Abstract
Aims
To evaluate the antitumor and antiangiogenic activity of metronomic ceramide analogs and their relevant molecular mechanisms.
Methods
Human endothelial cells [human dermal microvascular endothelial cells and human umbilical vascular endothelial cell (HUVEC)] and pancreatic cancer cells (Capan-1 and MIA PaCa-2) were treated with the ceramide analogs (C2, AL6, C6, and C8), at low concentrations for 144 hours to evaluate any antiproliferative and proapoptotic effects and inhibition of migration and to measure the expression of caveolin-1 (CAV-1) and thrombospondin-1 (TSP-1) mRNAs by real-time reverse transcription-polymerase chain reaction. Assessment of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and Akt phosphorylation and of CAV-1 and cyclin D1 protein expression was performed by ELISA. Maximum tolerated dose (MTD) gemcitabine was compared against metronomic doses of the ceramide analogs by evaluating the inhibition of MIA PaCa-2 subcutaneous tumor growth in nude mice.
Results
Metronomic ceramide analogs preferentially inhibited cell proliferation and enhanced apoptosis in endothelial cells. Low concentrations of AL6 and C2 caused a significant inhibition of HUVEC migration. ERK1/2 and Akt phosphorylation were significantly decreased after metronomic ceramide analog treatment. Such treatment caused the overexpression of CAV-1 and TSP-1 mRNAs and proteins in endothelial cells, whereas cyclin D1 protein levels were reduced. The antiangiogenic and antitumor impact in vivo of metronomic C2 and AL6 regimens was similar to that caused by MTD gemcitabine.
Conclusions
Metronomic C2 and AL6 analogs have antitumor and antiangiogenic activity, determining the up-regulation of CAV-1 and TSP-1 and the suppression of cyclin D1.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States and in Europe [1]. Current therapy for pancreatic cancer involves surgery and chemotherapy, but metastatic disease is resistant to most cytotoxic chemotherapeutic agents and to novel targeted therapies [2]. Gemcitabine is the main chemotherapeutic agent used to treat advanced adenocarcinoma of the pancreas (even though it does not prolong significantly the survival of patients), and more effective therapeutic options are urgently needed to improve the response and the survival of patients with this cancer. Among the emerging therapeutic approaches, metronomic chemotherapy (MC), the continuous administration of low-dose chemotherapeutic drugs, is one interesting option because of its good tolerability and the low costs associated with palliative treatments [3–5]. Such a regimen has been shown to have antiangiogenic activity and to inhibit tumor growth in vivo [6–8]. Moreover, the immunomodulating activity of low-dose cyclophosphamide seems to contribute to the antitumor effect of MC on established rat lymphomas and sarcomas [9]. Furthermore, metronomic paclitaxel preclinically enhanced the antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis [10]. Clinically, metronomic low-dose cyclophosphamide induced stable tumor-specific T-cell responses, which correlated with improved clinical outcome in advanced-stage breast cancer patients [11]. In the clinic, the benefits of MC have been demonstrated in a number of phase II clinical trials with a broad range of malignancies at advanced stages [12–14]. Interestingly, very few preclinical and clinical data are available on MC for pancreas cancer, and almost all such studies only evaluated metronomic gemcitabine [15–17].
Ceramide, a sphingosine-based lipid molecule, has attracted considerable interest for tumor treatment because of its emerging role as an intracellular proapoptotic molecule [18]. Ceramides are second messengers that play an important role in regulating cell growth, differentiation, and the cell death program [19,20]. Furthermore, the dynamic balance between the levels of ceramide and sphingosine-1-phosphate is an important factor that determines whether a cell survives and proliferates or whether it dies [21]. Ceramide induces proapoptotic mechanisms by activating ceramide-activated phosphatases and ceramide-activated kinases and by regulating protein kinase C (PKC), Akt, and Bcl-2 [22–24]. Indeed, tumor cells with low levels of endogenous ceramides are resistant to apoptosis, and tumor cell lines with a defect in ceramide generation are resistant to radiation-induced apoptosis [25]. Moreover, the glycosylation of ceramide, through the glucosylceramide synthase enzyme, confers resistance to doxorubicin in human breast cancer cells [26,27] and potentiates cellular multidrug resistance [28,29].
Endogenous ceramides are unable to cross the cell membrane, and therefore, they cannot be used as anticancer agents. Ceramide analogs, however, are able to pass through the cell membrane and to mimic the effects of endogenous ceramide. Such analogs have been developed in the past; in particular, C2 and C6 ceramide analogs have been investigated over the years as new potential drugs for cancer chemotherapy [30]. C2 and C6 ceramide analogs induce apoptosis in leukemic cell lines [31,32], and moreover, they proved to mimic ceramide by inducing apoptosis and by inhibiting proliferation of tumor cells but at concentrations that were considered to be too high for their development as potential antiproliferative agents [33]. Other promising ceramide analogs, such as the AL6 compound, which has a wide spectrum of cellular activities, have recently been synthesized [34], and furthermore, AL6 has also been successfully tested on pancreatic tumor cells [35].
In the present study, we tested the hypothesis that metronomic ceramide analogs can have an impact on the proliferation and on apoptosis of endothelial cells and that they can also inhibit tumor growth and tumor angiogenesis. To the best of our knowledge, this is the first time that such a hypothesis has been tested. We also present the results of our investigation into the possible mechanisms by which ceramide analog treatments can inhibit tumor growth.
Materials and Methods
Materials, Drugs, and Cell Lines
Recombinant human vascular endothelial growth factor (rhVEGF) and recombinant human epidermal growth factor (rhEGF) were from PeproTech EC Ltd. (London, United Kingdom). Cell culture media MCDB13, Dulbecco's modified Eagle's medium, and RPMI, FBS, horse serum, l-glutamine, penicillin, streptomycin, and gentamicin were purchased from Gibco (Gauthersburg, MD). Type A gelatin from porcine skin, supplements, and all other chemicals not listed in this section were obtained from Sigma Chemical Co. (St Louis, MO). Sterile plastics for cell culture were purchased from Costar (Cambridge, MA).
AL6 ceramide analog was a generous gift from Bracco S.p.A. (Milan, Italy), and C2, C6, and C8 ceramide analogs were purchased from Calbiochem (Darmstadt, Germany). The chemical structures of these compounds are shown in Figure 1. The compounds were dissolved to form a stock solution of 10 mM in 50% DMSO and 50% ethanol for in vitro and in vivo studies. Gemcitabine (Ely Lilly and Company, Indianapolis, IN) for in vivo studies was purchased from the hospital pharmacy. Human dermal microvascular endothelial cells (HMVEC-d) were obtained from Clonetics (San Diego, CA); human umbilical vascular endothelial cells (HUVECs) were obtained from the American Type Culture Collection (Manassas, VA). Endothelial cells were maintained in MCDB131 culture medium supplemented with 10% heat-inactivated FBS, l-glutamine (2 mM), heparin (10 U/ml), EGF (10 ng/ml), and basic fibroblast growth factor (5 ng/ml). The human pancreatic tumor cell lines MIA PaCa-2 and Capan-1 were purchased from the American Type Culture Collection and maintained in 10% FBS-Dulbecco's modified Eagle's medium and RPMI medium, respectively. Media were supplemented with l-glutamine (2 mM). HUVEC, HMVEC-d, MIA PaCa-2, and Capan-1 cells were routinely grown in 75-cm2 tissue culture flasks and kept in a humidified atmosphere of 5% CO2 at 37°C. Cells were harvested when they were in a log phase of growth, using a solution of 0.25% trypsin-0.03% EDTA, and thereafter, they were maintained at the above-described cell culture conditions for all subsequent experiments.
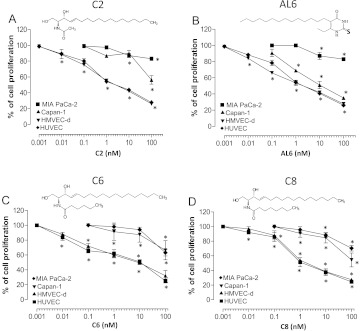
Figure 1.
Effect of ceramide analogs (A) C2, (B) AL6, (C) C6, and (D) C8 on in vitro cell proliferation. The antiproliferative effects of each drug was studied using continuous exposures (144 hours) to the drugs of HMVEC-d, HUVEC, Capan-1, and MIA PaCa-2 cells. Symbols and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
In Vitro Studies
Cell proliferation assay and apoptosis measurements. Endothelial (HUVEC and HMVEC-d; 1 x 103 cells/well) and tumor (MIA PaCa-2 and Capan-1; 0.5 x 103 cells/well) cells were plated onto 24-well sterile plastic plates (1% gelatin coated for the endothelial cells) and allowed to attach overnight. Cells were treated with ceramide analogs C2, AL6, C6, and C8 (0.001–100 nM) or with vehicle alone continuously for 144 hours in 1 ml of medium. For all samples, the media were changed every 24 hours [8]. At the end of the treatment, viable cells were quantified using an ADAM-MC cell counter (Digital Bio, NanoEnTek Inc., Seoul, South Korea). The data are presented as the percentage of the vehicle-treated cells. The concentration of drug that reduced cell proliferation by 50% (IC50) versus controls was calculated by nonlinear regression fit of the mean values of the data obtained in triplicate experiments (at least nine wells for each concentration).
To quantify the degree of apoptosis induced by the drug treatments, HMVEC-d, HUVEC, MIA PaCa-2, and Capan-1 cells were treated continuously for 144 hours with ceramide analogs (C2, AL6, C6, and C8) at a concentration corresponding to the experimental IC50, or at a lower concentration, or with vehicle alone. At the end of the experiment, cells were collected and the Cell Death Detection ELISA Plus Kit (Roche, Basel, Switzerland) was used as per the manufacturer's instruction. The OD was determined using a Multiskan Spectrum microplate reader (Thermo Labsystems, Milan, Italy) set to 405 nm (with a wavelength correction set to 490 nm). All experiments were repeated three times with at least three replicates per sample.
Migration assay. Migration assays were performed as previously described [36]. Briefly, viable HUVEC and MIA PaCa-2 cells were seeded at a density of 8.4 x 104 cells in 500 µl of serum-free MCDB131 in the upper chamber of 8-µm pore modified Boyden chamber (Becton Dickinson, Franklin Lakes, NJ). Cells were treated for 4 hours [36,37] with the ceramide analog C2 or AL6 at a concentration corresponding to the experimental 144-hour IC50 and at 100 pM in HUVEC or at 10 nM in MIA PaCa-2 cells to study only the effects on the migration process without impairing the cell viability. Vehicle alone was used as a control. Cells were allowed to migrate in 250 µl of medium with or without added rhVEGF or rhEGF (100 ng/ml) as a chemoattractant for endothelial and cancer cells, respectively; after 4 hours, the membranes were fixed, stained with hematoxylin, and then mounted onto slides. The pictures of the migrated cells were acquired as 512 x 1024 pixel images at 400x magnification using a digital microscope Axioplan2 (Carl Zeiss, Oberkochen, Germany; see Figure W1) and processed by image analysis software (ImageJ v.1.45s, National Institutes of Health, Bethesda, MD). Twenty different regions were analyzed in each slide to calculate the number of migrated cells, and the results are presented as a percentage of the controls. The experiments were performed in triplicate.
Detection of Akt and extracellular signal-regulated kinases 1 and 2 phosphorylation, caveolin-1, and cyclin D1 levels in endothelial and cancer cells by ELISA after metronomic ceramide analog treatments. HUVEC and HMVEC-d (10 x 104 cells/well) and MIA PaCa-2 and Capan-1 (5 x 104 cells/well) were treated continuously for 144 hours with ceramide analogs (C2, AL6, C6, and C8) at a concentration corresponding to the experimental IC50 of cell proliferation and at a lower concentration. At the end of the drug exposure, cells were harvested and immediately frozen in liquid nitrogen. Cells were lysed as per manufacturer's instructions. Each sample was then assayed for human extracellular signal-regulated kinases 1 and 2 (ERK1/2) and Akt phosphorylation by the Phospho-Detect ERK1/2 (pThr185/pTyr187) ELISA Kit and the PhosphoDetect Akt (pThr308) ELISA Kit (Calbiochem) and normalized by total protein ERK1/2 and Akt concentration, respectively. To measure the protein levels of human caveolin-1 (CAV-1) and human cyclin D1, we used the CAV-1 ELISA Kit and the Cyclin D1 ELISA Kit (USCN Life Science & Technology, Wuhan, China) and normalized by total protein concentration. The OD was determined using the Multiskan Spectrum microplate reader set to 450 nm. All the absorbance values were plotted as a percentage of signal relative to that of control cells (i.e., vehicle only). All experiments were repeated three times with a least three replicates per sample.
Immunohistochemical analysis for CAV-1 in endothelial cells. The procedure for immunohistochemistry was performed as previously described [38] on HUVECs treated with AL6 or with vehicle alone. Endogenous peroxidase activity was blocked by incubating the slides in 1% hydrogen peroxide and methanol for 10 minutes. After blocking of nonspecific staining with normal serum, the slides were incubated with primary antibody anti-CAV-1 rabbit polyclonal antibody (Chemicon International, Temecula, CA; 1:200 dilution), then with biotinylated secondary antibody (1:500 dilution for 30 minutes) and the avidin-biotin complex (Vector Laboratories, Burlingame, CA, for 30 minutes). The 3,3′-diaminobenzidine tetrahydrocloride was used as chromogen. Negative controls were obtained by omitting the primary antibody.
Real-time reverse transcription-polymerase chain reaction analysis of human CAV-1 and TSP-1 gene expression. To evaluate the expression of CAV-1 and thrombospondin-1 (TSP-1) mRNAs, we grew HUVEC and HMVEC-d (at 10 x 104 cells/well) and MIA PaCa-2 and Capan-1 (at 5 x 104 cells/well) cells in their respective media and treated them continuously for 144 hours with ceramide analogs (C2, AL6, C6, and C8) at concentrations corresponding to the experimental IC50 for cell proliferation, as well as at lower concentrations, or with vehicle alone. Next, in brief, total RNA (1 µg) was reverse transcribed at 37°C for 1 hour in a 100-µl reaction volume containing 0.8 mM deoxynucleotide mix (deoxyribonucleotide triphosphates [dNTPs]), 200 U of Moloney murine leukemia virus reverse transcriptase, 40 U of RNase inhibitor, and 0.05 µg/ml random primers. The resulting cDNA was diluted (2:3) and then amplified by quantitative reverse transcription-polymerase chain reaction using the Applied Biosystems 7900HT sequence detection system (Applied Biosystems, Foster City, CA). CAV-1 (Assay ID Hs00184697_m1) and TSP-1 (Assay ID00170236_m1) were purchased from Applied Biosystems. The PCR thermal cycling conditions and the optimization of primer concentrations were as per the manufacturer's instructions. Amplifications were normalized to glyceraldehyde-3-phosphate dehydrogenase, and the quantitation of gene expression was performed using the ΔΔCt calculation, where Ct is the threshold cycle. The amount of target, normalized to the endogenous control and relative to the calibrator (i.e., vehicle-treated control cells), was calculated using the formula 2-ΔΔCt.
Human TSP-1 detection in conditioned media by ELISA. HUVECs and HMVEC-d were treated continuously for 144 hours with ceramide analogs at a concentration corresponding to the experimental IC50 of cell proliferation, as well as to lower concentrations of the drug, or with vehicle alone. To measure the amount of secreted TSP-1 at the end of the incubation, we discarded the medium of each well and replaced it with serum-free medium for 4 hours, as previously described [39]. Each sample was then assayed for TSP-1 protein using the ELISA Quantikine Kit (R&D Systems, Minneapolis, MN) and normalized by total protein concentration in each sample. The OD was determined using the Multiskan Spectrum microplate reader set to 450 nm. All experiments were independently repeated six times, with at least nine samples for each tested drug concentration.
In Vivo Studies
Animals. CD nu/nu male mice, weighing 20 to 25 g, were supplied by Charles River (Milan, Italy) and were allowed unrestricted access to sterile food and water. Housing and all procedures involving animals were performed according to the protocol approved by the Academic Committee for Animal Experimentation of the University of Pisa, in accordance with the European Community Council Directive 86-609, recognized by the Italian government, on animal welfare. Each experiment employed the minimum number of mice needed to obtain statistically meaningful results.
MIA PaCa-2 tumor xenografts in nu/nu mice and drug treatments. MIA PaCa-2 cell viability was assessed by trypan blue dye exclusion, and on day 0, a 1.3 x 106 ± 5% inoculum of cells/mouse was implanted subcutaneously in a 0.2-ml volume of serum-free media between the scapulae of each mouse. Animal weights were monitored and, upon the appearance of a visible subcutaneous tumor mass, tumor dimensions were measured every 2 days in two perpendicular directions using calipers. Tumor volume (mm3) was defined as follows: [(w1 x w1 x w2) x (π/6)], where w1 and w2 are the largest and smallest tumor diameters (mm), respectively. The mice were then randomized into groups of six animals. To treat established tumors, we first allowed the xenografts to grow for 15 days. Thereafter, gemcitabine, C2, and AL6 were administered as follows: 1) group 1, gemcitabine given at the maximum tolerated dose (MTD; as a positive control)—two cycles of 120 mg/kg four times at 3-day intervals [40]; 2) groups 2 and 3 were treated with either ceramide analog C2 (group 2) or with AL6 (group 3) given everyday at a dose of 0.5 mg/kg per day; 3) group 4 (control group) was injected intraperitoneally (i.p.) with vehicle alone [saline solution and 1.9% DMSO and 1.9% ethanol (vol/vol)]. The experiment was terminated 57 days after the inoculation of the tumor cells. Mice were sacrificed by an anesthetic overdose.
In a separate experiment, dosing began 16 days after cell inoculation. Gemcitabine, C6, or C8 was then administered as follows: 1) group 1, gemcitabine at the MTD; 2) groups 2 and 3 were treated with ceramide analog C6 (group 2) or C8 (group 3) given everyday at a dose of 0.5 mg/kg per day; 3) group 4 (control group) was injected i.p. with vehicle alone [saline solution and 1.9% DMSO and 1.9% ethanol (vol/vol)]. Three groups were terminated on day 27 (groups 2, 3, and 4), and one group on day 35 (group 1) after tumor cell implantation.
Immunohistochemistry of Ki67 and microvessel density on MIA PaCa-2 tumor tissue samples. After surgical resection at the end of the in vivo experiment, tumor tissue samples from all the different treatment groups were fixed in 10% phosphate-buffered formalin for 12 to 24 hours and then embedded in paraffin for histology and immunohistochemistry. Five-micrometer sections were stained with hematoxylin-eosin for histologic analysis. Adjacent sections were cut for immunohistochemistry as previously described [41] using the following primary antibodies: anti-Ki67 clone MIB-1 (1:50 dilution; Zymed Laboratories Inc., South San Francisco, CA) as a cellular marker for proliferation [42] and the rat anti-mouse CD31 (1:100 dilution; PharMingen, San Diego, CA) to evaluate microvascular density. Negative controls were obtained by replacing the primary antibody with nonimmune serum. Nuclear staining was considered positive for MIB-1. The degree of positivity was evaluated by calculating the percentage of immunoreactive cells in a minimum count of 500 cells [43]. To calculate microvessel density, we selected the three most vascularized areas of the tumor (“hot spots”) and obtained the mean values by counting the vessels. A single microvessel was defined as a discrete cluster of cells positive for CD31 staining, with no requirement for the presence of a lumen. Microvessel counts were performed at x200 (x20 objective lens and x10 ocular lens; 0.74 mm2 per field) [39]. All parameters were determined independently by three expert pathologists (P.V., A.G.N., and G.F.), and discordant cases were solved by simultaneous review.
Statistical Analysis
Analysis by analysis of variance followed by the Student-Newman-Keuls test was used to assess the statistical differences in the in vitro and in vivo data. P values lower than .05 were considered significant. Statistical analyses were performed using the GraphPad Prism software package version 5.0 (GraphPad Software Inc., San Diego, CA).
Results
In Vitro Studies
Metronomic ceramide analogs preferentially inhibit proliferation and induce apoptosis in endothelial cells. The 144-hour exposure to ceramide analogs (C2, AL6, C6, and C8) inhibited cell proliferation of HMVEC-d and HUVEC in a concentration-dependent manner (Figure 1); the calculated IC50 values in HMVEC-d were 4.99 ± 3.60 nM for C2 (Figure 1A), 4.85 ± 3.14 nM for AL6 (Figure 1B), 28.95 ± 17.57 nM for C6 (Figure 1C), and 3.56 ± 0.92 nM for C8 (Figure 1D). Analogously, in HUVECs, the IC50s were 6.21 ± 3.05 nM for C2 (Figure 1A), 4.37 ± 1.30 nM for AL6 (Figure 1B), 23.54 ± 8.14 nM for C6 (Figure 1C), and 2.88 ± 0.91 nM for C8 (Figure 1D). In contrast, the ceramide analogs did not significantly affect the proliferation of MIA PaCa-2 and Capan-1 cell lines at these low concentrations (Figure 1), with the observed IC50s being higher than 100 nM (the only exception noted was for AL6 in Capan-1 cells, IC50 = 12.90 ± 8.56 nM, Figure 1B).
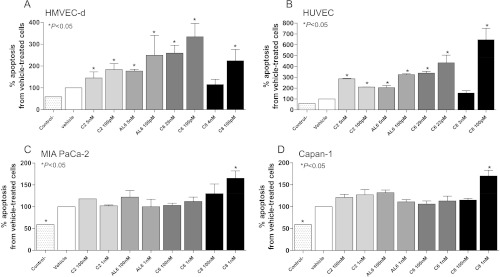
Apoptosis was significantly increased in both HMVEC-d (Figure 2A) and HUVEC (Figure 2B). Interestingly, the increasing concentrations of ceramide analogs (C2, AL6, C6, and C8) induced smaller percentages of apoptotic cells in the endothelial cells HMVEC-d (Figure 2A) and HUVEC [with the only exception of C2 (5 nM and 100 pM); Figure 2B], whereas the proliferation was a clearly concentration-dependent effect. These apoptotic effects were not enhanced in the MIA Paca-2 cancer cell line (Figure 2C) nor in the Capan-1 cell line (Figure 2D) after treatment with ceramide analogs.
Figure 2.
Proapoptotic effects of ceramide analogs C2, AL6, C6, and C8 on proliferating (A) HMVEC-d, (B) HUVEC, (C) MIA PaCa-2, and (D) Capan-1 cells, after 144 hours of treatment. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls. Control- stands for the negative control of the ELISA kit.
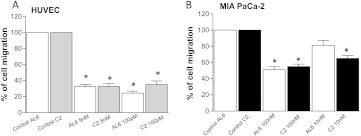
Metronomic ceramide analogs AL6 and C2 inhibit cell migration in endothelial and cancer cells. Figure 3A shows the significant inhibition of VEGF-induced migration of HUVECs treated at low concentrations of AL6 and C2 ceramide analogs. The chemoattractant activity of VEGF on endothelial cells was significantly blocked by both drugs tested, in particular by AL6 at 100 pM (Figure 3A), which showed the greatest inhibition against HUVECs (24.33 ± 1.45% of migrated cells vs 100% of controls; P < .05). Inhibition of EGF-induced MIA PaCa-2 cancer cell migration was not significant at low concentrations of AL6, although it was statistically different at higher concentrations (i.e., 100 nM; 51 ± 2.08% of migrated cells vs 100% of controls; P < .05; Figure 3B). C2 inhibited cancer cell migration at higher concentrations and at a lower rate when compared to the effects observed with the endothelial cells (Figure 3B).
Figure 3.
Effects of low-dose treatment with ceramide analogs C2 and AL6 on (A) endothelial cell and (B) cancer cell migration. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
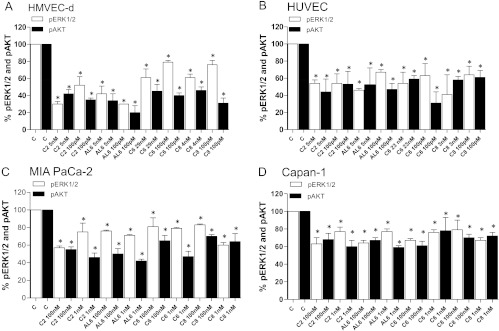
Phosphorylation of ERK1/2 and Akt is inhibited after exposure to metronomic ceramide analogs. Figure 4A shows the strong inhibition of ERK1/2 and Akt phosphorylation in HMVEC-d microvascular endothelial cell line after metronomic exposure to ceramide analogs. This inhibition is more evident for phosphorylated Akt (pAkt) with all analogs tested but, in particular, in samples treated with C2 and AL6. Differently, in HUVECs, the percentage of inhibition of pAkt and phosphorylated ERK1/2 was superimposable (Figure 4B). The decrease in phosphorylation of ERK1/2 and Akt was also observed in the two cancer cell lines MIA PaCa-2 (Figure 4C) and Capan-1 (Figure 4D), but these effects were seen at higher concentrations for all the compounds tested. However, the reduction in phosphorylation in the samples treated with C2 and AL6 was less pronounced than that observed in endothelial cells.
Figure 4.
Modulation of Akt (pThr308) and ERK1/2 (pThr185/pTyr187) phosphorylation by ceramide analogs C2, AL6, C6, and C8 in (A) HMVEC-d, (B) HUVEC, (C) MIA PaCa-2, and (D) Capan-1 cells after 144 hours of treatment. pAkt and phosphorylated ERK1/2 concentrations were measured by ELISA and normalized to total Akt and ERK1/2 protein concentration, respectively. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
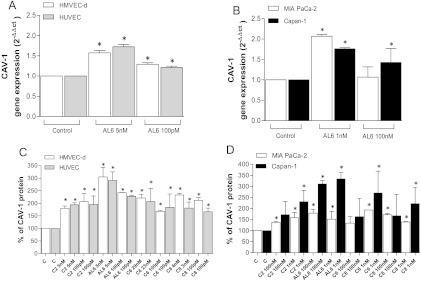
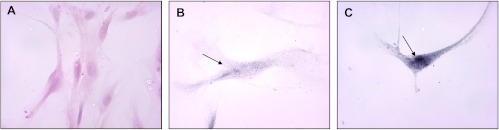
Metronomic ceramide analogs upregulate CAV-1 in endothelial and cancer cells. To investigate the possible mechanisms underlying the antiproliferative and proapoptotic effects of metronomic ceramide analogs, real-time PCR analysis and protein quantification were performed to evaluate the expression of CAV-1. Interestingly, up-regulation of CAV-1 (Figure 5) was found in both endothelial and tumor cells after treatment with ceramide analogs. Indeed, CAV-1 gene expression was significantly increased by AL6 treatment in endothelial cells (Figure 5A) and in cancer cells (Figure 5B). To verify that the increase of CAV-1 mRNA in treated cells resulted in an actual increase in relative protein levels, we quantified CAV-1 by ELISA in the lysate of vehicle-treated and of drug-treated cells. In HMVEC-d and HUVECs, there was a significant increase of CAV-1 after 144-hour treatment with all four ceramide analogs (Figure 5C), with a particularly significant increase in the cells treated with AL6 (5 nM). This up-regulation of CAV-1 protein was also observed in the tumor cell lines (although to a different extent in the MIA Paca-2 and Capan-1 cells at higher drug concentrations, and such differences were not always statistically significant; Figure 5D). In addition, we performed CAV-1 immunohistochemistry on vehicle-treated and on AL6-treated HUVECs. The histology of HUVECs (Figure 6A) confirmed the typical morphology of endothelial cells grown as monolayers (i.e., large-sized cells with evident pseudopodia), whereas CAV-1 immunohistochemistry in vehicle-treated cells revealed the staining to be at both cytoplasmic and membrane levels (Figure 6B). A considerable increase in the amount of protein was found in AL6-treated cells (Figure 6C) when compared with the immunoreactivity of controls, confirming the increased level of expression observed by the reverse transcription-polymerase chain reaction and ELISA methods.
Figure 5.
CAV-1 gene expression in (A) endothelial and (B) pancreatic cancer cell lines exposed to AL6, or to vehicle alone, for 144 hours. Gene expression was quantified by the formula 2-ΔΔCt. CAV-1 protein concentration in cell lysates after exposure to C2, AL6, C6, and C8 or to vehicle alone for 144 hours in (C) endothelial and (D) pancreatic cancer cell lines. CAV-1 concentration was measured with an ELISA kit and normalized to total protein concentration. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
Figure 6.
(A) HUVEC control cells stained with hematoxylin (x20), (B) HUVEC vehicle-treated control (x20) positive for CAV-1 protein, and (C) HUVEC treated with AL6, at 4 nM, for 144 hours (x20). Arrow points to positive immunoreactivity for CAV-1. The primary antibody dilution used was 1:200.
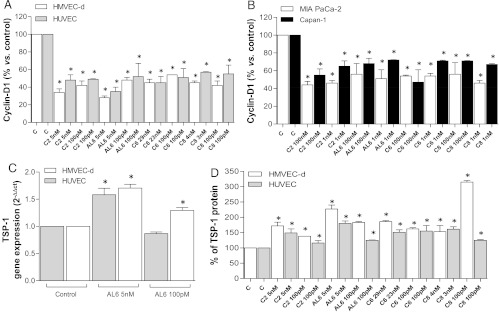
Metronomic ceramide analogs decrease cyclin D1 in endothelial and cancer cells. Intracellular levels of protein cyclin D1 were found to be reduced in both endothelial cell lines after treatment with metronomic ceramide analog compounds, an effect that was found to be statistically significant. In particular, a 144-hour treatment with C2 and AL6 caused a marked reduction of cyclin D1 levels (e.g., 28 ± 2% AL6 (5 nM) vs 100% of controls in HMVEC-d; Figure 7A). This reduction was also found, although to a lesser extent, in the two cancer cell lines, MIA PaCa-2 and Capan-1 (Figure 7B). Interestingly, AL6 (at 100 nM) caused a reduction of 56 ± 12% and 68 ± 6% versus 100% of controls for MIA PaCa-2 and Capan-1 cells, respectively.
Figure 7.
Cyclin D1 protein concentrations in cell lysates after exposure to C2, AL6, C6, and C8 or with vehicle alone for 144 hours in (A) endothelial and (B) pancreatic cancer cell lines. (C) TSP-1 gene expression (2-ΔΔCt) in endothelial cell lines (HUVEC and HMVEC-d) exposed to AL6 or to vehicle alone for 144 hours. (D) TSP-1 secretion in the conditioned media of HMVEC-d and HUVECs exposed to C2, AL6, C6, and C8 or to vehicle alone for 144 hours. TSP-1 concentrations in conditioned media were measured by ELISA and normalized to total protein concentration. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
TSP-1 mRNA and TSP-1 protein secretion are increased by metronomic ceramide analogs. Real-time PCR analysis was performed to evaluate the expression of TSP-1 in endothelial and cancer cells. This analysis showed a significant increase in TSP-1 mRNA expression in endothelial cells after treatment with AL6 (5 nM). Thus, we observed this effect on both HMVEC-d (1.706 ± 0.07 vs 1 of controls) and HUVECs (1.583 ± 0.119 vs 1 of controls; Figure 7C). In MIA PaCa-2 and Capan-1 tumor cells, the expression of TSP-1 was unaltered after prolonged administration of 100 nM AL6 (data not shown).
To determine whether the increase in TSP-1 mRNA led to increased TSP-1 secretion into the conditioned media, we analyzed media from our samples by ELISA. We found that higher levels of TSP-1 were detectable in the conditioned media of drug-treated endothelial cells (Figure 7D) compared with vehicle-treated controls. In particular, a marked increase was found in HMVEC-d (227 ± 13% vs 100% of controls) treated with AL6 (5 nM) and a similar result (180 ± 8% vs 100% of controls) was obtained with HUVECs (Figure 7D).
In Vivo Studies
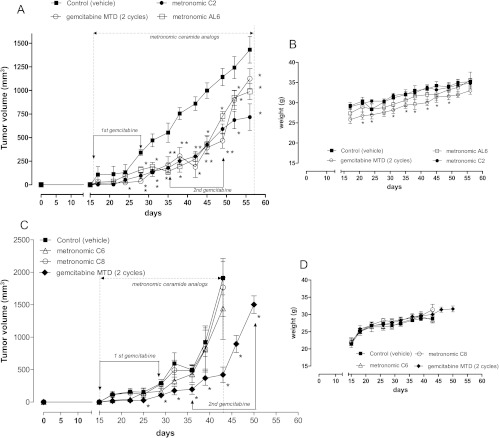
Metronomic C2 and AL6 inhibit tumor growth in vivo. In the ceramide-treated groups (C2 and AL6, 0.5 mg/kg per day i.p.), the tumor growths were markedly inhibited for more than 25 days after the beginning of metronomic treatment. For example, after 27 days of treatment, the average of C2-treated and AL6-treated tumors was 301 and 273 mm3 versus 860 mm3 of controls (corresponding to a 65% and a 68% inhibition), respectively. In the remaining 15 days, these tumors relapsed. However, by the time the treatment was terminated, the C2 and AL6 groups average tumor volumes were 717 and 988 mm3, respectively, compared to 1431 mm3 in the control group (which corresponds to a percentage of growth inhibition of 50% for C2 and 31% for AL6, compared to controls; Figure 8A). Similar antitumor activity was observed in mice treated with MTD gemcitabine, which we used as a positive control. For MTD gemcitabine, by the time the treatment was terminated, the average tumor volume was 1125 mm3 (i.e., a 21% growth inhibition compared to controls; Figure 8A). All compounds throughout the duration of the treatment did not cause any appreciable weight loss (Figure 8B). In contrast, despite the promising in vitro data we obtained, the metronomic C6 and C8 administration (0.5 mg/kg per day i.p.) did not cause much tumor inhibition in vivo compared to MTD gemcitabine (Figure 8C). In this experiment, C6 and C8 did not cause any significant weight loss (Figure 8D).
Figure 8.
(A) Antitumor effect of 1) MTD gemcitabine, two cycles of 120 mg/kg four times at 3-day intervals i.p.; 2) metronomic ceramide analog C2 everyday at 0.5 mg/kg per day i.p.; 3) metronomic ceramide analog AL6 everyday at 0.5 mg/kg per day i.p.; and 4) vehicle alone [saline solution and 1.9% DMSO and 1.9% ethanol (vol/vol)] i.p. on MIA PaCa-2 cell tumor xenotransplanted in CD nu/nu mice. (B) Body weight of MIA PaCa-2 tumor-bearing control mice and of mice treated with metronomic C2, AL6, and MTD gemcitabine schedules. (C) Antitumor effect of 1) MTD gemcitabine, 2) metronomic ceramide analog C6, 3) metronomic ceramide analog C8, and 4) vehicle alone. (D) Body weight of control mice and mice treated with metronomic C6, C8, and MTD gemcitabine schedules. Symbols and bars indicate mean ± SEM. P < .05 versus vehicle-treated controls.
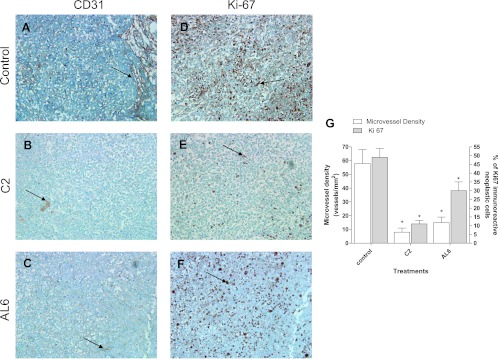
Metronomic C2 and AL6 significantly decrease microvessel density and Ki67 staining in MIA PaCa-2 xenograft tumor. Histologic staining with hematoxylin and eosin of subcutaneous MIA PaCa-2 cells was consistent with pancreatic adenocarcinoma (data not shown). Well-defined CD31 immunoreactivity was found to be localized in endothelial cells inside the vehicle-alone-treated tumors (Figure 9A). Microscopy analysis showed a clear reduction of microvessels in the C2-treated and AL6-treated tumors (Figure 9, B and C, respectively). Whereas control tumor xenografts showed a diffuse, strong, and easily detectable immunoreactivity to Ki67 (Figure 9D), a marked decrease in relative cell proliferation (i. e., a lower Ki67 staining) was observed in the C2-treated and AL6-treated tumors compared to vehicle-treated controls (Figure 9, E and 9F, respectively). In addition, compared to controls, metronomic C2 and AL6 treatments resulted in a significant decrease in the microvessel count and in the percentage of Ki67-positive cells after the quantification of all available samples (Figure 9G).
Figure 9.
Representative images of immunohistochemistry of mouse microvessel density in MIA PaCa-2 xenografts in (A) vehicle-treated mice (control group) and (B) in metronomic C2-treated and (C) metronomic AL6-treated mice. Representative images of immunohistochemistry of Ki67 in MIA PaCa-2 xenografts in (D) vehicle-treated mice (control group) and in (E) metronomic C2 and (F) metronomic AL6 groups of mice. Arrows point to positively stained cells. Original magnification, x200. (G) Quantification of microvessel density and Ki67 immunostaining in MIA PaCa-2 tumor xenografts treated with vehicle alone, metronomic C2, or metronomic AL6, at the end of the in vivo experiment. Columns and bars, mean values ± SD, respectively. *P < .05 versus vehicle-treated controls.
Discussion
MC has aroused a great deal of interest among investigators and oncologists recently. That was possibly a direct consequence of promising preclinical results and encouraging clinical data [3,5,7]. Moreover, the use of MC seems to eliminate or to greatly reduce the usual toxic effects observed when chemotherapy is administered at the MTD. This aspect increases patients' compliance, thus improving their quality of life—a key element in the palliative treatment of metastatic cancer such as that of the pancreas. This is of considerable interest not only for the ongoing pancreatic cancer trials such as the ones that include the testing of metronomic cyclophosphamide (clinicaltrial.gov" NCT00727441) but also for the planning of new pancreas cancer metronomic treatments in the future. In this regard, this tumor type has been clinically shown to have a significant inherent resistance to chemotherapeutic drugs and to anti-VEGF treatments [1]; thus, a different therapeutic approach is urgently needed on the basis of novel mechanism of actions. MC appears to act in part by the inhibition of tumor neovascularization [6–8]. On this basis, we decided to explore the possibility that not only the classic chemotherapeutic drugs [7] but also other compounds such as ceramide analogs administered at metronomic doses might have (similar) antitumor and antiangiogenic effect in vitro and in vivo. Ceramide analogs have been investigated for their antiproliferative and proapoptotic effects in human tumor cells, including pancreatic tumor cells [30,35,44], showing interesting effects but at high concentrations and at doses that unfortunately did not permit their further clinical development. This study demonstrates, for the first time, the antitumor and antiangiogenic effect of metronomic ceramide analogs (C2, AL6, C6, and C8), both in vitro and in vivo. This study highlights the possibility that these compounds (especially C2 and AL6), when administered at nontoxic concentrations and for prolonged periods of time, have preferential antiproliferative and proapoptotic effects on activated endothelial cells. Thus, on the basis of our data, we can define the in vitro metronomic ceramide analog schedule as the frequent administration of low concentrations of ceramide analogs that affect the proliferation of endothelial cells but not the proliferation of cancer cells (approximately 5%, or less, of the administered concentrations that determine, after 144 hours, the inhibition of 50% of cancer cell proliferation). The effects of exogenous cell-permeable short-chain ceramide analogs resulted in a striking decrease in endothelial cell proliferation and increase of apoptosis. These events are likely associated with perturbations in diverse cell signaling pathways, including the inactivation of Akt through its dephosphorylation. The decrease in pAkt has been described previously after the use of standard concentration (e.g., micromolar) of ceramide analogs, such as C6, in cancer cells [45]. Moreover, previous in vitro studies showed that sphingosine-1-phosphate, a ceramide antagonist, protected endothelial cells in culture from radiation-induced apoptosis [46] through the activation of the Akt pathway [47]. On the basis of our data, the metronomic ceramide analogs may induce apoptosis by two distinct mechanisms: 1) directly by decreasing the cellular levels of pAkt and 2) by upregulating TSP-1. With regard to the latter, TSP-1 up-regulation has been described previously as a mediator of the effects of MC [7,48]. In addition, the up-regulation of TSP-1 has been linked to the inhibition of Akt phosphorylation as detailed in a study by Bussolati et al. [49], who reported that the inhibition of Akt activation by phosphatidylinositol 3-kinase (PI3K) inhibitors is concomitant with the synthesis and the release of TSP-1 by tumor endothelial cells, in agreement with a previous report by Niu et al. [50]. In that study, loss of Akt signaling was found to correlate with a gradual increase in TSP-1 levels in endothelial cells. Indeed, TSP-1 has a direct impact on the remodeling of the vascular endothelium, inducing caspase-dependent apoptosis through the CD36 receptor. Moreover, TSP-1 is able to bind and sequestrate proangiogenic growth factors and cytokines secreted by nonendothelial cells, such as basic fibroblast growth factor and VEGF, thus reducing endothelial cell survival, migration, and vascular sprouting [51]. Interestingly, in our study, the secretion of TSP-1 protein was found increased in the conditioned medium of ceramide analog-treated endothelial cells versus vehicle-treated controls. However, the percentage of such increase was similar, and not significantly different, between lower and higher metronomic concentrations. This suggests a possible relevant role of the proapoptotic effects of the increased TSP-1 in the lower doses rather than at the higher ones, where inhibition of cell proliferation is more relevant to the antiendothelial effects of metronomic ceramide analogs.
Our data suggests that the possible molecular mechanism for inhibition of endothelial cell proliferation by metronomic ceramide analogs is by a significant decrease of cyclin D1 and through the inhibition of ERK1/2 phosphorylation. How do ceramide analogs affect cyclin D1 expression? The data obtained seem to suggest that the significant increase in the CAV-1 mRNA and protein expression, after low-dose ceramide analog treatment, could play an important role in the observed cyclin D1 down-regulation. Caveolin proteins serve as the structural components of caveolae, while also functioning as scaffolding proteins, capable of recruiting numerous signaling molecules to caveolae—as well as regulating their activity [52]. Moreover, it has been demonstrated that CAV-1 can act as a tumor suppressor in the mammary gland. In fact, CAV-1 is downregulated in the majority of mammary tumor cell lines, and in these cell lines, recombinant reexpression of CAV-1 potently inhibit their proliferation, their anchorage-independent growth, and their invasiveness [53–55]. More importantly, it has been shown that the loss of CAV-1 is associated with cyclin D1 up-regulation and with ERK1/2 hyperactivation [56,57]. Thus, Hulit et al. [58] showed that CAV-1 expression levels inversely correlated with cyclin D1 levels in cells. Expression of antisense CAV-1 increased cyclin D1 levels, whereas CAV-1 overexpression inhibited the expression of cyclin D1 gene, suggesting that cyclin D1 promoter activity is selectively repressed by CAV-1 [58]. These previously published data are consistent with our findings, and we can therefore propose that metronomic ceramide analogs may inhibit endothelial cell proliferation through this mechanism. However, it has been also shown that ceramide can induce cell cycle arrest through the simultaneous activation of stress-activating protein kinases and the inhibition of ERK [59,60]. In that respect, ceramide seems to competitively inhibit the ability of diacylglycerol-stimulated PKC. to disrupt the formation of a Raf/MEK/ERK signalosome, an event that is also associated with growth arrest [61].
In this study, we also sought to evaluate the antitumor and antiangiogenic activity of metronomic ceramide analogs in vivo. From our previous experience [62] and from data of previously published studies [34], we decided to treat animals with an MTD gemcitabine regimen, as a positive control, and with the metronomic ceramide analog schedules of 0.5 mg/kg per day. Our results show that metronomic schedule of all four compounds was very well tolerated when compared with the gemcitabine MTD regimen. Indeed, no significant animal weight loss was observed compared to the control group, which is consistent with our previously published results with MC in gastrointestinal tumor models [39,63,64]. Another major finding of our in vivo experiments was the demonstration of both antitumor and antiangiogenic activities of the metronomic administered C2 and AL6 compounds in subcutaneously implanted pancreatic tumors. Indeed, these ceramide analogs significantly delay tumor growth with kinetics that is consistent with the inhibitory effect seen with gemcitabine MTD treatment. Moreover, both microvessel density and proliferation rate were markedly decreased in tumors treated with metronomic C2 and AL6 schedules when compared to control group, a result consistent with our in vitro data. Interestingly, not all ceramide analogs tested in this study showed a significant in vivo effect in tumor xenografts. The C6 and C8 compounds did not show any significant activity in vivo, although they were administered at the same doses and with the same schedules as the other drugs. This is in spite of the fact that they showed similar in vitro data to that seen with the C2 and AL6 compounds. These data seem to suggest different pharmacokinetic characteristics of C6 and C8 analogs (e.g., tissue distribution), probably because of their different chemical structures rather than different pharmacodynamic properties. Unfortunately, to our knowledge, for short-chain cell-permeable ceramide analogs (such as C2, C6, and C8), there are no described in vivo pharmacokinetic studies. However, Stover and Kester [65] have described the 3[H]-C6 in vitro kinetics in breast cancer cells. The ceramide analog C6 reached the maximum intracellular concentration after 6 to 8 hours from the start of treatment and maintained measurable intracellular concentrations for almost another 10 hours. Future pharmacokinetic studies will probably be necessary to rationally explain the differences in antitumor efficacy that we observed.
In conclusion, metronomic ceramide analogs show significant antitumor and antiangiogenic activity in the absence of significant toxicity. These data could renew interest in translational cancer research using this class of compounds. Indeed, the potential benefit of ceramide-based MC in cancer is based on the ability of exogenous short-chain ceramide analogs to induce apoptosis and inhibition of proliferation/migration in endothelial cells. In that regard, exogenous ceramide analogs may synergistically augment the antiangiogenic and antitumor activity of other MC schedules. For example, low-dose metronomic oral dosing of LY2334737 [66], a prodrug of gemcitabine, was recently shown to have antitumor activity with a concomitant increase in intratumoral blood flow, an effect that may be of benefit for the delivery of drugs to tumors such as pancreatic cancer. Furthermore, one already reported effective combination is that of ceramide analogs plus paclitaxel. Thus, paclitaxel induction of apoptosis was found to be synergistically enhanced by C6 ceramide analog in nanoemulsion formulations [67]. Moreover, the identification of endothelial cell as a target population of ceramide analogs should allow the design of even more specific or potent ceramide analogs, or mimetics, for cancer therapy. For example, the potential to incorporate short-chain ceramide analogs into conventional or cationic liposomal delivery systems may not only improve efficiency of transfer but also may serve to augment the antiangiogenic effects of these compounds.
Supplemental Materials and Methods
Acknowledgments
We thank Prof. Alfredo Falcone, Dr Fotios Loupakis, Dr Giacomo Allegrini, and Prof. Franco Bocci for their suggestions. We thank Natzidielly Lerma and Courtney L. Becerril for their precious help in editing the manuscript.
Abbreviations
- CAV-1
caveolin-1
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- HMVEC-d
human dermal microvascular endothelial cells
- HUVEC
human umbilical vascular endothelial cell
- MC
metronomic chemotherapy
- MTD
maximum tolerated dose
- TSP-1
thrombospondin-1
- VEGF
vascular endothelial growth factor
Footnotes
The study has been funded, in part, by the Italian Association for Cancer Research (AIRC).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Asuthkar S, Rao JS, Gondi CS. Drugs in preclinical and early-stage clinical development for pancreatic cancer. Expert Opin Investig Drugs. 2012;21:143–152. doi: 10.1517/13543784.2012.651124. [DOI] [PubMed] [Google Scholar]
- 2.Matthaios D, Zarogoulidis P, Balgouranidou I, Chatzaki E, Kakolyris S. Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology. 2011;81:259–272. doi: 10.1159/000334449. [DOI] [PubMed] [Google Scholar]
- 3.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, Del Tacca M. Cyclophosphamide-methotrexate ‘metronomic’ chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2005;16:1243–1252. doi: 10.1093/annonc/mdi240. [DOI] [PubMed] [Google Scholar]
- 5.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 6.Laquente B, Vinals F, Germa JR. Metronomic chemotherapy: an antiangiogenic scheduling. Clin Transl Oncol. 2007;9:93–98. doi: 10.1007/s12094-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 8.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 9.Rozados VR, Mainetti LE, Rico MJ, Zacarias Fluck MF, Matar P, Scharovsky OG. The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol Res. 2010;18:601–605. doi: 10.3727/096504010x12777678141662. [DOI] [PubMed] [Google Scholar]
- 10.Chen CA, Ho CM, Chang MC, Sun WZ, Chen YL, Chiang YC, Syu MH, Hsieh CY, Cheng WF. Metronomic chemotherapy enhances antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis. Mol Ther. 2010;18:1233–1243. doi: 10.1038/mt.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353–362. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allegrini G, Di Desidero T, Barletta MT, Fioravanti A, Orlandi P, Canu B, Chericoni S, Loupakis F, Di Paolo A, Masi G, et al. Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers. Angiogenesis. 2012;15:275–286. doi: 10.1007/s10456-012-9260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelius T, Rinard K, Filleur S. Oral/metronomic cyclophosphamidebased chemotherapy as option for patients with castration-refractory prostate cancer: review of the literature. Cancer Treat Rev. 2011;37:444–455. doi: 10.1016/j.ctrv.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Italiano A, Toulmonde M, Lortal B, Stoeckle E, Garbay D, Kantor G, Kind M, Coindre JM, Bui B. “Metronomic” chemotherapy in advanced soft tissue sarcomas. Cancer Chemother Pharmacol. 2010;66:197–202. doi: 10.1007/s00280-010-1275-3. [DOI] [PubMed] [Google Scholar]
- 15.Tran Cao HS, Bouvet M, Kaushal S, Keleman A, Romney E, Kim G, Fruehauf J, Imagawa DK, Hoffman RM, Katz MH. Metronomic gemcitabine in combination with sunitinib inhibits multisite metastasis and increases survival in an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2010;9:2068–2078. doi: 10.1158/1535-7163.MCT-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cham KK, Baker JH, Takhar KS, Flexman JA, Wong MQ, Owen DA, Yung A, Kozlowski P, Reinsberg SA, Chu EM, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103:52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laquente B, Lacasa C, Ginesta MM, Casanovas O, Figueras A, Galan M, Ribas IG, Germa JR, Capella G, Vinals F. Antiangiogenic effect of gemcitabine following metronomic administration in a pancreas cancer model. Mol Cancer Ther. 2008;7:638–647. doi: 10.1158/1535-7163.MCT-07-2122. [DOI] [PubMed] [Google Scholar]
- 18.Perry DK, Kolesnick RN. Ceramide and sphingosine 1-phosphate in anti-cancer therapies. Cancer Treat Res. 2003;115:345–354. doi: 10.1007/0-306-48158-8_14. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Kolmakova A, Miller M. The role of the phospholipid sphingomyelin in heart disease. Curr Opin Investig Drugs. 2006;7:219–228. [PubMed] [Google Scholar]
- 20.Claria J. Regulation of cell proliferation and apoptosis by bioactive lipid mediators. Recent Pat Anticancer Drug Discov. 2006;1:369–382. doi: 10.2174/157489206778776961. [DOI] [PubMed] [Google Scholar]
- 21.Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 23.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 24.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 25.Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 26.Liu YY, Han TY, Giuliano AE, Cabot MC. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem. 1999;274:1140–1146. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 27.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 28.Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 29.Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50:104–114. doi: 10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macchia M, Barontini S, Bertini S, Di Bussolo V, Fogli S, Giovannetti E, Grossi E, Minutolo F, Danesi R. Design, synthesis, and characterization of the antitumor activity of novel ceramide analogues. J Med Chem. 2001;44:3994–4000. doi: 10.1021/jm010947r. [DOI] [PubMed] [Google Scholar]
- 31.Geley S, Hartmann BL, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. FEBS Lett. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis WD, Grant S, Kolesnick RN. Ceramide and the induction of apoptosis. Clin Cancer Res. 1996;2:1–6. [PubMed] [Google Scholar]
- 33.Kolesnick R, Hannun YA. Ceramide and apoptosis. Trends Biochem Sci. 1999;24:224–225. doi: 10.1016/s0968-0004(99)01408-5. [DOI] [PubMed] [Google Scholar]
- 34.Macchia M, Bertini S, Fogli S, Giovannetti E, Minutolo F, Rapposelli S, Danesi R. Ceramide analogues in apoptosis: a new strategy for anticancer drug development. Farmaco. 2003;58:205–211. doi: 10.1016/S0014-827X(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 35.Giovannetti E, Leon LG, Bertini S, Macchia M, Minutolo F, Funel N, Alecci C, Giancola F, Danesi R, Peters GJ. Study of apoptosis induction and deoxycytidine kinase/cytidine deaminase modulation in the synergistic interaction of a novel ceramide analog and gemcitabine in pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids. 2010;29:419–426. doi: 10.1080/15257771003730193. [DOI] [PubMed] [Google Scholar]
- 36.Bocci G, Danesi R, Del Tacca M, Kerbel RS. Selective antiendothelial effects of protracted low-dose BAL-9504, a novel geranylgeranyl-transferase inhibitor. Eur J Pharmacol. 2003;477:17–21. doi: 10.1016/j.ejphar.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- 38.Tahir SA, Ren C, Timme TL, Gdor Y, Hoogeveen R, Morrisett JD, Frolov A, Ayala G, Wheeler TM, Thompson TC. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9:3653–3659. [PubMed] [Google Scholar]
- 39.Bocci G, Falcone A, Fioravanti A, Orlandi P, Di Paolo A, Fanelli G, Viacava P, Naccarato AG, Kerbel RS, Danesi R, et al. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer. 2008;98:1619–1629. doi: 10.1038/sj.bjc.6604352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braakhuis BJ, Ruiz van Haperen VW, Boven E, Veerman G, Peters GJ. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22:42–46. [PubMed] [Google Scholar]
- 41.Viacava P, Naccarato AG, Bocci G, Fanelli G, Aretini P, Lonobile A, Evangelista G, Montruccoli G, Bevilacqua G. Angiogenesis and VEGF expression in pre-invasive lesions of the human breast. J Pathol. 2004;204:140–146. doi: 10.1002/path.1626. [DOI] [PubMed] [Google Scholar]
- 42.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Viacava P, Bocci G, Tonacchera M, Fanelli G, DeServi M, Agretti P, Berti E, Goletti O, Aretini P, Resta ML, et al. Markers of cell proliferation, apoptosis, and angiogenesis in thyroid adenomas: a comparative immunohistochemical and genetic investigation of functioning and nonfunctioning nodules. Thyroid. 2007;17:191–197. doi: 10.1089/thy.2006.0175. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett. 2008;260:88–95. doi: 10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Zhu QY, Wang Z, Ji C, Cheng L, Yang YL, Ren J, Jin YH, Wang QJ, Gu XJ, Bi ZG, et al. C6-ceramide synergistically potentiates the anti-tumor effects of histone deacetylase inhibitors via AKT dephosphorylation and α-tubulin hyperacetylation both in vitro and in vivo. Cell Death Dis. 2011;2:e117. doi: 10.1038/cddis.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnaud S, Niaudet C, Pottier G, Gaugler MH, Millour J, Barbet J, Sabatier L, Paris F. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007;67:1803–1811. doi: 10.1158/0008-5472.CAN-06-2802. [DOI] [PubMed] [Google Scholar]
- 47.Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, Fuks Z, Gaugler MH, Kolesnick R, Paris F. Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res. 2010;70:9905–9915. doi: 10.1158/0008-5472.CAN-10-2043. [DOI] [PubMed] [Google Scholar]
- 48.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bussolati B, Assenzio B, Deregibus MC, Camussi G. The proangiogenic phenotype of human tumor-derived endothelial cells depends on thrombospondin-1 downregulation via phosphatidylinositol 3-kinase/Akt pathway. J Mol Med (Berl) 2006;84:852–863. doi: 10.1007/s00109-006-0075-z. [DOI] [PubMed] [Google Scholar]
- 50.Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie-2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti-angiogenic molecule, thrombospondin-1. Cancer Biol Ther. 2004;3:402–405. doi: 10.4161/cbt.3.4.735. [DOI] [PubMed] [Google Scholar]
- 51.Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets. 2008;9:851–862. doi: 10.2174/138945008785909347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 53.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 54.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, Lisanti MP. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem. 2000;275:20717–20725. doi: 10.1074/jbc.M909895199. [DOI] [PubMed] [Google Scholar]
- 56.Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003;14:1027–1042. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 58.Hulit J, Bash T, Fu M, Galbiati F, Albanese C, Sage DR, Schlegel A, Zhurinsky J, Shtutman M, Ben-Ze'ev A, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 59.Ruvolo PP. Ceramide regulates cellular homeostasis via diverse stress signaling pathways. Leukemia. 2001;15:1153–1160. doi: 10.1038/sj.leu.2402197. [DOI] [PubMed] [Google Scholar]
- 60.Coroneos E, Wang Y, Panuska JR, Templeton DJ, Kester M. Sphingolipid metabolites differentially regulate extracellular signal-regulated kinase and stress-activated protein kinase cascades. Biochem J. 1996;316(pt 1):13–17. doi: 10.1042/bj3160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bourbon NA, Yun J, Berkey D, Wang Y, Kester M. Inhibitory actions of ceramide upon PKC-ε/ERK interactions. Am J Physiol Cell Physiol. 2001;280:C1403–C1411. doi: 10.1152/ajpcell.2001.280.6.C1403. [DOI] [PubMed] [Google Scholar]
- 62.Bocci G, Fioravanti A, Orlandi P, Bernardini N, Collecchi P, Del Tacca M, Danesi R. Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br J Cancer. 2005;93:319–330. doi: 10.1038/sj.bjc.6602720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fioravanti A, Canu B, Ali G, Orlandi P, Allegrini G, Di Desidero T, Emmenegger U, Fontanini G, Danesi R, Del Tacca M, et al. Metronomic 5-fluorouracil, oxaliplatin and irinotecan in colorectal cancer. Eur J Pharmacol. 2009;619:8–14. doi: 10.1016/j.ejphar.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 64.Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut. 2012 doi: 10.1136/gutjnl-2011-301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 66.Francia G, Shaked Y, Hashimoto K, Sun J, Yin M, Cesta C, Xu P, Man S, Hackl C, Stewart J, et al. Low-dose metronomic oral dosing of a prodrug of gemcitabine (LY2334737) causes antitumor effects in the absence of inhibition of systemic vasculogenesis. Mol Cancer Ther. 2012;11:680–689. doi: 10.1158/1535-7163.MCT-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desai A, Vyas T, Amiji M. Cytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulations. J Pharm Sci. 2008;97:2745–2756. doi: 10.1002/jps.21182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.