Abstract
Positron emission tomography (PET) is an attractive imaging tool to localize and quantify tracer biodistribution. ImmunoPET with an intact mAb typically requires two to four days to achieve optimized tumor-to-normal ratios. Thus, a positron emitter with a half-life of two to four days such as zirconium-89 [89Zr] (t1/2: 78.4 h) is ideal. We have developed an antibody-based, long-lived immunoPET tracer 89Zr-Desferrioxamine-p-SCN (Df-Bz-NCS)-rituximab (Zr-iPET) to image tumor for longer durations in a humanized CD20-expressing transgenic mouse model. To optimize the radiolabeling efficiency of 89Zr with Df-Bz-rituximab, multiple radiolabelings were performed. Radiochemical yield, purity, immunoreactivity and stability assays were carried out to characterize the Zr-iPET for chemical and biological integrity. This tracer was used to image transgenic mice that express the human CD20 on their B cells (huCD20TM). Each huCD20TM mouse received a 7.4MBq /dose. One group (n=3) received 2 mg/kg pre-dose (blocking) of cold rituximab 2 h prior to 89Zr-iPET; the other group (n=3) had no pre-dose (non-blocking). Small animal PET/CT was used to image mice at 1, 4, 24, 48, 72, and 120 h. Quality assurance of the 89Zr-iPET demonstrated NCS-Bz-Df: antibody ratio (c/a: 1.5 ± 0.31), specific activity (0.44 - 1.64 TBq/mol), radiochemical yield (>70%), and purity (>98%). The Zr-iPET immunoreactivity was >80%. At 120 h, Zr-iPET uptake (% ID/g) as mean ± STD for blocking and non-blocking groups in spleen was 3.2 ± 0.1 % and 83.3 ± 2.0 % (p value < 0.0013.). Liver uptake was 1.32 ± 0.05% and 0.61 ± 0.001% (p value < 0.0128) for blocking and non-blocking, respectively. The small animal PET/CT image shows the spleen specific uptake of Zr-iPET in mice at 120 h after tracer injection. Compared to the liver, the spleen specific uptake of Zr-iPET is very high due to the expression of huCD20. We optimized the radiolabeling efficiency of 89Zr with Df-Bz-rituximab. These radioimmunoconjugate lots were stable up to 5 days in serum in vitro. The present study showed that 89Zr is well suited for mAbs to image cancer over extended period of time (up to 5 days).
INTRODUCTION
Many monoclonal antibodies (mAbs) and mAb fragments are currently under clinical investigation due to their excellent potential for cancer diagnosis, therapy, and other pathological conditions 1, 2. The food drug administration (FDA) has approved several of these mAbs for cancer therapy including rituximab, panitumumab, bevacizumab, cetuximab, 90Y-ibritumomab tiuxetan, and 131I-tositumomab. The annual sales of these mAbs were estimated approximately $ 20 billion 3, 4. However, very few mAbs have been applied for positron emission tomography (PET) imaging. PET is an attractive molecular imaging tool to visualize and quantify tumor using targeting molecules 2, 5, 6. Compared to SPECT imaging, PET has many advantages including increased sensitivity. MAbs labeled with positron radionuclides (immunoPET) are a powerful molecular imaging strategy to track, visualize, and measure the tumor gene expression, since immunoPET can characterize and quantify antigen expression specific to a tumor type whereas FDG measures glucose metabolism.
To develop immunoPET agent(s) using intact mAbs or mAb-fragments, an appropriate positron emitter with a half-life of 2-4 days would be ideal. 89Zirconium (89Zr) is one of the most promising radionuclides with which to develop immunoPET agents with intact antibodies for in vivo imaging of cancer 1-4. The physical properties of 89Zr (t1/2 = 78.4h, Eγ = 908.97keV) are well suited for use in a monoclonal antibody-based imaging agent. The relatively low translational energy (distance traveled by the positron before annihilation with an electron) of the emitted positron from 89Zr (Rave.(β+) = 1.18mm) results in high resolution 89Zr images comparable to those observed with the 18F and 64Cu radionuclides Rave.(β+) = 0.69 and 0.70 mm, respectively5-7. Furthermore, routine commercial availability of 89Zr worldwide makes this radionuclide broadly available for research and clinical applications.
To prepare a systemically stable immunoPET agent the choice of chelator to load the radionuclide is an important criterion. For 89Zr labeling desferrioxamine (Df) is a well known chelator that provides stable complexes with zirconium and has been safely used in clinical studies for many years 8-11. ImmunoPET images and quantification results of 89Zr–mAb have been reported in both preclinical 12-16 and clinical studies 8-10, 17, 18. In these studies, results indicated neither pharmacokinetic changes nor a specific accumulation of 89Zr–mAb in non-target organs, except liver and kidneys. The only potential concern is the high radiation dose administered to the patient, which is inherent to the use of long-lived positron emitters like 89Zr and 124I. This might preclude repeated application of 89Zr–immunoPET 9.
The purpose of the current study was to develop and evaluate an antibody-based PET imaging agent for Non-Hodgkin’s lymphoma (NHL) to monitor lymphoma therapy. NHL is the fifth most common cancer in the US with 60,000 new cases and approximately 20,000 deaths each year. There are several known associations and genetic disorders that may play a role in the etiology of NHL. These include genetic abnormalities, environmental factors, viruses, immunodeficiency states, and connective-tissue disorders19. Most (80 to 85%) NHLs arise from B cells; the remainder arises from T cells or natural killer cells. Either precursor or mature cells may be involved. Overlap exists between leukemia and NHL because both involve proliferation of lymphocytes or their precursors. Although substantial progress has been made in understanding the molecular pathogenesis of several forms of NHL, prognostic information is based on morphology and histology.
To overcome these limitations, we developed, optimized and evaluated a novel PET radiopharmaceutical, of an antibody based (rituximab) tracer using 89Zr radionuclide chelated to p-isothiocyanatobenzyl-desferrioxamine B (Df-Bz-NCS). This tracer was evaluted using a humanized transgenic B-cell lymphoma mouse (huCD20TM) model. In this model the spleen is one of the key sites for B cells sequestration. These splenic B cells over-express CD20 antigens, and thus in our study the imaging agent accumulates in the spleen. The CD20 antigen is a 32-kDa non-glycosylated phosphoprotein, which is commonly expressed by human lymphoma cells a target in radioimmunotherapy and over-expressed on the surface of mature B cells 20, 21. The huCD20TM model is thus an excellent model for predicting the targeting capacity of the 89Zr-rituximab tracers. The rationale for this study is the fact that Rituxan, Bexxar, and Zevalin are all antibodies known to target the CD20 antigen in B-cell NHL. Several research groups including our own have, attempted to develop rituximab based immunoPET agents to target the CD20 antigen both in pre-clinical and clinical models 22-24. For example, Olafsen et al. developed immunoPET agents for imaging CD20-positive lymphomas in preclinical models using 64Cu-DOTA-minibody and 124I-scFv-Fc fragment; these immunoPET agents produced high-contrast images in vivo 23. In another study Tran et al. performed a clinical trial to target human CD20 using 124I-rituximab in five patients for detection of inflamed joints in rheumatoid arthritis 24. Although these are only pilot studies, they show encouraging results.
In this report we describe the preparation, quality assurance (QA) methods, and results of a pre-clinical study to evaluate the novel 89Zr-immunoPET (ZriPET) radiopharmaceutical against human CD20 antigen expressed in humanized transgenic mice prior to clinical translation into NHL patients.
MATERIALS AND METHODS
Reagents, antibodies, and radiochemicals
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. P-isothiocyanatobenzyl-desferrioxamine (Df-Bz-NCS) was purchased from Macrocyclics (Dallas, TX, USA). The MAb rituximab (Rituxan; 10 mg/ml) directed against the human CD20 was purchased from Stanford hospital pharmacy (Stanford University, Stanford). Rituximab is a high-affinity chimeric monoclonal antibody of the IgG1 subtype (human κ light chain and human γ1 heavy chain), which recognizes humanized B cell CD20. 89Zr (t1/2=78.4 h, β+=22.6%; ~2.7 GBq/ml in 1 M oxalic acid) was obtained from the University of Wisconsin.
Cell lines and instruments
The CD20+ B-cell lymphoma cell line Ramos was obtained from the American Type Culture Collection (ATCC number: CRL-1555). The CD20-Jurkat cells were obtained from ATCC (TIB-152) as well. Ramos cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (4.5 μg/L glucose), Jurkat cells in MEM/Ham’s F-12 (1:1), and 1% non-essential amino acids. All media were supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, 100 units/mL penicillin, 100 μg streptomycin, and 0.25 μg/mL fungizone. All media and additives were obtained from Invitrogen Corporation (Carlsbad, CA USA).
High performance liquid chromatography (HPLC) was performed on HPLC-Ultimate 3000 with an ultraviolet detector and an online radioactivity detector. The system used a SEC 3000 LC column (300 × 7.8 mm) with 5 μm hydrophilic bonded silica support and 400 Å pore size (Phenomenex, Torrance, CA 90501-1430, USA) Mass spectrometry (AB SCIEX TOF/TOF 5800) was performed at Stanford University and operated in linear mode with sinapinic acid as matrix.
Preparation of immunoconjugate
Optimization of chelate conjugation to antibody
To optimize the Df-Bz-NCS conjugation with rituximab and bind 1-2 chelates per antibody, the conjugation reaction was performed under nine different conditions. These experiments used three different pH conditions and three sets of chelate concentrations were tested. Briefly, 20 μL of a 50 μM solution of rituximab (7.5 mg/mL) was added to each reaction vial followed by addition of 2-10 μL of 0.1 M Na2CO3 at pH 8, 8.5, and 9.0 in triplicate. Three separate reaction vials from each pH condition received 20 μL of Df-Bz-NCS of 250, 375, and 500 μM concentrations in DMSO. At each pH condition the final chelate concentrations of Df-Bz-NCS was 5-fold, 7.5-fold and 10-fold molar excess over rituximab concentrations. Finally all reaction tubes were brought to 50 μL total volume using 0.1 mol/L sodium phosphate buffer (pH 8). Excess Df-Bz-NCS was then removed by 30 kDa membrane dialysis using slide-A-lyzer of 0.2 mL volume (Pierce, USA) with 0.1 M sodium acetate buffer (pH 7.0) as dialysis buffer. The conjugation experiments were repeated twice.
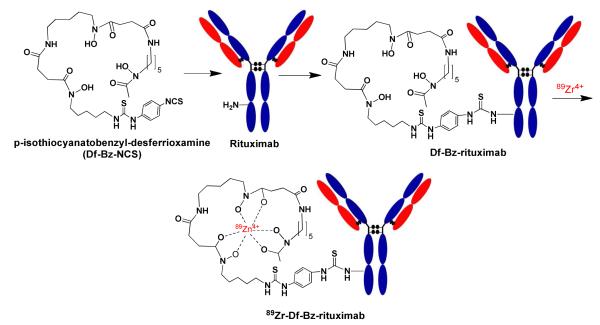
For the pre-clinical study, the Df-Bz-rituximab was prepared as a separate lot. An aqueous solution of rituximab (0.1 mM; 350 μL) was buffer exchanged with 0.1M Na2CO3 (pH 9.0) and then mixed with 5-fold molar excess of Df-Bz-NCS (5 mM; 35 μL of DMSO) in a 1 mL Eppendorf vial. The final reaction mixture was made up to 0.5 mL (pH = 8.5) using 0.1 mol/L sodium phosphate buffer. The reaction was kept for 1h at room temperature. Excess Df-Bz-NCS was then removed by 30-kDa membrane dialysis using slide-A-lyzer (Pierce, USA) and buffer exchanged into 0.1 M sodium acetate buffer (pH 7.0) for 89Zr labeling. The immunoconjugates were concentrated by centrifugation-dialysis to 0.1 to 0.2 mg/mL and stored at -80°C. The number of chelators (c) coupled per antibody (a) i.e. c/a molecule was estimated with MALDI-TOF MS by comparison of rituximab and Df-Bz-rituximab 15.
Optimization of radiolabeling
Based on previous reports 25 we further refined the radiolabeling conditions to create immunoconjugates with high specific activity and radiochemical yields. Labeling of Df-Bz-rituximab (c/a = 1.5) using 89Zr was optimized for two variables: a) pH conditions (pH = 6.5, 7.0 and 7.5) and b) labeling reaction time (45 and 60 minutes) at 37° C. The reaction was performed in a 1.5 ml Eppendorf tube in the following sequence: First, 89Zr (37 MBq; 250 μl) and 2.5 M Na2CO3 (50 μl) were added. After 3 min, 10 mM ammonium acetate buffer (150 μl, pH 7.0), Df-Bz-rituximab (150 μl, 10 μg), and 10mM ammonium acetate buffer (150 μl, pH 7.0) were added. After incubation (45 minutes), 0.1 M diethylenetriaminepentaacetic acid (DTPA, pH 7.0) was added to a final concentration of 5 mmol/L for 15 minutes to scavenge unchelated 89Zr in the reaction mixture. Based on the labeling optimization for pre-clinical study, the reaction mixture was kept at pH 7. 0 for 60 minutes. The Df-Bz-NCS-mAb (20 – 50μg; 100 μl) and 89Zr (74–190 MBq; 500 μl) and the other reagents were added as mentioned above. Purification of the radioimmunoconjugate (25 μg in 500 μL volume) was achieved by size exclusion chromatography on a Phenomenex SEC 3000 column (Torrance, CA, USA) in PBS buffer [0.1 mol/L NaCl, 0.05 mol/L sodium phosphate (pH 7.4)] at a flow rate of 1.0 mL/min. The radioimmunoconjugate peak (retention time at 8.5 minute) corresponding to antibody was collected, and concentrated using Amicon Ultra-15 (Millipore, USA) device and centrifuged at 3,000 × g for 15 minutes. The final product was filtered through a 0.2 micron filter into a sterile vial.
Immunoreactivity and stability of the radiolabeled immunoconjugates
Each lot of the radioimmunoconjugate was evaluated for the immunoreactive fraction and immunoreactivity by cell-binding assays as described in previous publications 5, 26. Briefly, Ramos (CD20 +) cells were suspended in microcentrifuge tubes at concentrations of 5.0, 4.0, 3.0, 2.5, 2.0, 1.5, and 0.5 × 106 cells/mL in 500 μL PBS (pH 7.4). Aliquots of 89Zr-Df-rituximab (50 μL of a stock solution of 0.37 kBq, [0.01 mCi] in 10 mL of 1% bovine serum albumin [BSA]; 0.37 kBq [1 μCi], 0.01 μg of mAb) were added to each tube (n=3; final volume 550 mL). After addition of tracer, the solutions were gently vortexed and incubated at 37°C. Two hours later, the solutions were centrifuged (300 g for 3 min.), resuspended, and washed twice with ice-cold PBS before removing the supernatant. Cells were then pelleted by centrifugation and the 89Zr-activity associated with the cell pellet was measured with a gamma counter (1470 WIZARD Automatic Gamma Counter; Perkin Elmer, Walthem, MA). The count data were background corrected and compared with the total number of counts in control samples. Competitive inhibition (blocking) assays were conducted by using the same procedure but with the addition of unmodified rituximab (50 μL, 0.2 mg/mL in 1% BSA, [1000-fold excess mAb; 10 μg]) to the 89Zr-Df-rituximab solutions.
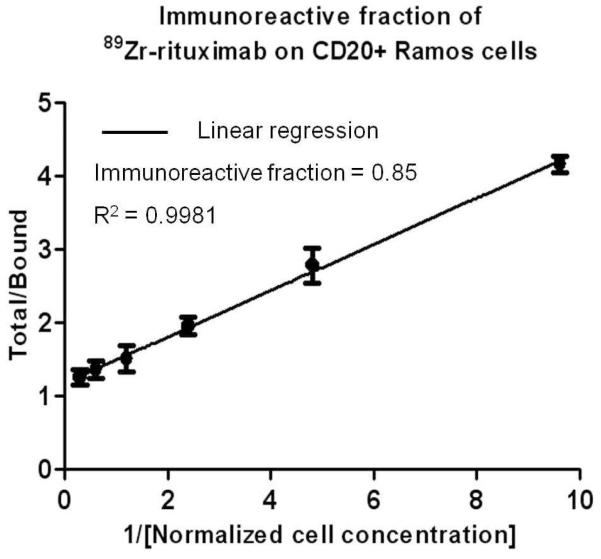
Immunoreactive fractions were determined by linear regression analysis of a plot of (total/bound) activity versus (1/[normalized cell concentration]), and calculated as 1/y-intercept. Stability of the labeled antibodies after incubation in human serum at 37°C was analyzed by Cellulose Acetate Electrophoresis (CAE) at 45 minutes and tested at 1, 4, 24, 48, 72 and 120 h 27, 28.
Animal studies
Animal studies were performed in compliance with approval from the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University. Nude mice (CD1-nu) from Charles River, Inc, and huCD20 transgenic mice (Genentech, South San Francisco) were purchased for the experiments 22, 29. Prior to the animal study huCD20 transgenic mice were screened to confirm the expression of CD20 positive targets by RT-PCR. The average weight of the mice was 25.0 ± 2.0 μg. Nude mice and two other groups of human CD20 positive transgenic mice (3 animals for each group) were imaged at 1, 4, 24, 48, 72, 96 and 120 h using small animal PET. All experimental mice received 89Zr-labeled radiopharmaceutical [200 μL, corresponding to 7.4 MBq, 2 μg of Df-Bz-rituximab] via tail vein injection. After radiotracer administration the animals were scanned at the time points indicated above. Results are reported as % injected dose per gram of tissue (%ID/g). Statistical analysis was done with Student’s t test (two-tailed, unequal variance).
Small animal PET imaging
Prior to the imaging experiments, the animals (nude and huCD20 transgenic mice) were lightly restrained and administered the dose of 89Zr-Df-Bz-rituximab (7.4 MBq / 2 μg Df-Bz-rituximab) via a lateral tail vein. At each time points (1, 4, 24, 48, 72, 96, and 120 hours) the animals were anesthetized and scanned as described before. PET was performed on a Siemens Inveon small-animal multimodality PET/CT system (Preclinical Solutions; Siemens Healthcare Molecular Imaging, Knoxville, TN). This PET/CT system combines two independently operating PET and CT scanners with excellent radial, tangential, and axial resolutions higher than 1.5 mm at the center of the field of view of the PET module. The CT raw images were acquired at 80 kVp at 500 μA, two bed position, half scan 220° of rotation, and 120 projections per bed position with a cone beam micro-x-ray source (50-ìm focal spot size) and a 2,048 × 3,072 pixel x-ray detector. CT raw data sets were reconstructed using Shepp-Logan filter and cone-beam filtered back-projection. On the basis of attenuation correction from the CT measurements, static PET scan was acquired with default settings of coincidence timing window of 3.4 ns and energy window of 350 to 650 keV. The first acquisition was started 60 min after the tracer injection and acquired for 5 min. We then performed 10 min acquisitions after 4, 24, 48 hours, and 20 min scans after 72, 96 and 120 hours after the tracer injection. The images were reconstructed with two-dimensional ordered-subset expectation maximization (OSEM 2D) algorithm 30. Image files were analyzed using open source software, a Medical Image Data Examiner (AMIDE) 31. For each small animal PET scan, three-dimensional regions of interest (ROIs) were drawn over the heart, liver, spleen, and whole body on decay-corrected whole-body images. The average radioactivity concentration in the ROI was obtained from the mean pixel values within the ROI volume. These data was converted to counts per milliliter per minute by using a predetermined conversion factor. The results were then divided by the injected dose to obtain an image region of interest–derived percentage injected dose per gram of tissue (%ID/g).
RESULTS
Preparation of Df-Bz-rituximab
The Df-Bz-rituximab immunoconjugate was prepared by coupling Df-Bz-NCS to the lysine groups of rituximab mAb (Scheme 1). Table 1A shows the various conditions of the immunoconjugation; various pH and molar concentrations of the chelates are listed in the table to optimize the immunoconjugation to achieve best chelates per Ab. The best immunoconjugation was achieved by the addition of a five-fold molar excess of Df-Bz-NCS over rituximab at pH of 8.5, incubated at 25°C for 45 min. These conditions resulted in a reproducible chelate/rituximab of 1.5 as assessed by MALDI-TOF. Although high concentrations of chelate (10-fold excess over mAb) provided maximum 2.6 c/a, we prefer to retain 5-fold molar excess to retain biological properties of the mAb as close to unmodified mAb.
Table 1.
Optimization of reaction conditions for Df chelate conjugation and radiochemistry to prepare 89Zr-Df-Bz-rituximab.
| aDf-Bz chelate conjugation to antibody | ||||
|---|---|---|---|---|
| pH | Time (minutes) | Mab (nmoles) |
Df-bz Chelates (excess nm fold) |
Chelates/Mab (c/a) |
|
| ||||
| 8 | 45 | 0.5 | 5 | 1.30 ± 0.14 |
| 8 | 45 | 0.5 | 7.5 | 1.55 ± 0.07 |
| 8 | 45 | 0.5 | 10 | 2.20 ± 0.14 |
| 8.5 | 45 | 0.5 | 5 | 1.55 ± 0.07 |
| 8.5 | 45 | 0.5 | 7.5 | 2.30 ± 0.14 |
| 8.5 | 45 | 0.5 | 10 | 2.35 ± 0.07 |
| 9 | 45 | 0.5 | 5 | 1.65 ± 0.21 |
| 9 | 45 | 0.5 | 7.5 | 2.30 ± 0.14 |
| 9 | 45 | 0.5 | 10 | 2.60 ± 0.57 |
| b Radiochemical yield optimization | ||
|---|---|---|
| Radiochemical yield (%) | ||
|
| ||
| 6.5 | 45 | 74.06 ± 3.51 |
| 7 | 45 | 76.25 ± 0.86 |
| 7.5 | 45 | 76.49 ± 0 |
| 6.5 | 60 | 76.16 ± 1.05 |
| 7 | 60 | 77.01 ± 2.16 |
| 7.5 | 60 | 76.78 ± 1.24 |
Temperatures: a= 25° C; b= 37° C
Radiolabeling of Df-Bz-rituximab
Table 1B shows the optimization of the 89Zr radiochemistry with Df-Bz-rituximab to achieve high radiochemical yield. Radiolabeling of Df-Bz-rituximab with 89Zr in NaHCO3 buffer (pH 7.0) yielded greater than 75% (Mean ± STD: 77.0 ± 2.1%). The highest radiochemical yield achieved was 77% at 37°C, of pH 7.0, at incubation for 60 minutes. Table 2 shows the quality assurance of the 89Zr-Df-Bz-rituximab PET tracer. Figure 1A shows the radiochemical purity was >95% (Mean ± STD: 98.0 ± 0.7%) determined by SEC.
Table 2.
Quality assurance of the 89Zr-Df-Bz-rituximab PET tracer.
| Characterization of 89Zr-Df-Bz-rituximab | ||
|---|---|---|
| Test # |
Test Specification | Results (Mean ±STD) |
| 1 | Chelate/Mab | 1.5 ± 0.3 |
| 2 | Radiochemical Yield (%) | 77.1 ± 2.1 |
| 3 | Final Product Yield (%) | 70 ± 2.8 |
| 4 | Specific Activity (TBq/mmole) | 5.1 - 6.0 ×102 |
| 5 | Purity by Radio HPLC (%) | 98 ± 0.7 |
| 6 | Purity by ITLC (%) | >95 |
| 7 | Purity by CAE 45 min (%) | >95 |
| 8 | Immunoreactivity (%) | 84.2 ± 2.8 |
Figure 1.
Purity and serum stability of the 89Zr-Df-rituximab: A. Radio-HPLC chromatogram PET tracer shows the purity of the tracer. The PET tracer was eluted by SEC 3000 column at a flow rate of 1 mL/min using PBS. Radioactivity of each of the 1 mL fractions was measured by a gamma counter and the data was used to plot the chromatogram. B. Serum stability of 89Zr-Df-rituximab assayed by cellulose acetate electrophoresis (CAE) at 45 min: The results of serum stability study of PET tracer tested by CAE are shown. CAE was performed for 45 min with barbital buffer (0.05 M, pH 8.6) at room temperature. Radioconjugate (50 μg) was mixed per ml of human serum and kept at 37°C for 3 days. At various time points (0, 24, 72, and 120 h), 2–10 μl samples were drawn and tested for stability on CAE. Radioactivity peaks in the figure correspond to radioconjugate in the serum at day 1 – 5, demonstrated radioconjugates are at identical migration distance.
Serum stability assay
Figure 1B shows the stability of the radiotracer in human serum over 5 days, tested by CAE at 45 minutes. The radio peaks in Figure 1B correspond to radiotracer at each time point. These results show that the tracer was stable in serum and greater than 98 % of the immunoconjugate was intact for up to 5 days.
Cell binding study
Figure 2 shows the binding fraction of the 89Zr-Df-Bz-rituximab tested with CD20 positive Ramos cells. The average immunoreactive fraction of 89Zr-Df-Bz-rituximab from each lot was 0.84 ± 0.7 (n = 6). The control experiment was performed with CD20 negative Jurkat cells, and CD20 positive Ramos cells pre-blocked with 100-fold excess of cold rituximab over 89Zr-rituximab. The immunoreactivity of the tracer was 84.2 ± 2.8 % (Mean ±STD) compared to the binding effect on same cells but pre-blocked with cold rituximab and Jurkat cells were 5 ± 1.6 % (Mean ±STD) and 4 ± 2.1 % (Mean ±STD) respectively, indicating the specific reactivity of the tracer.
Figure 2.
Live cell binding assay for the determination of the immunoreactive fraction of an 89Zr-rituximab tracer using Ramos cells. The assay was set up using six concentrations of Ramos cells in 1:2 dilutions from 3.3 to 0.1 million cells/mL, and the final concentrations of tracer was 10 ng/ml.Cells were used at various concentrations to infinite antigen excess (1/y-intercept). A plot of (X axis: total/bound) activity against (Y axis: 1/normalized cell concentration; mL/million) provided immunoreactive fraction (0.84 ± 0.7). X axis of graph represents cell concentrations (ml/million) 0.3, 0.6, 1.2, 2.4, 4.8 and 9.62, the corresponding immunoreactive fractions (mean ± SD) represent Y axis are 1.25 ± 0.11, 1.35 ± 0.12, 1.5 ± 0.18, 1.95 ± 0.12, 2.78 ± 0.24 and 4.16 ± 0.11 respectively. The data represented in the graph are corrected with subtraction of immunoreactive fractions on pre-blocked cells [non-specific binding (mean ± SD) = 0.008 ± 0.001] with cold rituximab prior to tracer application.
Small animal PET and CT imaging
To evaluate antibody based PET tracer targeting anti-CD20 B-cell lymphoma, we used a huCD20TM model that mimics a human B-cell lymphoma tumor and provides a clearance pattern such that rituximab recognize CD-20 antigens on B-cells in human lymphoma patients. The in vivo targeting ability of 89Zr-Df-Bz-rituximab in huCD20TM was demonstrated at various time points post tail-vein injection of radiopharmaceutical, with and without blocking of CD20 antigens by cold rituximab. Each mouse was imaged at various time points (1, 4, 24, 48, 72, 96 and 120 h) after tracer injection of 7-8 MBq of 89Zr-Df-Bz-rituximab (2-3 μg of rituximab).
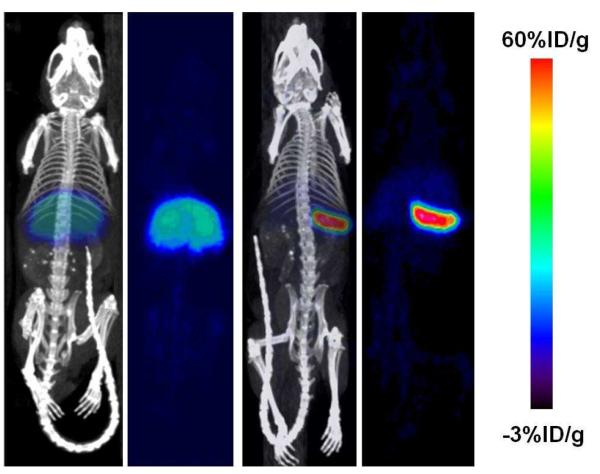
Figure 3 - 4 shows the immunoPET images of huCD20TM using the 89Zr-Df-Bz-rituximab tracer. Figure 3A PET images were obtained at 4, 24, 48, 72, 96 and 120 h after injection of tracer. At each time points PET/CT images were obtained from the control huCD20TM (Figure 3 top row: blocking = pre-blocked with 100-fold excess cold rituximab over tracer mass 2 h prior to tracer injection); and experimental huCD20TM (Figure 3 bottom row: Non-blocking = tracer alone). Figure 3B, images shown is huCD20images of just CT and co-registered with PET/CT for organ delineation. Organs marked as H = heart, L = liver and the spleen is marked with a yellow arrow. From these images it is evident that 89Zr-Df-Bz-rituximab had uptake primarily in the spleen, a major site for B cells, which express CD20 antigen. Figure 4 shows immunoPET images of huCD20TM at the maximum intensity projection of 89Zr-Df-Bz-rituximab after 5 days of post injection of the tracer. The spleen and liver uptake (%ID/g) is shown in Figure 5 at 120 h post injection compared with the pre-dose group (human CD20 antigens were pre-blocked with cold rituximab). The radiopharmaceutical uptake (%ID/g) in the pre-blocked (n=3) and unblocked (n=3) groups is 3.19 ± 0.14, and 83.3 ± 1.99 for spleen; 1.32 ± 0.05, and 0.61 ± 0.01 for liver, respectively.
Figure 3.
ImmunoPET images of 89Zr-Df-rituximab on huCD20TM model. The representative images were scanned (5-20 minutes) at 4 - 120 h after tracer injection (7.4MBq/ 2-3μg of rituximab). Panels A-B, top (blocked = Pre-dose; control) and (non-blocked = no pre-dose; experiment) bottom rows are huCD20TM coronal images of pre-blocked and unblocked, respectively. Panel A are a mouse that received 4 mg/kg cold rituximab 2 h prior to tracer injection and panel B are mouse that received the PET tracer only. Panel C shows the CT and PET/CT images at 120 h after tracer injection for organ identification. Spleen is indicted by the yellow arrow. The other major organs are as marked (H= heart and L= liver). The color bar shows tracer %ID/g.
Figure 4.
ImmunoPET images of huCD20TM after 120 h post injection with maximum intensity projections (MIP) shows mouse with and without pre dose of rituximab. The 89Zr-Df-rituximab uptake by spleen is very high compared to pre-blocked mouse by cold rituximab. ImmunoPET tracer was stable even after 120 h and no PET signals were observed in bone marrow.
Figure 5.
Quantitative analysis of organs uptake estimated from the ROI of the images. Data shown are tracer uptake value at 120 h post injection by liver (gray bar), and spleen (black bar) of huCD20 mice with and without pre-dose groups. Y axis is %ID/gram and X axis shows comparison of control and experiment groups. Tracer uptake by the liver is similar for both study groups, and the spleen uptake for the group without pre-dose is > 50 times higher than that with the pre-dose group, confirming the specificity of the tracer. The data represent the mean ± STD of 3 mice per group.
DISCUSSION
To monitor NHL therapy we have developed a novel immunoPET tracer using 89Zr radionuclide that chelated to desferrioxamine (Df) linked to rituximab. This work was conducted in an effort to eventually translate the methods developed here for human lymphoma patient management through PET imaging. In this report we present in vitro characterization and pre-clinical evaluation of 89Zr-Df-rituximab tracer in a huCD20TM mouse model, which expresses the human CD20. Previously, we have developed a 64Cu-DOTA-rituximab immunoPET tracer 22 that received FDA approval for a physician sponsored IND (#104995) for safety and clearance and is currently being tested in a clinical trial. We are also interested in developing immunoPET tracers for radioimmunotherapy monitoring with longer half-life radionuclides for comparable half-life of antibody (2-5 days). To match the half-life of antibody, currently very few long half-life PET radionuclides are available for use in clinical studies; i.e., 124I, and 89Zr. Although iodination (124I) of antibodies for PET is simple, it has widespread limitations of lower resolution and enzymatic de-iodination 32, 33.
Recently, 89Zr-immunoPET tracers are being increasingly used in clinical studies 34, 35. This is because the low production cost, purity, stability and extended half-life period of the 89Zr radionuclides make it a perfect choice to link to an antibody for targetable PET tracers. On the other hand GMP grade Df-Bz-NCS bi-functional chelator is also commercially available for 89Zr labeling for clinical studies. The Df-Bz-NCS ligand has already shown good biological performance when used in protein conjugation of radionuclides such as 68Ga, 66Ga, 225Ac, 177Lu and lead radionuclides 36, 37. Furthermore, currently both Df-Bz-NCS conjugated antibodies and 89Zr-labeled antibodies are in clinical trials after FDA approval. Although, few studies have shown that DTPA can be used for the chelation of 89Zr ions; it shows demetalation in vivo and hence Df continues to remain the best chelator for 89Zr 38-40. Thus in our study, Df-Bz-NCS was utilized to prepare Df-Bz-rituximab according to published procedures 25, 41.
In the present report, we optimized the reaction conditions for conjugation of Df and radiolabeling of 89Zr to chelate for high radiochemical yield and specific activity specifically for the rituximab antibody. The conjugation optimization yielded maximum 2.6 chelates per antibody (Table 1) at pH 9.0, with 10-fold molar excess ratio after 45 minutes; although, chelate ratio per antibody was dictated by the number of lysine molecules per antibody in our case the c/a is comparable to the reported result elsewhere using different antibody 41. Although we have achieved 2.6 c/a, we realize that too many lysine group modifications may alter the biodistribution from the parent molecule 42, 43. Hence we restrict our chelate conjugation to not more than 2.0 per antibody for the use of pre-clinical studies. Similarly, the radiolabeling optimization yielded maximum 77.0 ± 2.16 (mean ± STD) at pH 7 and after purification the final product purity was > 98% monomeric (Figure 1A and Table 2).
The 89Zr-Df-Bz-rituximab conjugate showed good in vitro stability for up to 5 days at 37°C in 0.9% saline, 50mM DTPA and human serum (Figure 1B). Our results confirmed with previous reports elsewhere that the Df-Bz-SCN linked immunoconjugates were stable over a 6-day period in serum at 37°C 36, 41. After five days less than 1.5% activity appeared at 5 cm in CAE at 45 minutes, which may correspond to 89Zr-transchelated protein in serum, possibly due to demetalation from the 89Zr-Df-rituximab (Figure 1B). Figure 2 shows the estimation of immunoreactive fraction of 89Zr-Df-Bz-rituximab used in the pre-clinical studies. The immunoreactive fraction of the 89Zr-Df-rituximab was measured by specific in vitro cellular association assays using CD20 positive Ramos cells prior to each pre-clinical study. The average immunoreactive fraction of 89Zr-Df-rituximab (Figure 2) was found to be 0.85 (n= 6). Control experiments were performed using the CD20 negative Jurkat cells (no CD20 expression) and Ramos cells pre-blocked with 100-fold excess of cold unmodified rituximab 1 h prior to tracer application, showed < 4 % binding. These data clearly demonstrated that the 89Zr-Df-rituximab tracer preparation was highly immunoreactive with specific binding effect on huCD20 expressing live cells.
ImmunoPET images of 89Zr-Df-rituximab were acquired on huCD20TM using the Inveon small animal CT-PET scanner at 0 - 120 h post injection presented in Figures 3 and 4. Figure 3A-B bottom row (non-blocking-huCD20TM) shows clearly that the tracer uptake in spleen is very high compared to the liver and other organs. In the same figure, the top row PET images pertaining to blocking-huCD20TM (control group) show that the tracer uptake was very low at all time points due to pre-blocking by cold rituximab. Figure 5 shows %ID/g of tracer uptake by organs of huCD20TM; a) liver and b) spleen from both non-blocked and blocked huCD20TM PET images (calculated from the region-of-interest) respectively. ROI quantification of these images showed that at 120 h post injection the absolute uptake of 89Zr-rituximab by spleen was 83.0 and 3.2 %ID/g in non-blocked - and blocked-huCD20TM mice respectively. These data show that 89Zr-Df-rituximab immuno-PET imaging provides a very high contrast image at CD20 expressing marker compared to the liver. The huCD20TM model we used in this study will mimic human patients with respect to targeting protein, which is identical to human CD20. Thus this study outcome would be more favorable for clinical translation.
To see a high contrast image we prepared tracer with high specific activity by using very low mass of antibody (2-3μg/dose; 80-120 μg/Kg) compared to studies carried out by others (0.2 mg/dose; 8 mg/kg) 36. This is because high mass rituximab may induce apoptosis, by complement-mediated lysis, and antibody-dependent cellular toxicity which will lead to eradication of B cells. To visualize optimal high contrast images over extended periods, the tracer affinity for CD20 target should be high and the tracer should be intact over longer times. Thus compared to other published studies our tracer with high specific activity showed very high contrast images over extended period of time. This was reflected in Figure 3B and 4 at 120 h where the immunoPET demonstrated very high contrast images for spleen of non-blocked-huCD20TM, compared to blocked-huCD20TM. ROI quantitative analysis of 89Zr-Df-rituximab uptake in spleen (Figure 5), of non-blocked-huCD20TM, was approximately 25-fold higher %ID/g than blocked-huCD20TM. Thus our study data observed from the high specific activity tracer may be useful for other mAbs and mAb-fragments for high imaging contrast and for imaging for extended periods of time.
Zirconium is known to bind plasma proteins 44 and is later deposited in mineral bone 45, 6. However, in our study we did not see bone uptake of the radionuclide originating from the breakdown of the 89Zr-antibody or any non-specific association of 89Zr to antibody which could then easily transchelate to plasma proteins compared to 89Zr bound to Df. The results reported here are consistent with previous investigations and confirm that 89Zr is a suitable radionuclide for labeling full, intact antibodies (150 kDa) 36. The 89Zr has the potential to solve many problems associated with various PET isotopes such as; a) PET isotopes of 64Cu, and 86Y have short half-life, b) 64Cu, 68Ga, 124I are demonstrated to have high uptake in background tissue, c) 124I are known for low in vivo stability (for any internalizing antibody-antigen constructs) and poor dosimetry. Contrary to the above mentioned isotopes, 89Zr-labeled mAbs can be used for both localizing tumors and measuring the long-term effects (5 days) of drug treatment from a single radiotracer injection. Such measurements cannot be achieved by using 64Cu,124I, and 99mTc radiolabeled rituximab 23, 24, 47. The image quality and chelate chemistry of 89Zr-Df-mab immunoPET tracers were well demonstrated by various PET imagers 36, 41, 48. For example studies pertaining to 89Zr-Df-trastuzumab represent one of the most promising radiotracers for non-invasive immunoPET measurements of HER2/neu expression in vivo 48.
CONCLUSIONS
We have prepared 89Zr-Df-rituximab with high specific activity, purity, stability, and immunoreactivity. The 89Zr-immuoPET tracer was shown to be stable for up to five days in human serum. The desferrioxamine chelates conjugation to antibody and radiolabeling of immunoconjugates were optimized in such a way that the biological activity of the antibody was intact. The pre-clinical studies of 89Zr-Df-rituximab with the huCD20TM model clearly indicated that this tracer is specifically targeted to huCD20 antigen and has potential to translate to patients. Compared to our previous immunoPET tracer (64Cu-DOTA-rituximab), 89Zr-Df-rituximab could be used to monitor and measure therapeutic effects over extended periods of time in both pre-clinical and clinical studies. Further, using 89Zr-Df-rituximab the pharmacodynamic effects of any novel drugs can potentially be studied along with patient response to drug treatment. Currently, optimization towards GMP preparation for an IND application to FDA and eventual clinical translation of 89Zr-Df-rituxiab are underway.
Supplementary Material
Scheme 1.
Schematic representation of immuno-PET tracer synthesis
Footnotes
SUPPORTING INFORMATION Additional information is available, such as method of 89 Zirconium productions, and ex vivo biodistribution study; biodistribution data of 89Zr-Df-rituximab from ROI of PET images and from ex-vivo organ uptake; and movie of immuno-PET tracer on huCD20TM. This information is available free of charge via the internet at http://pubs.acs.org/.
REFERENCES
- (1).Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–57. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- (2).Wu AM. Antibodies and antimatter: the resurgence of immuno-PET. J Nucl Med. 2009;50:2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- (3).Lawrence S. Billion dollar babies--biotech drugs as blockbusters. Nat Biotechnol. 2007;25:380–2. doi: 10.1038/nbt0407-380. [DOI] [PubMed] [Google Scholar]
- (4).Maggon K. Monoclonal antibody “gold rush”. Curr Med Chem. 2007;14:1978–87. doi: 10.2174/092986707781368504. [DOI] [PubMed] [Google Scholar]
- (5).Nayak TK, Brechbiel MW. Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges. Bioconjug Chem. 2009;20:825–41. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12:1379–89. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- (7).Boswell CA, Brechbiel MW. Development of radioimmunotherapeutic and diagnostic antibodies: an inside-out view. Nucl Med Biol. 2007;34:757–78. doi: 10.1016/j.nucmedbio.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Borjesson PK, Jauw YW, Boellaard R, de Bree R, Comans EF, Roos JC, Castelijns JA, Vosjan MJ, Kummer JA, Leemans CR, Lammertsma AA, van Dongen GA. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. 2006;12:2133–2140. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- (9).Borjesson PKE, Jauw YWS, de Bree R, Roos JC, Castelijns JA, Leemans CR, van Dongen GAMS, Boellaard R. Radiation dosimetry of zirconium-89-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med. 2009;50:1828–1836. doi: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- (10).Dijkers E, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009;50:974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- (11).Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE, van Dongen GA. p-Isothiocyanatobenzyl-deferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:250–259. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, Lambin P. Disparity between in vivo EGFR expression and zirconium-89-labeled cetuximab uptake assessed by PET. J. Nucl. Med. 2008;50:123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- (13).Brouwers A, Verel I, Van Eerd J, Visser G, Steffens M, Oosterwijk E, Corstens F, Oyen W, Van Dongen G, Boerman O. PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother. Radiopharm. 2004;19:155–163. doi: 10.1089/108497804323071922. [DOI] [PubMed] [Google Scholar]
- (14).Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, Brouwers AH, van Dongen GA, Perk LR, Lub-de Hooge MN. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007;48:1313–1319. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- (15).Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- (16).Verel I, Visser GW, Boerman OC, van Eerd JE, Finn R, Boellaard R, Vosjan MJ, Stigter-van Walsum M, Snow GB, van Dongen GA. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiopharm. 2003;18:655–661. doi: 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- (17).Perk LR, Visser OJ, Stigter-van Walsum M, Vosjan MJ, Visser GW, Zijlstra JM, Huijgens PC, van Dongen GA. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:1337–1345. doi: 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- (18).Zalutsky MR. Potential of immuno-positron emission tomography for tumor imaging and immunotherapy planning. Clin. Cancer Res. 2006;12:1958–1960. doi: 10.1158/1078-0432.CCR-06-0405. [DOI] [PubMed] [Google Scholar]
- (19).Fisher SG, Fisher RI. The epidemiology of non-Hodgkin’s lymphoma. Oncogene. 2004;23:6524–6534. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- (20).Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–66. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- (21).Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular Immunology. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- (22).Natarajan A, Gowrishankar G, Nielsen CH, Wang S, Iagaru A, Goris ML, Gambhir SS. Positron Emission Tomography of (64)Cu-DOTA-Rituximab in a Transgenic Mouse Model Expressing Human CD20 for Clinical Translation to Image NHL. Mol Imaging Biol. 2012 doi: 10.1007/s11307-011-0537-8. DOI: 10.1007/s11307-011-0537-8. [DOI] [PubMed] [Google Scholar]
- (23).Olafsen T, Betting D, Kenanova VE, Salazar FB, Clarke P, Said J, Raubitschek AA, Timmerman JM, Wu AM. Recombinant anti-CD20 antibody fragments for small-animal PET imaging of B-cell lymphomas. J Nucl Med. 2009;50:1500–8. doi: 10.2967/jnumed.108.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tran L, Huitema AD, van Rijswijk MH, Dinant HJ, Baars JW, Beijnen JH, Vogel WV. CD20 antigen imaging with 124I-rituximab PET/CT in patients with rheumatoid arthritis. Hum Antibodies. 2011;20:29–35. doi: 10.3233/hab20110239. [DOI] [PubMed] [Google Scholar]
- (25).Vosjan MJ, Perk LR, Visser GWM, Budde M, Jurek P, Kiefer GE, van Dongen GAMS. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protocols. 2010;5:739–743. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- (26).Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- (27).Adams GP, DeNardo SJ, Deshpande SV, DeNardo GL, Meares CF, McCall MJ, Epstein AL. Effect of mass of 111In-benzyl-EDTA monoclonal antibody on hepatic uptake and processing in mice. Cancer Res. 1989;49:1707–11. [PubMed] [Google Scholar]
- (28).Saludes JP, Natarajan A, DeNardo SJ, Gervay-Hague J. The Remarkable Stability of Chimeric, Sialic Acid-derived alpha/delta-Peptides in Human Blood Plasma. Chemical Biology & Drug Design. 2010;75:455–460. doi: 10.1111/j.1747-0285.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–26. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- (30).Irmler IM, Opfermann T, Gebhardt P, Gajda M, Brauer R, Saluz HP, Kamradt T. In vivo molecular imaging of experimental joint inflammation by combined (18)F-FDG positron emission tomography and computed tomography. Arthritis Res Ther. 2010;12:R203. doi: 10.1186/ar3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–7. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- (32).Geissler F, Anderson SK, Press O. Intracellular catabolism of radiolabeled anti-CD3 antibodies by leukemic T cells. Cell Immunol. 1991;137:96–110. doi: 10.1016/0008-8749(91)90060-o. [DOI] [PubMed] [Google Scholar]
- (33).Shih LB, Thorpe SR, Griffiths GL, Diril H, Ong GL, Hansen HJ, Goldenberg DM, Mattes MJ. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J Nucl Med. 1994;35:899–908. [PubMed] [Google Scholar]
- (34).Dijkers EC, Munnink T. H. Oude, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–92. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- (35).Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50:974–81. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- (36).Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE, van Dongen GA. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging. 2010;37:250–9. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang ZF, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl Med Biol. 2003;30:725–31. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- (38).Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–39. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Meijs WE, Haisma HJ, Klok RP, van Gog FB, Kievit E, Pinedo HM, Herscheid JD. Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J Nucl Med. 1997;38:112–8. [PubMed] [Google Scholar]
- (40).Meijs WE, Herscheid JD, Haisma HJ, Pinedo HM. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int J Rad Appl Instrum A. 1992;43:1443–7. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- (41).Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E, Vandlen R, Darwish M, Junutula JR, Williams S-P, Marik J. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nuclear Medicine and Biology. 2010;37:289–297. doi: 10.1016/j.nucmedbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- (42).van Gog FB, Visser GW, Klok R, van der Schors R, Snow GB, van Dongen GA. Monoclonal antibodies labeled with rhenium-186 using the MAG3 chelate: relationship between the number of chelated groups and biodistribution characteristics. J Nucl Med. 1996;37:352–62. [PubMed] [Google Scholar]
- (43).van Gog FB, Visser GW, Stroomer JW, Roos JC, Snow GB, van Dongen GA. High dose rhenium-186-labeling of monoclonal antibodies for clinical application: pitfalls and solutions. Cancer. 1997;80:2360–70. [PubMed] [Google Scholar]
- (44).Mealey J., Jr. Turn-over of carrier-free zirconium-89 in man. Nature. 1957;179:673–4. doi: 10.1038/179673a0. [DOI] [PubMed] [Google Scholar]
- (45).Fletcher CR. The radiological hazards of zirconium-95 and niobium-95. Health Phys. 1969;16:209–20. doi: 10.1097/00004032-196902000-00011. [DOI] [PubMed] [Google Scholar]
- (46).Shiraishi Y, Ichikawa R. Absorption and retention of 144 Ce and 95 Zr-95 Nb in newborn, juvenile and adult rats. Health Phys. 1972;22:373–8. doi: 10.1097/00004032-197204000-00009. [DOI] [PubMed] [Google Scholar]
- (47).Stopar TG, Mlinaric-Rascan I, Fettich J, Hojker S, Mather SJ. (99m)Tc-rituximab radiolabelled by photo-activation: a new non-Hodgkin’s lymphoma imaging agent. Eur J Nucl Med Mol Imaging. 2006;33:53–9. doi: 10.1007/s00259-005-1838-4. [DOI] [PubMed] [Google Scholar]
- (48).Holland JP, Caldas-Lopes E, Divilov V, Longo VA, Taldone T, Zatorska D, Chiosis G, Lewis JS. Measuring the pharmacodynamic effects of a novel Hsp90 Inhibitor on HER2/neu expression in mice using 89Zr-DFO-Trastuzumab. PLoS ONE. 2010;5:e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.