Abstract
First-line treatment of small cell lung cancer (SCLC) with combination chemotherapy consisting of cis-diamminedichloroplatinum(II) (cisplatin) and etoposide is frequently followed by early relapses and a dismal prognosis. Survival of a fraction of tumor cells and development of chemoresistance may be influenced by an initial cellular stress response against the administered xenobiotics. Therefore, we compared the short-term effects of cisplatin and non-cross-resistant bis-[(p-methoxybenzyl)cyclopentadienyl] titanium(IV) dichloride (Titanocene Y) on phosphorylation of 46 sites of a total of 38 signaling proteins in tumor suppressor protein 53 (p53)-wild-type NCI-H526 SCLC cells. The functional significance of selected kinases for the cytotoxicity of both drugs was tested using specific inhibitors and an activator. The cisplatin-induced cellular stress response involved activation of p38α mitogen-activated protein kinase, whereas Titanocene Y-triggered signaling affected c-Jun N-terminal kinase. Phosphorylation of adenosine monophosphate (AMP)-activated protein kinase α1 (AMPKα1) was increased by both drugs, which promoted cell survival, as indicated by results obtained using AMPK inhibitor compound C and AMPK activator 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside. This is in good agreement with previous reports, where AMPKα1 was demonstrated to represent an important factor for the sensitivity to cisplatin in colon and ovarian cancers, most likely by induction of autophagy. Thus, AMPKα1 constitutes a potential target to be exploited for chemotherapeutic treatment of SCLC to circumvent resistance to metal-based compounds.
Introduction
Current mainstay of treatment of patients with advanced cancer is chemotherapy, and cis-diamminedichloroplatinum(II) (cisplatin), introduced into the clinics in the 1970s, is still a well-proven anticancer agent [1]. The established standard of therapy for patients with small cell lung cancer (SCLC), the leading cause of cancer deaths worldwide, is to use a platinum-based agent like cisplatin along with a second chemotherapeutic drug, such as etoposide, docetaxel, gemcitabine, pemetrexed, or vinorelbine [2]. However, crucial drawbacks of platinum-based drugs like cisplatin for anticancer therapy that limit their applicability are side effects such as nephrotoxicity, emetogenesis, neurotoxicity, and occurrence of both inherent and acquired resistance to the drug [3,4]. Resistance may be caused by various mechanisms, i.e., inhibition of drug uptake, inactivation by binding to metallothioneins or glutathione, which is linked to increased export of the cisplatin-glutathione conjugates by multidrug resistance protein 2, or increased repair or replicative bypass of the cisplatin-DNA adducts, respectively [3,5]. The cellular effect of cisplatin consists in formation of interstrand and intrastrand DNA cross-links, which creates double-strand DNA breaks and subsequently activates the cellular damage response. Occurrence of bulky adduct-containing DNA is sensed by the phosphoinositide-3 kinases DNA-dependent protein kinase, ataxia telangiectasia mutated, and ataxia telangiectasia mutated and Rad3-related, respectively [6]. DNA damage signaling is commonly transduced by cascades downstream of phosphoinositide-3 kinase/AKT, tumor suppressor protein 53 (p53), and the mitogen-activated protein kinases (MAPKs), i.e., extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), also termed stress-activated protein kinase, and p38α/β/γ MAPKs [3,7]. Whereas cisplatin triggers induction of ERK, JNK, and p38 MAPK signaling in tumor cells, JNK and p38 MAPK are differentially regulated in response to other platinum-based drugs. Downstream of p38 MAPK or JNK, phosphorylated p53 activates caspases and induces apoptotic cell death [8,9]. Generally, p53 controls both the G2/M and G1 cell cycle checkpoints and mediates growth arrest, when DNA has sustained damage, and activates DNA repair proteins. If the damage proves to be irreparable programmed cell death is initiated by p53 [3,10].
To meet the demand for novel drugs that offer the therapeutic potential to circumvent cellular resistance, coordination complexes with central atoms apart from platinum, in particular transition metals like titanium or ruthenium, lanthanum, copper, or silver have been developed for in vitro and in vivo investigations [11]. A subclass of organometallic compounds termed “metallocenes,” characterized by a transition metal (M) central atom linked to cyclopentadienide (Cp-/C5H5-) ligands and the basic formula Cp2M, has gained increasing interest as promising anticancer agents [12]. Ring-substituted derivatives of titanocene dichloride (Cp2TiCl2) have demonstrated considerable cytotoxicity in a broad spectrum of tumors in vitro and in vivo, revealing highest activity for bis-[(p-methoxybenzyl) cyclopentadienyl] titanium(IV) dichloride (Titanocene Y) with an IC50 value of 5.5 εM, lying within the range of the IC50 value of cisplatin (3.3 εM) [13–15].Whereas cisplatin is known to damage DNA directly, the mode of action of titanocenes has not been straightened out so far [15]. It has been proposed that titanocenes may interfere with gene transcription by direct interaction of titanium with DNA. In this regard, expression of genes involved in cellular zinc uptake was downregulated and transcription of the members of the metallothionein 1 family was induced, as illustrated by treatment of NCI-H526 SCLC cells with the derivative bis-[N,N-dimethylamino-2(N-methylpyrrolyl) methylcyclo-pentadienyl] titanium (IV) dichloride (Titanocene C) and whole-genome gene expression analysis [16]. Disturbed zinc homeostasis may then be followed by cell cycle arrest and apoptosis because of defects in transcription factors and accumulation of misfolded proteins in the ER. Our present study aims at the elucidation of important signal transduction proteins in NCI-H526 cells in response to short-term exposition to Titanocene Y in comparison to cisplatin and to define cellular pathways, which could be responsible for the absence of cross-resistance between the two compounds. Still, in the case of cisplatin as the standard drug for the treatment of SCLC, only two reports describe its effects on signaling phosphoproteins, i.e., JNK and c-Jun in the SCLC cell line SHP77 and the AKT survival pathway in SW2, H82, and U1285 cells, respectively [17,18].
Materials and Methods
Materials
Unless noted otherwise, all chemicals were purchased from Sigma- Aldrich (St Louis, MO). The test compounds Titanocene Y (Figure 1) and Titanocene C were synthesized and kindly provided by Prof. M. Tacke (Dublin, Ireland) [19]. Stock solutions of Titanocene Y and cisplatin were prepared in DMSO and phosphate-buffered saline (PBS), respectively, and stored at -20°C.
Figure 1.
Molecular structure of Titanocene Y.
The kinase inhibitors KRIBB3, SP600125, AR-A014418, PF-573228, SC-68376, compound C, and Src inhibitor-1 were used to investigate the functional roles of heat shock protein 27 (Hsp27), JNK, glycogen synthase kinase 3α/β (GSK-3α/β), focal adhesion kinase (FAK), p38α MAPK, AMP-activated protein kinase α (AMPKα), and Src kinases, respectively. 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) was applied as an activator of AMPK.
Cell Line and Culture Conditions
The NCI-H526 SCLC cell line was obtained from the American Type Culture Collection (Rockville, MD). Cells were grown in suspension in RPMI-1640 bicarbonate medium (Seromed, Berlin, Germany) supplemented with 10% FBS (Seromed), 4 mM glutamine, and antibiotics (10x stock formulated to contain ∼5000 U of penicillin, 5 mg of streptomycin, and 10 mg/ml neomycin; Sigma-Aldrich), checked for mycoplasma contamination (Mycoplasma PCR ELISA; Roche Diagnostics, Vienna, Austria), and subcultured twice a week. DNA profiling by short tandem repeat analysis of the NCI-H526 cells proved their identity to the American Type Culture Collection specifications, and the yeast p53 functional assay revealed expression of fully active p53 (functional assay of separated alleles in yeast FASAY; data not shown).
Chemosensitivity Assays
Cells (1 x 104) in 100 µl of medium per well were distributed in 96-well microtiter plates (Greiner, Kremsmuenster, Austria) and test substances were added in another 100 µl. The test compounds were serially diluted in two-fold steps in triplicate. The microtiter plates were incubated under tissue culture conditions (37°C, 5% CO2, 95% humidity) for 4 days and cell viability was measured using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (EZ4U; Biomedica, Vienna, Austria). This assay quantifies mitochondrial activity of the cells and therefore their viability by generation of a formazan dye from tetrazolium salt through mitochondrial reduction. Optical density was measured using a microplate reader (Eurogenetics, Brussels, Belgium) at 450 nm with an empty well as reference. Values obtained from control wells containing cells and media alone were set to 100% proliferation.
To test for effects of selected kinase inhibitors and an AMPK activator on cytotoxic activities of cisplatin and Titanocene Y, we performed cytotoxicity tests of these compounds as described above using cells incubated either in medium alone or in medium supplemented with fixed concentrations of kinase inhibitors/activator. The doses of the kinase inhibitors had been selected according to their IC80 values determined previously. The percentage of viable cells in medium without inhibitor/activator was subtracted from the percentage of viable cells in the presence of the inhibitor/activator, and the mean value of these differences over the whole concentration range tested is presented as inhibitory/stimulatory activity of JNK inhibitor SP600125, p38α MAPK inhibitor SC-68376, GSK-3 inhibitor AR-A014418, AMPK inhibitor compound C, and AMPK activator AICAR, respectively.
Cell Cycle Analysis
Cells (1 x 106 per well) were incubated with the test compounds in six-well plates for 4 days. Harvested cells were washed with PBS and fixed with 70% ethanol at -20°C for 30 minutes, washed again, transferred into staining solution (20 µg/ml propidium iodide (PI), 5 µg/ml ribonuclease A, and 0.05% Nonidet P40 in PBS), and incubated at room temperature overnight. Washed cells were analyzed by acquisition of 1 x 104 cells in flow cytometry (Cytomics FC500; Beckman Coulter, Krefeld, Germany) at excitation and emission wavelengths of 488 and 675 nm, respectively. The proportion of apoptotic sub-G1 cells was obtained from the logarithmic PI histograms and percentages of cells in cell cycle phases G1/0 (resting), S (DNA synthesis), and G2M (mitotic) were calculated from the linear PI histograms using the MultiCycle AV software (Phoenix Flow Systems, San Diego, CA). Experiments were done in duplicate.
Apoptosis Assay
The occurrence of apoptotic/necrotic cells was assessed by annexin V (AnnV)/PI double staining experiments. Cells (5 x 105), incubated either in medium alone or pretreated with the respective test substance, were harvested and labeled using the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit from Sigma-Aldrich according to the manufacturer's instructions. Following incubation at room temperature for 10 minutes, cells were washed with PBS, labeled with PI (final concentration of 10 µg/ml), and immediately analyzed by flow cytometry. Proportions of intact/viable, apoptotic, late apoptotic/necrotic, or necrotic cells were obtained as AnnV-/PI-, AnnV+/PI-, AnnV+/PI+, or AnnV-/PI+ dot plots, respectively.
Indirect Immunofluorescence Tests
To test for expression of p53, we harvested cells by trypsinization. PBS washed cells were fixed in 70% ethanol at -20°C for 30 minutes and washed again (Eppendorf centrifuge, 12,000 rpm, 5 minutes) before staining with the respective antibody. Native cells (4 x 105) were incubated with primary mouse monoclonal antibody DO-1 in medium (1:50) in microtiter plates (Greiner) at 4°C overnight. Thereafter, cells were stained with goat anti-mouse FITC-labeled IgG secondary antibody at a dilution of 1:50 (4°C, 20 minutes), washed again, and analyzed by flow cytometry. Antigen expression was calculated as relative fluorescence intensity = mean fluorescence antibody/mean fluorescence control.
Determination of Patterns of Protein Phosphorylation
Relative protein phosphorylation levels of 38 selected proteins were obtained by profiling of 46 specific phosphorylation sites using the Proteome Profiler Human Phospho-Kinase Array Kit ARY003 from R&D Systems (Minneapolis, MN) according to the manufacturer's instructions. Briefly, cells were rinsed with PBS, 1 x 107 cells/ml lysis buffer was solubilized under permanent shaking at 4°C for 30 minutes, and aliquots of the lysates were stored frozen at -80°C. Membranes with spotted catcher antibodies were incubated with diluted cell lysates at 4°C overnight. Thereafter, cocktails of biotinylated detection antibodies were added at room temperature for 2 hours. Phosphorylated proteins were revealed using streptavidin-HRP/chemiluminescence substrate (SuperSignal West Pico, Thermo Fisher Scientific, Rockford, IL) and autoradiography films (Amersham Hyperfilm ECL, GE Healthcare Life Sciences, Buckinghamshire, United Kingdom). The resulting spots were scanned and images were quantified using the ImageQuant TL v2005 software (Amersham Biosciences, Buckinghamshire, United Kingdom) and Microsoft Excel software. The specific phosphorylation sites of the proteins investigated in this study covered by the proteome profiler array are listed in Table 1.
Table 1.
Specific Phosphorylation Sites of Proteins Covered by the Proteome Profiler Human Phospho-Kinase Array Kit (ARY003).
| Phosphoprotein | Phosphorylation Site |
| Akt | Ser473 |
| Akt | Thr308 |
| AMPKα1 | Thr174 |
| AMPKα2 | Thr172 |
| Chk-2 | Thr68 |
| c-Jun | Ser63 |
| CREB | Ser133 |
| eNOS | Ser1177 |
| ERK1/2 | Thr202/Tyr204, Thr185/Tyr187 |
| FAK | Tyr397 |
| Fgr | Tyr412 |
| Fyn | Tyr420 |
| GSK-3α/β | Ser21/Ser9 |
| Hck | Tyr411 |
| Hsp27 | Ser78/Ser82 |
| JNK pan | Thr183/Tyr185, Thr221/Tyr223 |
| Lck | Tyr394 |
| Lyn | Tyr397 |
| MEK1/2 | Ser218/Ser222, Ser222/Ser226 |
| MSK1/2 | Ser376/Ser360 |
| p27 | Thr157 |
| p27 | Thr198 |
| p38α | Thr180/Tyr182 |
| p53 | Ser15 |
| p53 | Ser392 |
| p53 | Ser46 |
| p70 S6 kinase | Thr229 |
| p70 S6 kinase | Thr389 |
| p70 S6 kinase | Thr421/Ser424 |
| Paxillin | Tyr118 |
| PLCγ-1 | Tyr783 |
| Pyk2 | Tyr402 |
| RSK1/2 | Ser221/Ser227 |
| RSK1/2/3 | Ser380/Ser386/Ser377 |
| Src | Tyr419 |
| STAT1 | Tyr701 |
| STAT2 | Tyr689 |
| STAT3 | Tyr705 |
| STAT4 | Tyr693 |
| STAT5a | Tyr694 |
| STAT5a/b | Tyr694/Tyr699 |
| STAT5b | Tyr699 |
| STAT6 | Tyr641 |
| TOR | Ser2448 |
| Yes | Tyr426 |
| β-Catenin |
CREB indicates cAMP-response element-binding protein; MSK1/2, mitogen- and stress-activated kinase 1/2; PLCγ-1, phospholipase C, gamma 1; RSK1/2, ribosomal S6 kinase 1/2.
Statistics
Statistical analysis was performed using two-tailed Student's t test for normally distributed samples (*P < .05 was regarded as statistically significant). Values are shown as mean ± SD or SEM.
Results
Cytotoxic Effects of Cisplatin, Titanocene C, and Titanocene Y in NCI-H526 Cells
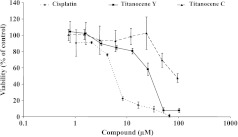
To compare the cytotoxic effects of the two titanocene derivatives Titanocene C and Titanocene Y with that of cisplatin, we incubated NCI-H526 cells with 8 two-fold dilutions of either 0.8 to 96.0 εM Titanocene C, 0.8 to 102.3 εM Titanocene Y, or 1 to 66.7 εM cisplatin for 4 days (Figure 2). The cells proved to be sensitive to both cisplatin (IC50: 12.0 ± 0.5 εM) and Titanocene Y (IC50: 30 ± 10 εM), whereas Titanocene C exhibited significantly lower cytotoxicity (IC50: 93 ± 7 εM). Activity ratios of the compounds amounted to 2.5 for Titanocene Y versus cisplatin and 7.8 for Titanocene C versus cisplatin. Thus, the cytotoxic activity of Titanocene Y was approximately three times higher than that of Titanocene C.
Figure 2.
Dose-response curves for cisplatin, Titanocene Y, or Titanocene C, respectively, obtained in NCI-H526 cells using MTT proliferation assays. Values are presented as mean ± SD (n = 3).
Effects of Cisplatin and Titanocene Y on Cell Cycle Distribution of NCI-H526 Cells
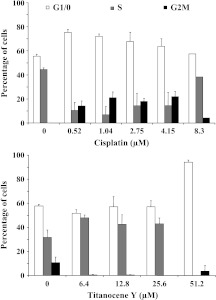
To assess alterations of cell cycle distribution induced by the test compounds, we treated NCI-H526 cells with two-fold dilutions of either 0.5 to 8.3 εM cisplatin or 0.8 to 102.4 εM Titanocene Y for 3 days and determined cell cycle distributions following PI staining of the cells by flow cytometry (Figure 3). Treatment of the cells with cisplatin resulted in a reduction of the proportion of cells in G1/0 and S phases and increases in the G2M phase fraction (except at a concentration of 8.3 εM cisplatin, owing to a low number of viable cells left). Unlike cisplatin, treatment with Titanocene Y led to significant growth arrest in S phase at a medium concentration as well as in G1/0 phase at 51.2 εM. Exceeding concentrations were not evaluated, because only a low number of viable cells was remaining following the incubation with the compounds.
Figure 3.
Cell cycle alterations induced by treatment of NCI-H526 cells with either cisplatin or Titanocene Y at the indicated concentrations. Values are presented as mean ± SD (n = 2). Differences to control cells were statistically significant for cisplatin for G1/0 phase for dilutions of 0. 52 to 2.75 εM versus control and for the S and G2M phases over the whole concentration range, except at 8.3 εM, versus control. Differences to control cells were statistically significant for Titanocene Y for G1/0 phase at 51.2 εM, for S phase over the whole concentration range, and for G2M phase for all concentrations, except 51.2 εM Titanocene Y.
AnnV Assay of the Induction of Apoptosis in Response to Cisplatin and Titanocene Y
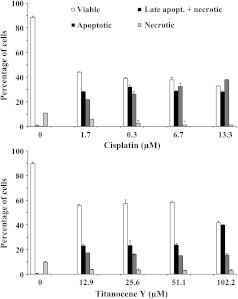
Investigation of the type of cell death was carried out by flow cytometric analysis of NCI-H526 cancer cells following incubation with cisplatin or Titanocene Y for 3 days and double labeling of the treated cells with AnnV-FITC and PI. Figure 4 shows the proportions of apoptotic or late apoptotic/necrotic cells, respectively, resulting from drug treatment. The data indicate significant induction of apoptotic cell death in NCI-H526 cells in response to 0.3 to 13.3 εM cisplatin and a similar but less pronounced induction of apoptosis in response to 12.9 to 51.1 εM Titanocene Y, with highest toxicity at 102.2 εM of this drug.
Figure 4.
AnnV assay of the induction of apoptotic cell death in NCI-H526 cells in response to cisplatin (top) and Titanocene Y (bottom). Columns depict the proportion of viable, apoptotic, late apoptotic/necrotic, and necrotic cells, respectively, following exposure to the indicated concentrations of the drugs. All differences in the percentage of populations to the untreated control cells are statistically significant for cisplatin and Titanocene Y. At a concentration of 102.2 εM Titanocene Y, the proportion of apoptotic cells was significantly increased in relation to the other concentrations tested.
Phosphorylation of Signaling Mediators in Response to Cisplatin and Titanocene Y
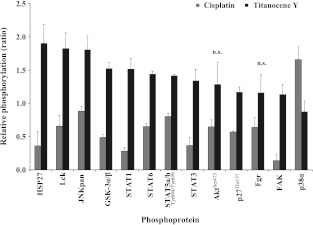
To identify cellular signal transduction pathways involved in the short-time effects of cisplatin and Titanocene Y on NCI-H526 cells, 46 specific Ser/Thr or Tyr phosphorylation sites of 38 selected proteins were analyzed by phosphoprotein arrays. Therefore, NCIH526 cells were incubated with either 65 εM cisplatin or 102 εM Titanocene Y for 6 hours and then subjected to analysis using the respective array. Levels of the proteins with markedly altered phosphorylation obtained by normalization of the values to untreated control cells are shown in Figure 5, A and B. The pattern of protein phosphorylation was found to be significantly different for the two test compounds, and a number of important signal transduction mediators were involved. Phosphorylation levels of the following proteins at their specific activation/inactivation sites were significantly increased by Titanocene Y (Figure 5): Hsp27 (1.90 ± 0.28-fold over control), lymphocyte-specific protein tyrosine kinase (Lck; 1.82 ± 0.24-fold over control), GSK-3α/β (1.52 ± 0.09-fold over control), signal transducers and activators of transcription (STAT1, 1.51 ± 0.17-fold over control; STAT6, 1.44 ± 0.04-fold over control; STAT5a/b, 1.41 ± 0.02-fold over control; and STAT3, 1.34 ± 0.17-fold over control), and p27Kip1 protein (p27-Thr157; 1.17 ± 0.08-fold over control). Protein kinase B (Akt-Ser473; 1.28 ± 0.34-fold over control), Fgr kinase (Fgr; 1.16 ± 0.27-fold over control), and FAK (1.13 ± 0.15-fold over control) exhibited increased phosphorylation that was not statistically significant.
Figure 5.
Relative levels of protein phosphorylation (ratios of phosphorylation of treated cells/untreated cells) for the panel of proteins exhibiting opposing modification in response to either cisplatin or Titanocene Y in NCI-H526 cells. With exception of p38α MAPK, all proteins revealed increased phosphorylation in response to Titanocene Y, whereas cisplatin yielded decreased or unchanged phosphorylation (all differences significant, except for Akt-Ser473 and Fgr). Phosphorylation levels of all proteins were different from a ratio r = 1.0, with exception of JNKpan for cisplatin and p38α MAPK for Titanocene Y, respectively.
In contrast to Titanocene Y, cisplatin reduced phosphorylation levels of Hsp27 (0.36 ± 0.21-fold over control), Lck (0.66 ± 0.16- fold over control), JNKpan (0.88 ± 0.08-fold over control), GSK-3α/β (0.49 ± 0.04-fold over control), Akt (Ser473; 0.65 ± 0.10-fold over control), STAT1 (0.28 ± 0.05-fold over control), STAT6 (0.65 ± 0.05-fold over control), STAT5a/b (0.80 ± 0.05-fold over control), STAT3 (0.37 ± 0.12-fold over control), p27 (Thr157; 0.57 ± 0.01-fold over control), Fgr (0.64 ± 0.15-fold over control), and FAK (0.14 ± 0.09-fold over control) significantly. Furthermore, phosphorylation of p38α MAPK was markedly increased in comparison to Titanocene Y. With exception of Akt, all differences between cisplatin- and Titanocene Y-treated cells were significant for this first group of phosphoproteins (Figure 5). Most strikingly, phosphorylation of the stress kinases JNK and p38α MAPK in response to the two drugs was opposed, i.e., each of those two kinases exhibited increased phosphorylation by only one of the compounds, whereas phosphorylation was not altered following treatment with the other drug. In detail, phosphorylation of JNK was enhanced 1.81 ± 0.2-fold over control in Titanocene Y-treated NCI-H526 cells but marginally affected by cisplatin, whereas p38α MAPK was 1.66 ± 0.19-fold over control higher phosphorylated by cisplatin treatment but barely sensitive to Titanocene Y.
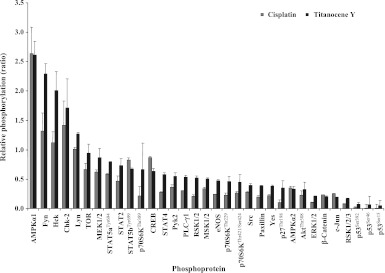
Furthermore, Figure 6 depicts proteins with single-direction phosphorylation changes in response to either Titanocene Y or cisplatin, respectively. In contrast to the first group of contrariwise modified phosphoproteins shown above, these remaining proteins exhibited concurrent changes in respect to increased phosphorylation of AMPKα1, Fyn, Hck, Chk-2, and Lyn and decreased phosphorylation of all remaining proteins. For both groups of protein phosphorylation patterns, all differences in the ratios between cisplatin- and Titanocene Y-treated NCI-H526 cells were statistically significant, with the exception of Akt and Fgr.
Figure 6.
Relative levels of protein phosphorylation (ratios of phosphorylation of treated cells/untreated cells) for the panel of proteins exhibiting concurrent modifications in response to either cisplatin or Titanocene Y in NCI-H526 cells.
Furthermore, a number of proteins exhibited parallel phosphorylation following incubation of the cells with either cisplatin or Titanocene Y. In detail, phosphorylation of AMPKα1 was 2.61-fold increased by cisplatin and 2.64-fold by Titanocene Y, respectively. Enhanced phosphorylation of Fyn (2.29 ± 0.17-fold over control), Hck (2.01 ± 0.32-fold over control), and Lyn (1.27 ± 0.03-fold over control) was significant in response to Titanocene Y; Fyn phosphorylation was increased following exposure to cisplatin (1.32 ± 0.31-fold over control); however, this change did not reach statistical significance. Similarly, Chk-2 phosphorylation was enhanced to 1.71 ± 0.49-fold over control by Titanocene Y and 1.42 ± 0.41-fold over control by cisplatin. TOR and MEK1/2 phosphorylation was significantly decreased after treatment with cisplatin (TOR, 0.66 ± 0.11-fold over control; MEK1/2, 0.63 ± 0.03-fold over control), whereas Titanocene Y affected these proteins only to a minor degree (mitogen-activated protein kinase kinase 1/2 [MEK1/2], 0.87 ± 0.15-fold over control; target of rapamycin [TOR], 0.94 ± 0.16-fold over control). All other phosphoproteins, except p70S6K in response to Titanocene Y, displayed decreased phosphorylation levels. Table 2 lists the remaining proteins where phosphorylation was regulated in parallel for both cisplatin and Titanocene Y. Analysis of all these proteins where phosphorylation was altered in the same direction by both cisplatin and Titanocene Y followed a linear regression (y = 1.0776x + 0.1598) and gave a significant correlation with a coefficient of R2 = 0.8524 (P < .001).
Table 2.
Relative Phosphorylation (Treatment/Control) of Proteins Regulated in Parallel in Response to Exposure of NCI-H526 Cells to Cisplatin and Titanocene Y.
| Parallel Regulation | Cisplatin | SD | Titanocene Y | SD | |
| Phosphorylated Protein | Mean | Mean | |||
| AMPKα1 | 2.64 | 0.44 | 2.61 | 0.23 | NS |
| Fyn | 1.32 | 0.31 | 2.29 | 0.17 | NS |
| Hck | 1.12 | 0.18 | 2.01 | 0.32 | NS |
| Chk-2 | 1.42 | 0.41 | 1.71 | 0.49 | NS |
| Lyn | 1.01 | 0.03 | 1.27 | 0.03 | * |
| TOR | 0.66 | 0.11 | 0.94 | 0.16 | NS |
| MEK1/2 | 0.63 | 0.03 | 0.87 | 0.15 | NS |
| STAT5a-Tyr694 | 0.58 | 0.02 | 0.80 | 0.01 | * |
| STAT2 | 0.47 | 0.08 | 0.74 | 0.11 | NS |
| STAT5b-Tyr699 | 0.83 | 0.03 | 0.67 | 0.02 | * |
| p70S6K-Thr389 | 0.22 | 0.16 | 0.67 | 0.45 | NS |
| CREB | 0.87 | 0.02 | 0.63 | 0.05 | * |
| STAT4 | 0.28 | 0.01 | 0.58 | 0.04 | * |
| Pyk2 | 0.36 | 0.04 | 0.55 | 0.06 | NS |
| PLC-γ1 | 0.30 | 0.01 | 0.54 | 0.03 | * |
| RSK1/2 | 0.21 | 0.02 | 0.52 | 0.03 | * |
| MSK1/2 | 0.34 | 0.02 | 0.50 | 0.02 | * |
| eNOS | 0.24 | 0.00 | 0.47 | 0.02 | * |
| p70S6K-Thr229 | 0.23 | 0.04 | 0.46 | 0.09 | NS |
| p70S6K-Thr421/Ser424 | 0.26 | 0.03 | 0.45 | 0.13 | NS |
| Src | 0.28 | 0.02 | 0.40 | 0.03 | * |
| Paxillin | 0.20 | 0.02 | 0.39 | 0.01 | * |
| Yes | 0.22 | 0.02 | 0.38 | 0.02 | * |
| p27-Thr198 | 0.11 | 0.04 | 0.35 | 0.12 | NS |
| AMPKα2 | 0.35 | 0.03 | 0.33 | 0.05 | NS |
| Akt-Thr308 | 0.23 | 0.07 | 0.32 | 0.13 | NS |
| ERK1/2 | 0.11 | 0.01 | 0.21 | 0.01 | * |
| β-Catenin | 0.23 | 0.00 | 0.21 | 0.01 | NS |
| c-Jun | 0.25 | 0.01 | 0.19 | 0.00 | NS |
| RSK1/2/3 | 0.08 | 0.00 | 0.17 | 0.01 | NS |
| p53-Ser392 | 0.02 | 0.01 | 0.08 | 0.03 | NS |
| p53-Ser46 | 0.01 | 0.07 | 0.07 | 0.14 | NS |
| p53-Ser15 | 0.01 | 0.07 | 0.05 | 0.10 | NS |
Ratios are presented as mean ± SD; NS, difference not significant.
Difference significant, P < .05.
Considerable decreases in phosphorylation of more than 90% of control were furthermore observed for p53 for treatment with either cisplatin or Titanocene Y (cisplatin: p53 Ser392, 0.02 ± 0.01-fold over control; p53 Ser46, 0.01 ± 0.07-fold over control; p53 Ser15, 0.01 ± 0.07-fold over control and Titanocene Y: p53 Ser392, 0.08 ± 0.03-fold over control; p53 Ser46, 0.06 ± 0.14-fold over control; p53 Ser15, 0.05 ± 0.10-fold over control).
Effects of Cisplatin and Titanocene Y on p53 Protein Level of NCI-H526 Cells
The p53 protein level of NCI-H526 cells was quantified by indirect immunofluorescence using the DO-1 mouse monoclonal antibody that recognizes an N-terminal epitope between amino acid residues 11 to 25 of wild-type and mutant p53. Comparisons between untreated and drug-treated SCLC cells revealed a reduction of the protein levels of p53 of 82% and 79% in response to the 6-hour exposure to cisplatin and Titanocene Y, respectively (data not shown).
Effects of Kinase Inhibitors and an AMPK Activator on Cytotoxic Activities of Cisplatin and Titanocene Y against NCI-H526 Cells
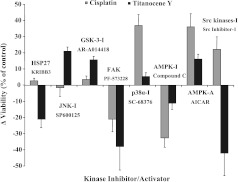
The functional roles of Hsp27, JNK, GSK-3α/β, FAK, p38α MAPK, AMPKα, and Src kinases in regard to the cytotoxic activities of cisplatin and Titanocene Y against NCI-H526 cells were tested using the inhibitors KRIBB3 (6.3 εM), SP600125 (12.0 εM), AR-A014418 (12.0 εM), PF-573228 (0.6 εM), SC-68376 (6.0 εM), compound C (1.25 εM), and Src inhibitor-1 (0.6 εM), as well as AICAR (5.0 εM), an agent that stimulates AMPK activity (Figure 7). IC50 values for the respective compound in NCI-H526 cells were >1.0 mM for AICAR, >50 εM for AR-A014418, SP600125, SC-68376, and KRIBB3, 6.3 εM for compound C, and 2.5 εM for both Src inhibitor-1 and PF-573228, respectively.
Figure 7.
Effects of selected phosphoprotein inhibitors on the cytotoxic activities of cisplatin and Titanocene Y. Assays were performed using NCI-H526 cells and the two drugs in the absence or presence of inhibitors (I) of Hsp27, JNK, GSK3α/β, FAK, p38α MAPK, AMPK, and Src, as well as AMPK activator (A) AICAR. Values represent the mean differences (± SEM) of cytotoxic activities in the presence and absence of the inhibitors/activator over six concentrations of cisplatin or Titanocene Y. A positive difference indicates increased survival of cells in the presence of the respective inhibitor/activator and the drug and a cell death-promoting role of the phosphoprotein, whereas a negative difference indicates an opposed effect on cell death of the respective phosphoprotein. All differences to controls (0% change) were statistically significant, except for KRIBB3, SP600125, and AR-A014418 in combination with cisplatin.
Inhibition of p38α MAPK and Src kinases as well as activation of AMPKα decreased the cytotoxic effect of cisplatin, which indicates a death-promoting activity of the two former kinases and a protective role for AMPKα. Accordingly, inhibition of AMPKα by compound C reduced the cytotoxicity of cisplatin, and a similar effect was detected for the inhibition of FAK. The cytotoxic effect of Titanocene Y was impeded by the activities of Hsp27, FAK, AMPKα, and Src kinases, whereas phosphorylation of JNK and GSK-3α/β resulted in increased cell death. Thus, the main kinases regulated differently, which promote the cytotoxicity of cisplatin and Titanocene Y, respectively, are p38α MAPK and JNK, whereas the main effectors of resistance seem to be FAK and AMPKα for both chemotherapeutics, as well as Hsp27 and Src kinases in the case of Titanocene Y.
Discussion
In two thirds of SCLC patients, disease has already disseminated at time of presentation, and although these tumors are highly sensitive to first-line cytotoxic therapy, the rapid appearance of chemoresistance/radioresistance results in early relapses and low survival rate [36]. Clinical trials of new agents have been disappointing, and still, platinum- and vepeside-based regimens are the standard therapy [2]. Cisplatin, displaying clinical activity against a wide variety of solid tumors, is one of the most potent antitumor agents employed [1]. The aim of the present work was to study the cisplatin-triggered signaling in the p53-wild-type SCLC cell line NCI-H526 derived from a bone metastasis of a patient before therapy in respect to pathways that may be involved in the development of resistance and to compare them with a titanocene derivative that does not exhibit cross resistance to platinum drugs in cancer cells [15]. We demonstrated that NCI-H526 cells were sensitive to both cisplatin and Titanocene Y displaying IC50 values of 12 and 30 εM, respectively. Alteration in cell cycle distribution induced by Titanocene Y, namely, arrest in the S and G1/0 phases, differs from that of cisplatin, resulting in G2M arrest mainly. Application of both chemotherapeutic drugs to NCI-H526 cells induced significant apoptotic cell death, with higher activity found for the platinum compound.
Next, we compared the short-term effects of both drugs on selected mediators of signal transduction to detect putative distinctions in their intracellular modes of action. Six-hour exposure of the cells with the indicated concentrations of the compounds for subsequent assessment using the phosphoprotein array aimed at matching with the expected highest plasma concentrations in patients. According to the literature, an intravenous bolus injection of 100 mg/m2 cisplatin leads to an immediate peak blood level of 20 εM and a decrease to 6.7 εM within 2 hours [20]. In contrast, pharmacokinetic studies of Titanocene Y are still lacking. However, the pharmacokinetics of a lyophilized formulation of titanocene dichloride (MKT4) gave a biologic plasma half-life of 22.8 ± 11.2 hours and a Cmax of approximately 30 εg/ml at a dose of 420 mg/m2 [21]. The NCI-H526 cells were treated for a period of 6 hours with concentrations of cisplatin or Titanocene Y that in both cases would have resulted in less than 10% of survival in MTT assays after 4 days of incubation. Analysis of the arrays of treated versus untreated cells revealed a set of inversely phosphorylated proteins with increased phosphorylation in response to Titanocene Y, whereas phosphorylation was reduced in response to cisplatin. Exceptions were elevated phosphorylation of p38α MAPK in case of cisplatin and basal phosphorylation levels of both p38α MAPK in response to Titanocene Y and JNK in response to cisplatin. In general, increased phosphorylation of Hsp27, Lck, JNK, GSK-3, STATs, and FAK is linked to higher resistance to platinum complexes and other chemotherapeutics. In detail, expression of Hsp27 and Hsp70, implicated in the mediation of antiapoptosis, was upregulated in cisplatin-resistant ovarian tumor cell lines [22,23]. Aberrant expression or activation of Lck was reported for both lymphoid and nonlymphoid malignancies [24]. Lck stimulates both tyrosine phosphorylation and DNA-binding activity of STAT5b, generally induced in a variety of tumor cells. Activated Lck protects murine hemopoietic BaF3 pro-B cells from interleukin-3 withdrawal-induced apoptosis and is involved in fractionated radiation-induced expansion of the CD133+ glioma-initiating cell population and in acquisition of resistance to cisplatin and etoposide in glioma cells [25].
The three major pathways of MAPKs, namely, those involving JNK, p38α MAPK, or extracellular signal-regulated kinase 1/2 (ERK1/2), respectively, control cell proliferation, differentiation, and cell death. JNK and p38α MAPK are key effectors of stress and inflammation responses evoked by a variety of physical, chemical, and biologic stress stimuli, whereas ERK1/2 is mostly induced by growth factors. Their role in the response to cisplatin is complex as these proteins, in most cases, are able to either induce or suppress apoptosis or to be irrelevant in this process [26]. Cisplatin, as well as the less effective isomer trans-platin, stimulated the activity of JNK in SCLC cells, i.e., the response to trans-platin was rapid and transient, whereas JNK activation by cisplatin was delayed and sustained [27,28]. One of the downstream targets of JNK is GSK-3α/β, a multifunctional Ser/Thr kinase negatively regulated by phosphorylation of Ser9 [29]. Occurrence of pGSK-3β-Ser9 cells was considerably enhanced in resistant CP70 compared to native A2780 cells [30]. Inhibition of GSK-3α/β as indicated by increased phosphorylation after treatment with Titanocene Y along with enhanced activity of JNK plays a role in induction of the observed apoptotic response of the NCI-H526 cells.
FAK protein expression was early downregulated in a testicular tumor germ cell line by cisplatin, which thus failed to activate the expression of the inhibitors of apoptosis, c-IAP-2 and XIAP, resulting in a defect in the abrogation of caspase activation [31]. Simultaneous knockdown of RelA and STAT5B sensitized tumor cells to carboplatin treatment most effectively. Similarly, the nuclear factorkappa B (NF-κB) inhibitor BMS-345541 as well as the STAT5 inhibitor dasatinib significantly enhanced sensitivity of the cells to carboplatin. Furthermore, silencing of p27 enhanced the cytotoxic effect of cisplatin, doxorubicin, and gemcitabine in both RMG-I and SMOV-2 ovarian cancer cells [32]. In conclusion, down-regulation of the protein phosphorylation in response to cisplatin in NCI-H526 cells points to induction of apoptotic cell death, whereas up-regulation of the phosphorylation of the same set of proteins following exposure to Titanocene Y seems to be in favor of a partially antiapoptotic response. The roles of JNK and p38α MAPK vary in different tumor entities, and thus, their respective implications in the cytotoxic effects of cisplatin or Titanocene Y cannot be generally deduced from this assessment of phosphorylation solely.
A second group of phosphoproteins showed concurrent alterations, namely, either increased or decreased phosphorylation, respectively, in response to cisplatin and Titanocene Y. Most prominently affected was AMPKα1, which regulates the energy balance system by monitoring the intracellular energy status and confers resistance to cisplatin [33–35]. Inhibition of the AMPK pathway sensitized human leukemia K562 cells to doxorubicin [36]. Similarly, AMPK was rapidly activated by cisplatin in AGS gastric cancer and HCT116 colon cancer cells, and compound C-mediated inhibition of AMPK in those cells as well as in HCT116 xenografts resulted in a remarkable increase in cisplatin-induced apoptosis [33]. From this, it was concluded that AMPK fulfills a pivotal function for protection against the cytotoxic effect of cisplatin. On the contrary, the AMPK activator metformin suppressed growth and metastasis of ovarian cancer cells with enhancement of the cytotoxicity of cisplatin in vivo [37,38]. Increases in cellular AMP and subsequent activation of AMPK in response to cisplatin were primarily attributed to cellular damage by reactive oxygen species (ROS). Furthermore, c-Src, a tyrosine kinase involved in tumor proliferation, migration, and angiogenesis, was reported to modulate the cytotoxic effect of cisplatin-induced DNA damage [39]. Treatment with dasatinib abrogated Src phosphorylation in the majority of non-SCLC cell lines tested, with modest effects on cell proliferation and survival. Moreover, cisplatin in combination with dasatinib exhibited higher cytotoxicity in five cell lines and blocked transcription of a panel of DNA repair genes. Additionally, p70S6K and AKT/mammalian target of rapamycin (mTOR) survival pathways were shown to play an important role in resistance to cisplatin in human ovarian cancer cells [40]. A yeast complementation assay using our NCI-H526 cell extracts proved functional p53 protein (data not shown). Furthermore, p53 underwent extensive degradation in response to both compounds cisplatin and Titanocene Y in these cells. Basically, levels of wild-type p53 are very low under normal conditions; however, p53 was found to be highly phosphorylated in untreated NCI-H526 cells and became dephosphorylated and degraded upon drug treatment. The present study aimed at a first assessment of a large panel of phosphoproteins, and because the protein content of the respective kinases was not measured, it has to be taken into account that the observed alterations may be either because of a variation in the number of phosphorylated sites or amount of expressed protein. However, according to published reports, treatment of different cell lines with cisplatin for up to 24 hours did not alter protein expression of p38α MAPK, JNK, and AMPKα [33,41,42].
In conclusion, most of the phosphoproteins that were hyperphosphorylated by Titanocene Y but hypophosphorylated by cisplatin in NCI-H526 cells seem to counteract or promote apoptosis, respectively, with the exception of JNK and p38α MAPK, which are activated by either cisplatin or Titanocene Y. To prove the specific roles of Hsp27, JNK, GSK-3α/β, FAK, p38α MAPK, AMPKα, and Src kinases in regard to survival of NCI-H526 cells, the effects of the respective kinase inhibitors along with an activator of AMPKα on the cytotoxicity of cisplatin and Titanocene Y were investigated. The experiments indicated that JNK, activated by Titanocene Y, transmits signals that result in NCI-H526 cell death, whereas p38α MAPK fulfills an identical function for cisplatin. After exposure of a panel of cell lines to cisplatin, doxorubicin, or taxol, respectively, it was merely cisplatin that was capable of activating p38 MAPK, with two- to threefold higher stimulation of p38 MAPK compared to JNK [43]. Therefore, the preferred induction of JNK versus p38α MAPK seems to be linked to the specific DNA damage in response to Titanocene Y, which does not involve double-strand breaks, in contrast to cisplatin [44]. In addition to AMPKα, FAK plays a prosurvival role for both cisplatin and Titanocene Y. The higher IC50 value of this titanocene compared to cisplatin may be because of the activation of Hsp27 and Src kinases, which have an antiapoptotic role. It should be stressed that the kinase inhibitors employed basically affect phosphorylation sites of the respective target kinases and not their level of protein expression.
In general, cisplatin like most metals, induces ROS and thus triggers cell death, apart from its DNA damaging effect [45,46]. ROS stimulate proapoptotic signaling molecules such as apoptosis signal-regulating kinase 1, JNK, and p38 MAPK [47]. Transient low-level activity of stress-activated protein kinases promotes cell proliferation, whereas persistent, high-level activity results in cell death. In the transformed cells, where basal stress signaling is potentiated compared to normal cells, additional stress stimuli induce substantially higher levels of ROS and stress kinase activity, which in turn augments the apoptotic response of those cells [48]. Because of an elevated antioxidant capacity in advanced cancer cells, the ROS levels decrease and stress kinase activity is suppressed, resulting in greater stress resistance.
Stimulation of AMPK in cells exposed to anticancer drugs leads to increased chemoresistance, and recent work has proposed a mechanism of counteracting the cytotoxic action of the drugs by this kinase. Dose- and time-dependent induction of autophagy was observed in human U251 glioma, rat C6 glioma, and mouse L929 fibrosarcoma cells after treatment with cisplatin, as demonstrated by up-regulation of autophagy-inducing protein beclin-1 and subsequent appearance of acidic autophagic vesicles [49]. Inhibition of autophagy with lysosomal inhibitors augmented cisplatin-triggered DNA fragmentation and apoptotic cell death markedly. The induction of autophagy in cisplatin-treated cells was preceded by activation of AMPK and concomitant down-regulation of mTOR-mediated phosphorylation of p70S6 kinase. siRNA-mediated knockdown of AMPK, just as the AMPK inhibitor compound C, increased cisplatin-induced cell death, whereas silencing of mTOR, as well as the AMPK activator metformin, protected cells from death in response to cisplatin.
In summary, activation of AMPK seems to play an important role in the antiapoptotic process in response to cisplatin and Titanocene Y in the NCI-H526 SCLC cell line, which had been derived from a bone metastasis from a patient before therapy. This is in good agreement with reports on the role of AMPK on the effects of cisplatin and doxorubicin in gastric and colon cancer cell lines, as well as a leukemia cell line, respectively [33,36]. For both metal-based compounds investigated in the present study, cisplatin and Titanocene Y, activation of AMPK was prominent and found to be a determinant of chemosensitivity of NCI-H526 SCLC cells to the respective compound, which was demonstrated functionally by the AMPK inhibitor compound C and the AMPK activator AICAR, respectively. AMPK-triggered autophagy may constitute a key mechanism, which protects a fraction of the SCLC cells from first-line chemotherapy with cisplatin and, presumably, other metal-based compounds and may thus account for early regrowth of resistant tumor cells. Thus, interference with AMPK activation may represent an interesting therapeutic target to improve the outcome of metal-based chemotherapy in different tumor entities. The efficacy of Titanocene Y seems to be retarded or hampered by a partial induction of antiapoptotic signaling, which, however, seems to be counterbalanced by proapoptotic pathways, ultimately resulting in significant anticancer toxicity at higher concentrations. Again, these findings underline differential mechanisms of action of cisplatin and Titanocene Y.
Acknowledgments
We would like to thank Eva-Maria Schuster for help in the characterization of the NCI-H526 cell line.
Footnotes
This project was funded in part (in Ireland) by the Higher Education Authority (HEA) through a PRTLI4 grant and the Medical Scientific Fund of the Mayor of the City of Vienna (No. 11016). The authors declare that they have no conflict of interest.
References
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Califano R, Abidin AZ, Peck R, Faivre-Finn C, Lorigan P. Management of small cell lung cancer: recent developments for optimal care. Drugs. 2012;72:471–490. doi: 10.2165/11597640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 4.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Kuo MT. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Met Based Drugs. 2010;2010:430939. doi: 10.1155/2010/430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp MG, Lindsey-Boltz LA, Sancar A. The DNA damage response kinases DNA-dependent protein kinase (DNA-PK) and ataxia telangiectasia mutated (ATM) are stimulated by bulky adduct-containing DNA. J Biol Chem. 2011;286:19237–19246. doi: 10.1074/jbc.M111.235036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hublarova P, Greplova K, Holcakova J, Vojtesek B, Hrstka R. Switching p53-dependent growth arrest to apoptosis via the inhibition of DNA damage-activated kinases. Cell Mol Biol Lett. 2010;15:473–484. doi: 10.2478/s11658-010-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathi R, Samadder T, Gupta S, Surolia A, Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol Cancer Ther. 2011;10:255–268. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- 9.Yano T, Itoh Y, Matsuo M, Kawashiri T, Egashira N, Oishi R. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. Apoptosis. 2007;12:1901–1909. doi: 10.1007/s10495-007-0110-8. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol. 2011;1:978312. doi: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbutcheon-Singh KB, Grant MP, Harper BW, Krause-Heuer AM, Manohar M, Orkey N, Aldrich-Wright JR. Transition metal based anticancer drugs. Curr Top Med Chem. 2011;11:521–542. doi: 10.2174/156802611794785226. [DOI] [PubMed] [Google Scholar]
- 12.Chavain N, Biot C. Organometallic complexes: new tools for chemotherapy. Curr Med Chem. 2010;17:2729–2745. doi: 10.2174/092986710791859306. [DOI] [PubMed] [Google Scholar]
- 13.Fichtner I, Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M. Antitumor activity of Titanocene Y in xenografted Caki-1 tumors in mice. Anticancer Drugs. 2006;17:333–336. doi: 10.1097/00001813-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Bannon JH, Fichtner I, O'Neill A, Pampillón C, Sweeney NJ, Strohfeldt K, Watson RW, Tacke M, Mc Gee MM. Substituted titanocenes induce caspase-dependent apoptosis in human epidermoid carcinoma cells in vitro and exhibit antitumour activity in vivo. Br J Cancer. 2007;97:1234–1241. doi: 10.1038/sj.bjc.6604021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olszewski U, Hamilton G. Mechanisms of cytotoxicity of anticancer titanocenes. Anticancer Agents Med Chem. 2010;10:302–311. doi: 10.2174/187152010791162261. [DOI] [PubMed] [Google Scholar]
- 16.Olszewski U, Claffey J, Hogan M, Tacke M, Zeillinger R, Bednarski PJ, Hamilton G. Anticancer activity and mode of action of Titanocene C. Invest New Drugs. 2011;29:607–614. doi: 10.1007/s10637-010-9395-5. [DOI] [PubMed] [Google Scholar]
- 17.Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, Simões-Wüst AP, Yousefi S, Simon HU, Stahel R, Zangemeister-Wittke U. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer. 2005;117:755–763. doi: 10.1002/ijc.21242. [DOI] [PubMed] [Google Scholar]
- 18.Levresse V, Marek L, Blumberg D, Heasley LE. Regulation of platinum-compound cytotoxicity by the c-Jun N-terminal kinase and c-Jun signaling pathway in small-cell lung cancer cells. Mol Pharmacol. 2002;62:689–697. doi: 10.1124/mol.62.3.689. [DOI] [PubMed] [Google Scholar]
- 19.Hogan M, Claffey J, Fitzpatrick E, Hickey T, Pampillón C, Tacke M. Synthesis and cytotoxicity studies of Titanocene C analogues. Met Based Drugs. 2008;2008:754358. doi: 10.1155/2008/754358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 21.Korfel A, Scheulen ME, Schmoll HJ, Gründel O, Harstrick A, Knoche M, Fels LM, Skorzec M, Bach F, Baumgart J, et al. Phase I clinical and pharmacokinetic study of titanocene dichloride in adults with advanced solid tumors. Clin Cancer Res. 1998;4:2701–2708. [PubMed] [Google Scholar]
- 22.Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett. 2001;168:173–181. doi: 10.1016/s0304-3835(01)00532-8. [DOI] [PubMed] [Google Scholar]
- 23.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Cooper JC, Yu CL. A constitutively active Lck kinase promotes cell proliferation and resistance to apoptosis through signal transducer and activator of transcription 5b activation. Mol Cancer Res. 2006;4:39–45. doi: 10.1158/1541-7786.MCR-05-0202. [DOI] [PubMed] [Google Scholar]
- 25.Kim RK, Yoon CH, Hyun KH, Lee H, An S, Park MJ, Kim MJ, Lee SJ. Role of lymphocyte-specific protein tyrosine kinase LCK in the expansion of glioma-initiating cells by fractionated radiation. Biochem Biophys Res Commun. 2010;402:631–636. doi: 10.1016/j.bbrc.2010.10.072. [DOI] [PubMed] [Google Scholar]
- 26.Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Cubero FJ, Zhao G, Trautwein C. JNK: a double-edged sword in tumorigenesis. Hepatology. 2011;54:1470–1472. doi: 10.1002/hep.24532. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 29.Choi CH, Lee BH, Ahn SG, Oh SH. Proteasome inhibition-induced p38 MAPK/ERK signaling regulates autophagy and apoptosis through the dual phosphorylation of glycogen synthase kinase 3β. Biochem Biophys Res Commun. 2012;418:759–764. doi: 10.1016/j.bbrc.2012.01.095. [DOI] [PubMed] [Google Scholar]
- 30.Cai G, Wang J, Xin X, Ke Z, Luo J. Phosphorylation of glycogen synthase kinase-3 beta at serine 9 confers cisplatin resistance in ovarian cancer cells. Int J Oncol. 2007;31:657–662. [PubMed] [Google Scholar]
- 31.Andjilani M, Droz JP, Benahmed M, Tabone E. Down-regulation of FAK and IAPs by laminin during cisplatin-induced apoptosis in testicular germ cell tumors. Int J Oncol. 2006;28:535–542. [PubMed] [Google Scholar]
- 32.Itamochi H, Yoshida T, Walker CL, Bartholomeusz C, Aoki D, Ishihara H, Suzuki N, Kigawa J, Terakawa N, Ueno NT. Novel mechanism of reduced proliferation in ovarian clear cell carcinoma cells: cytoplasmic sequestration of CDK2 by p27. Gynecol Oncol. 2011;122:641–647. doi: 10.1016/j.ygyno.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Hwang JT, Yun H, Chi SG, Lee SJ, Kang I, Yoon KS, Choe WJ, Kim SS, Ha J. Inhibition of AMP-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. J Biol Chem. 2008;283:3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 34.Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP kinase signaling determines whether c-Jun N-terminal kinase promotes survival or apoptosis during glucose deprivation. Carcinogenesis. 2009;30:529–537. doi: 10.1093/carcin/bgn259. [DOI] [PubMed] [Google Scholar]
- 35.Alexander A, Walker CL. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett. 2011;585:952–957. doi: 10.1016/j.febslet.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q, Shen B, Zhang B, Zhang W, Chin SH, Jin J, Liao DF. Inhibition of AMP-activated protein kinase pathway sensitizes human leukemia K562 cells to nontoxic concentration of doxorubicin. Mol Cell Biochem. 2010;340:275–281. doi: 10.1007/s11010-010-0428-3. [DOI] [PubMed] [Google Scholar]
- 37.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–491. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Ceppi P, Papotti M, Monica V, Lo Iacono M, Saviozzi S, Pautasso M, Novello S, Mussino S, Bracco E, Volante M, et al. Effects of Src kinase inhibition induced by dasatinib in non-small cell lung cancer cell lines treated with cisplatin. Mol Cancer Ther. 2009;8:3066–3074. doi: 10.1158/1535-7163.MCT-09-0151. [DOI] [PubMed] [Google Scholar]
- 40.Peng DJ, Wang J, Zhou JY, Wu GS. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun. 2010;394:600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bragado P, Armesilla A, Silva A, Porras A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 42.Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J Biol Chem. 2003;278:23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 43.Losa JH, Parada Cobo C, Viniegra JG, Sánchez-Arevalo Lobo VJ, Ramón y Cajal S, Sánchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22:3998–4006. doi: 10.1038/sj.onc.1206608. [DOI] [PubMed] [Google Scholar]
- 44.Erxleben A, Claffey J, Tacke M. Binding and hydrolysis studies of antitumoural titanocene dichloride and Titanocene Y with phosphate diesters. J Inorg Biochem. 2010;104:390–396. doi: 10.1016/j.jinorgbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Florea A-M, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jungwirth U, Kowol CR, Keppler BK, Hartinger CG, Berger W, Heffeter P. Anticancer activity of metal complexes: involvement of redox processes. Antioxid Redox Signal. 2011;15:1085–1127. doi: 10.1089/ars.2010.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001;21:6913–6926. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]