2 was observed in the PDAC of KrasG12D/+ mice fed control diets. However, administration of 1000 and 2000 ppm NO-aspirin diet resulted in a significant decrease in COX-2 protein expression levels in the pancreas (Figure 4, D–F).
Figure 4.
Effect of NO-aspirin on cell proliferation (A–C) and COX-2 (D–F) in pancreatic tumors. Immunohistochemical analysis was performed with paraffin-embedded and microsectioned pancreatic tissues as described in Materials and Methods. Immunohistochemical analysis of PCNA (A–C) and COX-2 (D–F) expression in PanIN lesions and in ductal cells. A significantly decreased expression of PCNA and COX-2 was seen with low-dose NO-aspirin treatment. (G) Significant differences in PIs were observed between the groups. (H) Effects of NO-aspirin on COX activity in the pancreas from mice as assessed with the radio-HPLC method. Values are means ± SEM, N = 6 per treatment group. A significant (P < .006 and 0.0003) inhibition of AA metabolites (PGs and TXB2) was observed in the NO-aspirin-treated group.
NO-Aspirin Inhibits Tumor COX Activity
A significant decrease was seen in total COX metabolites in the pancreatic tumor of mice fed low-dose NO-aspirin diet, as determined with radio-HPLC analysis. However, 2000 ppm NO-aspirin showed lesser inhibition. As shown in Figure 4H, mean total PGs and TXB2 levels in control and 1000 and 2000 ppm treated pancreas were 62.4, 36.43 (P < .0003), and 52.7 (15.5%; P < .01) pmol/mg protein per minute, respectively (Figure 4H).
Inhibition of β-Catenin, iNOS, PCNA, COX-2, Cyclin D1, and Bcl-2 and Increase of p21, p38, and p53 by NO-Aspirin
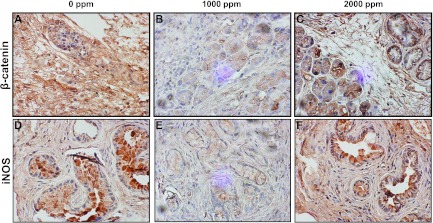
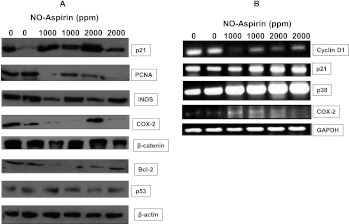
Protein analysis by IHC and Western immunoblot analysis and/or reverse transcription-PCR clearly demonstrated that pancreatic tissues from NO-aspirin-fed mice had significantly lower expression of β-catenin (Figures 5, A–C, and 6A), iNOS (Figures 5, D–F, and 6A), PCNA, COX-2, and Bcl-2 compared with pancreatic tissues from mice fed control diet (Figures 4, D–F, and 6, A and B). Importantly, up-regulation of p21, p38, and p53 expressions were observed in the pancreatic tumor tissues of NO-aspirin-fed mice compared with control diet-fed mice (Figure 6, A and B). The Western blot results have been further confirmed by quantitative analysis performed by densitometry of the blots (Figure W1).
Figure 5.
Effect of NO-aspirin on β-catenin (A–C) and iNOS (D–F) in pancreatic tumors. Immunohistochemical analysis was performed with paraffin-embedded and microsectioned pancreatic tissues as described in Materials and Methods. (A–C) Immunohistochemical analysis of β-catenin, PCNA, and iNOS (D–F) expression in PanIN lesions and in ductal cells. A significantly decreased expression of β-catenin and iNOS expression was seen with low-dose NO-aspirin treatment.
Figure 6.
(A) Effect of NO-aspirin on protein expression levels of p21, PCNA, iNOS, COX-2, β-catenin, Bcl-2, and p53 expression in PDAC. Protein expression was analyzed by Western blot analysis as described in the text. (B) Effect of NO-aspirin on the mRNA expression levels of cyclin D1, p21, p38, and COX-2 as determined with reverse transcription-PCR.
Discussion
Epidemiological and clinical studies, as well as experimental studies, suggest that NSAIDs are effective agents for cancer prevention. COX-2 is overexpressed in both animal and human pancreatic cancers, and several preventive and therapeutic strategies revolve around COX-2 as an important molecular target for chemoprevention [31]. Hence, NSAIDs and COX-2-selective inhibitors have been widely tested for cancer prevention, including that of pancreatic cancer. For example, Funahashi et al. found that the selective COX-2 inhibitor nimesulide delays the progression of pancreatic cancer precursor lesions in the KrasG12D mouse [32]. However, the gastrointestinal toxicities and cardiovascular risks accompanying these agents have prompted a search for novel approaches/agents devoid of unwanted side effects but with similar or higher efficacies. In the present study, we have demonstrated the chemopreventive effects of NO-aspirin against formation of PanIN lesions and their progression to PDAC in p48Cre/+-LSL-KrasG12D/+ mice. This mouse model captures many significant features of human pancreatic cancer development including aspects of histology, molecular biology, and tumor biology and has been well justified for use in chemoprevention studies [8,29]. The delay of PanIN progression to PDAC in the p48Cre/+-LSL-KrasG12D/+ mice shows the potential of NO-aspirin to be an effective chemopreventive agent. We did not observe a dose dependency. In fact, the lower dose of NO-aspirin appeared to be more protective than the higher dose. The reasons for the lack of better inhibitory effects at higher doses needs further investigation.
Results also suggest that NO-aspirin delays the progression from PanIN-1 and PanIN-2 lesions to PanIN-3 and PDAC (Figure 3). Because only PanIN-3 are classified as carcinoma in situ, delaying the progression toward PDAC suggests a major impact on cancer development. Of great interest has been the observation that the long-term administration of NO-aspirin is not associated with overt toxicity. Similar to the observations described here, we have shown previously that an epidermal growth factor receptor (EGFR) inhibitor and atorvastatin inhibit Kras-activated development of PanINs and PDAC [8,29]. This is the first report on the effect of NO-aspirin against pancreatic cancer in a mutant Kras mouse model. However, tumor efficacy effects of NO-aspirin are not new; previously, NO-aspirin was tested for efficacy against chemically induced pancreatic tumors in a hamster model [33] and colon cancers in rats [34]; both studies supported the chemopreventive property of this agent. Furthermore, low-dose NO-aspirin inhibited spread of the carcinoma by 97% (Figure 2E), suggesting that this agent may block invasiveness within the pancreas and further metastasis to nearby organs. The bioassay protocol using PDAC as an end point limited the comparative analysis of overall survival and rate of metastasis in control diet and treatment groups. However, loss of 20% of the mice on the control diets but not in the treated groups supports the notion that NO-aspirin may increase survival by limiting metastasis.
An indirect insight into the mechanism underlying the cell kinetic changes induced by NO-aspirin is provided by our study of the expression of p21WAF1/CIP1 [35]. p21 is activated by p53 to produce cell cycle arrest in response to DNA damage. p21WAF1/CIP1 blocks cell cycle progression, both by acting as a general inhibitor of cyclin dependent kinase (Cdk)/cyclin complexes and by inhibiting DNA replication by binding to PCNA, a subunit of DNA polymerase. We observed a significant increase in p53 and p21 expressions in the low-dose-treated NO-aspirin and reduced expression of tumor promoting markers cyclin D1, β-catenin, COX-2, PCNA, and iNOS. Aspirin and NO-aspirin treatments of colon cancer cells activated the p38 [mitogen-activated protein kinase (MAPK)] pathway along with their respective downstream transcription factors such as c-Jun and activating transcription factor 2 (ATF-2) [36,37]. Aspirin-induced p38 activation precedes rapid ubiquitin-dependent cyclin D1 degradation [37]. Similarly, we observed a dose-dependent increase in p38 and a decrease in cyclin D1 mRNA expression in this study, suggesting that NO-aspirin causes inhibition of cyclin D1 through the p38 MAPK pathway (Figure 6B).
We have previously reported that NO-NSAIDs including NO aspirin have strong inhibitory activity against colon carcinogenesis associated with suppression of COX, iNOS, and β-catenin levels in colon tumors [34]. Here, NO-aspirin showed a good suppression of iNOS, COX-2, and β-catenin protein expressions (Figures 4, D–F, 5, and 6A) corresponding to inhibition of pancreatic tumorigenesis, thereby confirming the previous results in colon cancer. The profound effects of NO-aspirin on cell proliferation, cell cycle, and cell death, combined with the role of MAPK cascades in these cellular events that are critical to carcinogenesis, confirm our findings that NO-aspirin acts, at least in part, by modulating this extensive signal transduction system. Thus, it is apparent that NO-aspirin exerts effects on multiple molecular targets in pancreatic cancer, perhaps more than the present study has revealed. The actual contribution of each of these changes to the chemopreventive effect of this agent remains to be determined. As argued elsewhere regarding the mechanism of action of this compound [19], the choice between mechanistic dominance (one pathway is sufficient for the effect) and mechanistic redundancy (effects on multiple pathways are required) is unresolved and our present study underscores this point.
In summary, our data indicate that NO-aspirin has significant potential for pancreatic cancer prevention and possibly for treatment.
Supplemental Materials and Methods
Acknowledgments
We thank the University of Oklahoma Health Sciences Center Rodent Barrier Facility staff. We also thank Dr Julie Sando for valuable suggestions and editorial help.
Footnotes
We acknowledge the support from the National Cancer Institute (N01CN-53300).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.American Cancer Society, author. Cancer Facts and Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–282. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Eckel F, Schneider G, Schmid RM. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15:1395–1410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed A, Janakiram NB, Lightfoot S, Gali H, Vibhudutta A, Rao CV. Early detection and prevention of pancreatic cancer: use of genetically engineered mouse models and advanced imaging technologies. Current Med Chem. 2012;19:3701–3713. doi: 10.2174/092986712801661095. [DOI] [PubMed] [Google Scholar]
- 5.Ren YX, Xu GM, Li ZS, Song YG. Detection of pointmutation in K-ras oncogene at codon 12 in pancreatic diseases. World J Gastroenterol. 2004;10:881–884. doi: 10.3748/wjg.v10.i6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 7.Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumor biology and progressing translational oncology. Gut. 2011 doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A, Janakiram NB, Li Q, Madka V, Ely M, Lightfoot S, Steele VE, Rao CV. EGFR inhibitor gefitinib prevents progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mice model. Cancer Prev Res. 2010;3:1417–1426. doi: 10.1158/1940-6207.CAPR-10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, Cole BF, Sandler RS, Haile RW, Anhen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 11.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 12.Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, Cameron JL, Hruben RH. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- 13.Hermanova M, Trna J, Nenutil R, Dite P, Kala Z. Expression of COX-2 is associated with accumulation of p53 in pancreatic cancer: analysis of COX-2 and p53 expression in premalignant and malignant ductal pancreatic lesions. Eur J Gastroenterol Hepatol. 2008;20:732–739. doi: 10.1097/MEG.0b013e3282f945fb. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KE, Johnson TW, Lazovich D, Folsom AR. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst. 2002;94:1168–1171. doi: 10.1093/jnci/94.15.1168. [DOI] [PubMed] [Google Scholar]
- 15.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of antiinflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menezes RJ, Huber KR, Mahoney MC, Moysich KB. Regular use of aspirin and pancreatic cancer risk. BMC Public Health. 2002;2:18. doi: 10.1186/1471-2458-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schernhammer ES, Kang JH, Chan AT, Michaud DS, Skinner HG, Giovannucci E, Colditz GA, Fuchs CS. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst. 2004;496:22–28. doi: 10.1093/jnci/djh001. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SC, Giovannucci E, Bergkvist L, Wolk A. Aspirin and nonsteroidal anti-inflammatory drug use and risk of pancreatic cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:2561–2564. doi: 10.1158/1055-9965.EPI-06-0574. [DOI] [PubMed] [Google Scholar]
- 19.Tan XL, Reid Lombardo KM, Bamlet WR, Oberg AL, Robinson DP, Anderson KE, Petersen GM. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res. 2011;4:1835–1841. doi: 10.1158/1940-6207.CAPR-11-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med. 2004;10:324–330. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Iconomou G, Kalofonos HP, Koutras A, Vagenakis AG, Rigas B. Pilot study of NO-donating aspirin in patients with pancreatic cancer pain. J Support Oncol. 2006:168. [PubMed] [Google Scholar]
- 22.Kashfi K, Ryann Y, Qiao LL, Williams JL, Chen J, Del-Soldato P, Traganos F, Rigas B. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells evidence of a tissue type-independent effect. J Pharmacol Exp Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 23.Yeh RK, Chen J, Williams JL, Baluch M, Hundley TR, Rosenbaum RE, Kalala S, Traganos F, Benardini F, del Soldato P, et al. NO-donating nonsteroidal antiinflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: a general pharmacological property? Biochem Pharmacol. 2004;67:2197–2205. doi: 10.1016/j.bcp.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Bak AW, McKnight W, Li P, Del Soldato P, Calignano A, Cirino G, Wallace JL. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998;62:PL367–PL373. doi: 10.1016/s0024-3205(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 25.Williams JL, Kashfi K, Ouyang N, del Soldato P, Kopelovich L, Rigas B. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APCMin/+) mice. Biochem Biophys Res Commun. 2004;313:784–788. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Kashfi K, Borgo S, Williams JL, Chen J, Gao J, Glekas A, Benedini F, Del Soldato P, Rigas B. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J Pharmacol Exp Ther. 2005;312:978–988. doi: 10.1124/jpet.104.075994. [DOI] [PubMed] [Google Scholar]
- 27.Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- 28.Cena C, Lolli ML, Lazzarato L, Guaita E, Morini G, McElroy SP, Megson IL, Fruttero R, Gasco A. Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin. J Med Chem. 2003;46:747–754. doi: 10.1021/jm020969t. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed A, Qian L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951–1962. doi: 10.1002/ijc.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed A, Janakiram NB, Qian L, Choi C, Zhang Y, Steele VE, Rao CV. Chemoprevention of colon and small intestinal tumorigenesis in APCMin/+ mice by licofelone, a novel dual 5-LOX/COX inhibitor: potential implications for human colon cancer prevention. Cancer Prev Res. 2011;4:2015–2026. doi: 10.1158/1940-6207.CAPR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann JR, DuBois RN. Cyclooxygenase-2 and gastrointestinal cancer. Cancer J. 2004;10:145–152. doi: 10.1097/00130404-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ, Eibl G. Delayed progression of pancreatic intraepithelial neoplasia in a conditional KrasG12D mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res. 2007;67:7068–7071. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, Kashfi K, Rigas B. Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res. 2006;66:4503–4511. doi: 10.1158/0008-5472.CAN-05-3118. [DOI] [PubMed] [Google Scholar]
- 34.Rao CV, Reddy BS, Steele VE, Wang CX, Liu X, Ouyang N, Patlolla JM, Simi B, Kopelovich L, Rigas B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther. 2006;5:1530–1538. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 35.Hershenson MB. p21Waf1/Cip1 and the prevention of oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2004;286:L502–L505. doi: 10.1152/ajplung.00336.2003. [DOI] [PubMed] [Google Scholar]
- 36.Hundley TR, Rigas B. Nitric oxide-donating aspirin inhibits colon cancer cell growth via mitogen-activated protein kinase activation. J Pharmacol Exp Ther. 2006;316:25–34. doi: 10.1124/jpet.105.091363. [DOI] [PubMed] [Google Scholar]
- 37.Thoms HC, Dunlop MG, Stark LA. p38-Mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res. 2007;67:1660–1669. doi: 10.1158/0008-5472.CAN-06-1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.