Abstract
Background
Alcoholism has been repeatedly associated with gray and white matter pathology. Although neuroimaging has shown alcoholism-related brain volume reductions and axonal compromise, the integrity of white matter volumes in chronic alcoholism has been challenging to measure on a regional level.
Methods
We first examined effects of alcoholism on cerebral white matter volumes by lobar and gyral subdivisions in 42 abstinent alcoholics and 42 control participants (split evenly by gender). We also examined cerebellar white matter and regions of the corpus callosum, as well as ventricular volumes. Next, relationships between white matter and ventricular volumes with measures of drinking patterns were assessed. Finally, an examination of early versus late abstinence was conducted. Within each examination, gender effects were explored.
Results
Differences in regional white matter volumes between alcoholics and controls were observed primarily in the corpus callosum, with a stronger group difference among men than among women. Years of heavy drinking had a strong negative impact on frontal and temporal white matter among alcoholic women, and on the corpus callosum among alcoholic men. Quantity of alcohol consumption was associated with smaller corpus callosum and larger ventricular volumes among alcoholic women, while abstinence duration was associated with larger corpus callosum volume among alcoholic men. Preliminary data indicated that alcoholic women showed stronger positive associations between sobriety duration and white matter volume than men within the first year of abstinence, while men showed this association more so than women after one year of abstinence.
Conclusions
Effects of drinking history on white matter and ventricular volumes vary by gender, with alcoholic women showing greatest sensitivity in frontal, temporal, ventricular, and corpus callosum regions, and alcoholic men showing effects mainly in the corpus callosum. Preliminary results indicate that recovery of white matter volume may occur sooner for women than for men.
Keywords: white matter, volumetric MRI, gender, corpus callosum, abstinence
INTRODUCTION
In neuropathology where white matter is affected, white matter volume loss likely contributes to impairments in cognition, emotion, and behavior. Neuroimaging has documented alcoholism-related abnormalities in gray matter (Fein et al., 2002; Makris et al., 2008), as well as in white matter microstructure (Harris et al., 2008; Pfefferbaum et al., 2009; Rosenbloom et al., 2003;Yeh et al., 2009) and macrostructure ( Demirakca et al., 2011; Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). This white matter damage could contribute to diminishment of communication among gray matter regions that normally function together as a network, leading to impairments that resemble disconnection syndromes (Geschwind, 1965). Indeed, numerous associations between white matter pathology and impairments in alcoholics have been reported for visuospatial abilities (Muller-Oehring et al., 2009), cognitive flexibility (Chanraud et al., 2009), executive functions (Chanraud et al., 2007), and balance and psychomotor speed (Pfefferbaum et al., 2010). Identification of regional variability in white matter loss can inform the etiology of these and other behavioral impairments in alcoholism.
While diffusion tensor imaging has allowed for precise localization of white matter pathology in alcoholism, white matter volume loss most often is assessed at a fairly gross level. In the present study, we employed a parcellation algorithm that allows for investigation of cortical white matter volumetrics on a finer scale (Salat et al., 2009) to confirm and more precisely localize previous reports of the relationship between prolonged alcohol abuse and white matter tissue volume in the corpus callosum, the cerebrum, and the cerebellum, as well as in the ventricles (Demirackca et al., 2011; Hommer et al., 2001; Kril et al., 1997; Oscar-Berman and Marinkovic, 2007; Pfefferbaum et al., 1997; Sullivan, 2003). We expected that control participants would have larger white matter volumes than alcoholics particularly in the corpus callosum and frontal lobes, as well as smaller ventricles. We further anticipated that among alcoholics, longer durations of alcoholism and higher quantities of alcohol consumed would be associated with smaller white matter volumes and larger ventricles. Additionally, because it has been demonstrated that there is potential for white matter regrowth following injury in adult brain tissue (Davies et al., 1997), and because recovery of white matter after periods of even brief abstinence among alcoholics has been reported (Agartz et al., 2003; Bartsch et al., 2007; Gazdzinski et al., 2010; Shear et al., 1994), we expected that longer durations of sobriety would be associated with larger white matter and smaller ventricular volumes. Because rates of recovery of white matter following abstinence from alcohol may change over time (Mon et al., 2011), we expected that the effect of sobriety duration would vary between early (less than one year) and later (one year or more) abstinence.
Finally, the issue of gender differences related to the impact of chronic alcoholism on white matter has yielded conflicting results, with studies suggesting greater impact on women (Hommer et al., 2001), greater impact on men (Pfefferbaum et al., 2001), regionally-specific gender differences (Pfefferbaum and Sullivan, 2002), or no gender differences (Demirakca et al., 2011). Differential findings likely are related to methodological differences among studies, especially participant characteristics such as drinking severity and length of abstinence. As such, we investigated white matter and ventricular volumes and their relationships with drinking variables separately in alcoholic men and women, as well as tested for gender interaction effects.
MATERIALS AND METHODS
Participants
Participants included 42 abstinent long-term alcoholics (21 women) and 42 demographically similar nonalcoholic control participants (21 women) (Table 1). All were right-handed, native English speakers recruited through flyers placed in treatment and after-care facilities and in public places (e.g., churches, stores), and advertisements placed in newspapers and web sites. Selection procedures for both groups included an initial telephone interview to determine age, education, health history, and history of alcohol and drug use. Eligible individuals were invited to the laboratory for further screening and evaluations. This study was reviewed and approved by our human studies Institutional Review Boards, and participants gave informed consent for participation.
Table 1.
Participant Demographics, Drinking History, and Intracranial Volume by Group and Gender
| All Alcoholics | All Controls | Alcoholic Women | Alcoholic Men | Control Women | Control Men | |
|---|---|---|---|---|---|---|
| n = 42 | n = 42 | n = 21 | n = 21 | n = 21 | n = 21 | |
| Agea (years) | ||||||
|
| ||||||
| Mean | 53.9 | 53.9 | 53.4 | 54.4 | 57.7 | 50.2 |
| SD | 11.0 | 12.4 | 11.4 | 10.8 | 13.6 | 10.1 |
| Range | 26.5 – 76.7 | 25.8 – 76.9 | 26.5 – 73.0 | 26.6 – 76.7 | 25.8 – 76.9 | 29.0 – 69.6 |
| Educationb (years) | ||||||
|
| ||||||
| Mean | 14.7 | 15.5 | 15.3 | 14.1 | 15.6 | 15.4 |
| SD | 2.0 | 2.0 | 2.0 | 1.9 | 2.3 | 1.6 |
| Range | 12 – 19 | 12 – 20 | 12 – 19 | 12 – 18 | 12 – 20 | 12 – 18 |
| WAIS-III Full Scale IQ | ||||||
|
| ||||||
| Mean | 110.3 | 111.6 | 110.1 | 110.5 | 111.2 | 112.0 |
| SD | 15.0 | 16.3 | 14.2 | 16.0 | 19.3 | 13.1 |
| Range | 72 – 140 | 79 – 152 | 72 – 137 | 81 – 140 | 79 – 142 | 90 – 152 |
| Duration of Heavy Drinkingc,d,e,f (years) | ||||||
|
| ||||||
| Mean | 17.4 | 0.0 | 14.3 | 20.5 | 0.0 | 0.0 |
| SD | 7.7 | 0.0 | 5.2 | 8.5 | 0.0 | 0.0 |
| Range | 5.0 – 35.0 | 0.0 | 6.0 – 25.0 | 5.0 – 35.0 | 0.0 | 0.0 |
| Quantity Frequency Indexc,d,e | ||||||
|
| ||||||
| Mean | 11.2 | 0.4 | 8.7 | 13.7 | 0.2 | 0.5 |
| SD | 8.8 | 0.6 | 5.8 | 10.5 | 0.5 | 0.7 |
| Range | 2.7 – 38.4 | 0.0 – 2.6 | 2.7 – 28.1 | 4.5 – 38.4 | 0.0 – 2.4 | 0.0 – 2.6 |
| Length of Sobrietyg,h,i,j (years) | ||||||
|
| ||||||
| Mean | 8.3 | 2.1 | 10.6 | 5.9 | 3.6 | 0.5 |
| SD | 10.3 | 6.4 | 11.1 | 8.8 | 8.5 | 1.3 |
| Range | 0.1 – 32.3 | 0.002 – 29.2 | 0.1 – 32.1 | 0.1 – 32.3 | 0.002 – 29.2 | 0.002 – 5.1 |
| Intracranial Volume (mm3)k,l,m | ||||||
|
| ||||||
| Mean | 1478101.9 | 1420734.2 | 1351291.0 | 1604912.7 | 1275862.9 | 1565605.5 |
| SD | 219635.9 | 248340.0 | 174922.0 | 185806.4 | 242111.5 | 154068.2 |
| Range | 974164.8 – 2063600.1 | 769279.7 – 1815425.1 | 974164.8 – 1600966.5 | 1333255.8 – 2063600.1 | 769279.7 – 1630917.6 | 1195513.5 – 1815425.1 |
Quantity Frequency Index scores are roughly equivalent to the average number of drinks consumed per day (last 6 months for controls, approximately last six months of drinking for alcoholics).
Control Women > Control Men, p ≤ .05.
Control Men > Alcoholic Men, p ≤ .05.
Alcoholics > Controls, p ≤ .001;
Alcoholic Women > Control Women, p ≤ .001;
Alcoholic Men > Control Men, p ≤ .001.
Alcoholic Men > Alcoholic Women, p ≤ .01.
Alcoholics > Controls, p ≤ .005;
Alcoholic Women > Control Women, p ≤ .05;
Alcoholic Men > Control Men, p ≤ .05;
Length of sobriety was not entered for 8 controls (5 men) who never drank alcohol.
Men > Women, p ≤ .001;
Alcoholic Men > Alcoholic Women, p ≤ .001;
Control Men > Control Women, p ≤ .001.
Participants were excluded if any source (i.e., Diagnostic Interview Schedule (DIS) (Robins et al., 1989), hospital records, or personal interviews) indicated neurological dysfunction (e.g., head injury with loss of consciousness greater than 30 minutes, stroke, epilepsy, seizures unrelated to alcohol withdrawal, or Korsakoff’s syndrome (Oscar-Berman, 2012), electroconvulsive therapy, current or untreated major psychiatric disease (e.g., schizophrenic disorders), poly-drug abuse, HIV, or if they failed screening for MRI scanning.
Participants completed a medical history interview, vision test, handedness questionnaire (Briggs and Nebes, 1975), and a battery of neuropsychiatric and neurological tests. All participants were administered the DIS to provide lifetime psychiatric diagnoses according to DSM-IV criteria (APA, 1994). Alcoholic participants met DSM-IV criteria for alcohol abuse or dependence, drank more than 21 drinks/week for at least five years, and had abstained from alcohol use for at least four weeks prior to testing. Participants also were given a structured interview to assess drinking history (Cahalan et al., 1969; MacVane et al., 1982), which permitted the calculation of a Quantity Frequency Index (QFI) score for each participant.
Drinking History Variables
We selected three variables to test correlational hypotheses with brain volumes: duration of heavy drinking (DHD), average amount of drinking as measured by Quantity Frequency Index (QFI) scores, and length of sobriety (LOS) (Table 1). Duration of heavy drinking is equivalent to the number of years during which alcoholic participants consumed 21 or more drinks per week. Quantity Frequency Index scores approximate the number of drinks consumed per day, and take into consideration the amount, type, and frequency of alcohol consumption either over the last six months (control participants), or over the six months preceding cessation of drinking (alcoholic participants). For the three alcoholic participants who did not drink heavily (i.e., >3 drinks/day on average) during those last six months, we assessed the six-month period during which they last drank heavily. Length of sobriety was calculated by subtracting the date of the participant’s last drink from the date of their MRI scan.
Image Acquisition and Analysis
Data were acquired on a 3T Siemens (Erlangen, Germany) MAGNETOM Trio Tim MRI scanner with a 12-channel head coil. High-resolution sagittal T1-weighted MP-RAGE scans (TR=2530 ms, TE=3.39 ms, flip angle=7°, FOV=256 mm, slice thickness=1.33 mm (128 slices), matrix=256 × 192) were collected for all participants. For most participants, two such volumes were collected and averaged to aid in motion correction. An auto-align localizer was employed to adjust the acquired slices such that they run parallel to an imaginary plane between the anterior and posterior commissures.
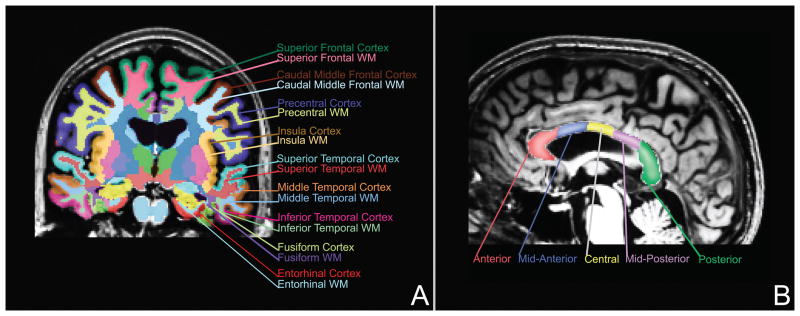
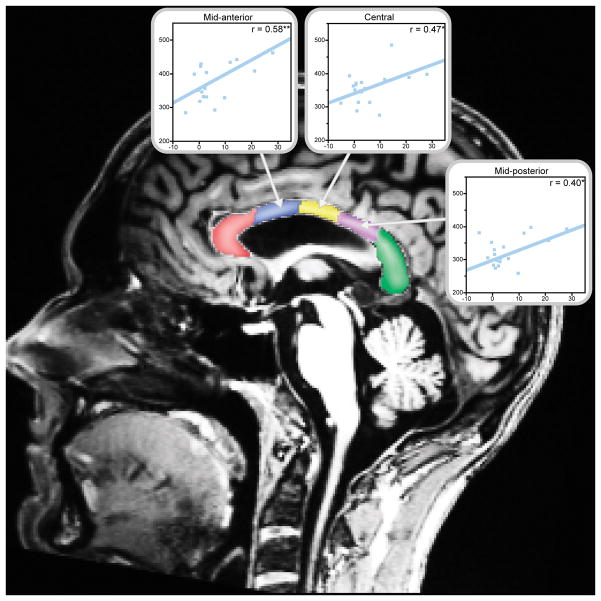
Scans were analyzed using the FreeSurfer processing stream, version 4.5.0 (http://surfer.nmr.mgh.harvard.edu). Cortically-associated white matter regions (Figure 1a) were defined using an automated parcellation procedure (Salat et al., 2009) that classifies volumes of cortical white matter by their overlying gyral subdivisions according to a cortical gray matter parcellation (Desikan et al., 2006; Fischl et al., 2004). The algorithm classifies white matter voxels as being associated with a particular cortical region by extending out 5 mm from the gray/white matter border, with a constraint not to extend into the centrum semiovale. For a complete listing of the 33 bilateral cortical white matter regions classified by this algorithm, see (Salat et al., 2009). Ventricular and anterior-posterior corpus callosum subdivision volumes (Figure 1b) were estimated using an atlas-based segmentation algorithm (Fischl et al., 2002). All individual cortical parcellations and subcortical segmentations were manually inspected to ensure accuracy of the classification algorithms.
Figure 1.
(A) An example of a subset of the gray matter and gyral-associated white matter regions generated by FreeSurfer are shown in a coronal slice. (B) Corpus callosum subdivisions generated by FreeSurfer (Fischl et al., 2002) are shown, with each region encompassing 20% of the anterior-posterior distance through the corpus callosum.
Statistical Analyses
Statistical analyses were performed using PASW Statistics version 17.0.3 (SPSS: An IBM Company) and JMP Pro version 9.0.1 (SAS Institute, Inc.). We employed a multilevel data analysis approach, wherein we conducted a series of statistical tests to investigate a set of research questions examining effects of alcoholism, drinking history, and gender on white matter and ventricular volumes. Since white matter volume can vary with age and head size (Salat et al., 2009; Westlye et al., 2010), all volumetric statistical analyses controlled for participants’ ages and estimated total intracranial volumes (Buckner et al., 2004).
Multilevel Region of Interest Approach
In addition to examination of total cerebral white matter volume, all statistical analyses were carried out on the first level in 8 major white matter and ventricular regions, each of which could be subdivided into a set of smaller component regions. These areas included the corpus callosum, white matter in the cerebellum, frontal, cingulate, temporal, parietal, and occipital lobes, and the ventricles. When a total/lobe-level region yielded significant findings, these were explored for further localization on a subsequent level by performing post-hoc testing of the component regions (Makris et al., 2008). For cortical regions, the subregions were the gyral-associated white matter subdivisions according to the gray matter parcellation as defined in the Desikan atlas (Desikan et al., 2006; Salat et al., 2009) (Figure 1a). The five subregions of the corpus callosum are shown in Figure 1b. These areas are arranged anterior-posterior, each covering 20% of the primary eigenaxis distance (approximately along the y-axis) through the corpus callosum. Ventricular component regions were left and right lateral and lateral inferior ventricles, and the third and fourth ventricles (Fischl et al., 2002; Fischl et al., 2004). When statistical tests for the total/lobe-level regions did not reach significance, further tests were not conducted to minimize type I error.
Evaluation of Gender Differences
To examine gender effects, statistical testing was done first among alcoholics considered regardless of gender, then in each group of alcoholic men and alcoholic women considered separately, and finally tests for gender interactions were conducted. Analysis of the combined gender group allowed us to test our hypotheses with greater statistical power, as well as highlight how reports from gender-mixed groups of alcoholics may be masking effects specific to (or stronger in) one gender.
Volumetric Statistical Testing
The first goal of this study was to determine which regions of the brain showed evidence for alcoholism-related white matter and ventricular volume abnormalities, and to determine the extent to which these effects were influenced by gender. To investigate alcoholism group, gender, and group by gender interaction effects on white matter and ventricular volumes, we first ran univariate analyses of variance (ANOVAs) including all participants in the study. Separate ANOVAs were then run examining group effects considering just men and just women. The results of these analyses are reported in Table 2 and in Figure 2.
Table 2.
Alcoholism Group Effects on White Matter (WM) and Ventricular Volumes
| ALC vs. NC | Women: ALC vs. NC | Men: ALC vs. NC | Group by Gender | |||||
|---|---|---|---|---|---|---|---|---|
| F1,78 | p | F1,38 | p | F1,38 | p | F1,78 | p | |
| Corpus Callosum | 11.02 | .001 | 3.29 | .08 | 6.24 | .02 | 0.21 | .65 |
| Anterior | 3.86 | .05 | 1.68 | .20 | 1.14 | .29 | 0.01 | .91 |
| Mid-Anterior | 8.64 | .004 | 2.56 | .12 | 4.74 | .04 | 0.24 | .62 |
| Central | 9.09 | .003 | 1.87 | .18 | 9.24 | .004 | 1.27 | .26 |
| Mid-Posterior | 11.69 | .001 | 2.00 | .17 | 13.45 | .001 | 2.24 | .14 |
| Posterior | 7.99 | .006 | 3.78 | .06 | 2.32 | .14 | 0.11 | .74 |
| Cerebellum WM | 1.06 | .31 | 0.67 | .42 | 0.63 | .43 | 0.01 | .91 |
| Total Cerebral WM | 0.46 | .50 | 0.07 | .79 | 0.58 | .45 | 0.06 | .81 |
| Frontal WM | 0.02 | .90 | 0.29 | .60 | 0.03 | .86 | 0.53 | .47 |
| Cingulate WM | 0.36 | .55 | 0.00 | .99 | 0.58 | .45 | 0.05 | .82 |
| Temporal WM | 0.30 | .59 | 0.03 | .87 | 0.88 | .35 | 0.58 | .45 |
| Parietal WM | 0.002 | .96 | 0.03 | .86 | 0.19 | .67 | 0.13 | .72 |
| Occipital WM | 0.86 | .36 | 1.05 | .31 | 0.21 | .65 | 0.12 | .74 |
| Ventricles | 1.66 | .20 | 0.28 | .60 | 2.79 | .10 | 1.25 | .27 |
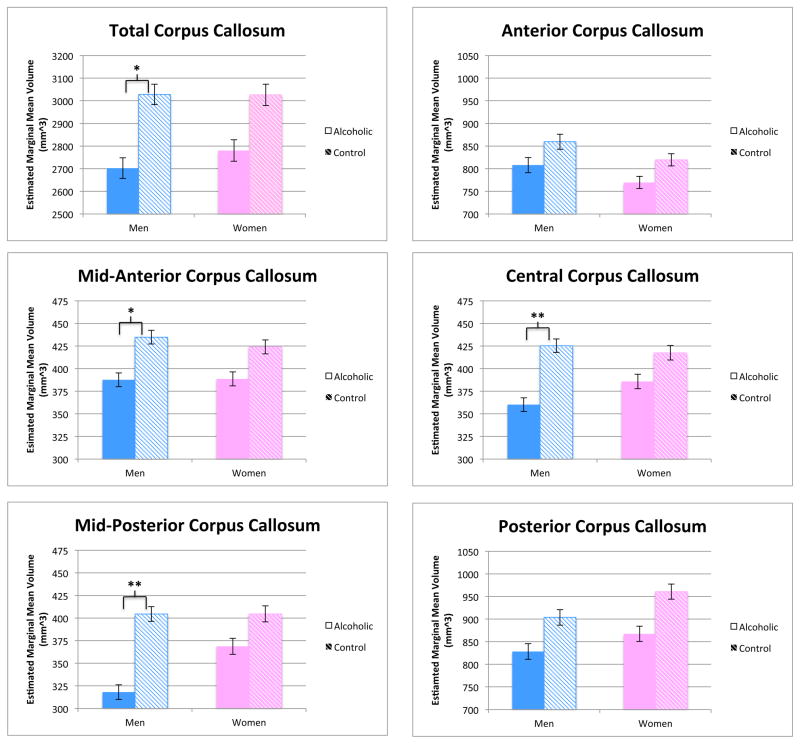
ALC = Alcoholics, NC = Nonalcoholic Controls. In all significant (bold font) Group findings, volumes were smaller among alcoholics than among controls. Age and intracranial volume were included as covariates in the ANOVA models. Significant Group effects on total/lobe-level regions (found for corpus callosum only) were explored to determine which component regions contributed to the overall region effect. Corpus callosum results are highlighted in Figure 2.
Figure 2.
Group differences in total, mid-anterior, central, and mid-posterior corpus callosum volumes were significant when comparing alcoholic and control men, but not alcoholic and control women (*p<0.05, **p<0.01). Volume difference calculations are adjusted for age and estimated total intracranial volume. Error bars represent S.E.M.
The second research goal was to determine the impact of three measures of alcoholism severity on white matter and ventricular volumes among alcoholics, and to explore how these effects interact with gender. To test this, three separate partial correlation analyses (one-tailed) between white matter and ventricular volumes and 1) duration of heavy drinking, 2) Quantity Frequency Index scores, and 3) length of sobriety were calculated for the entire group of alcoholics, as well as in alcoholics broken out by gender. For each grouping of alcoholics, individuals whose drinking history metrics were outliers (Tukey extreme points) were excluded from correlations for that measure. Outliers existed only for Quantity Frequency Index scores, which led to the exclusion of four participants from the overall alcoholic group: three alcoholic men and one alcoholic woman. When considering the alcoholic men and women as subgroups, the outliers consisted of two men and one woman. When a partial correlation was significant for one gender but not the other, a Fisher r-to-z test (two-tailed) was conducted to determine whether the partial correlation values differed significantly by gender. The results of these analyses are reported in Tables 3 and 4, as well as in Figures 3, 4, and 5.
Table 3.
Partial Correlations of Drinking Variables with All Total/Lobe-Level White Matter (WM) and Ventricular Volumes
| All Alcoholics | Alcoholic Men | Alcoholic Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DHD | QFI | LOS | DHD | QFI | LOS | DHD | QFI | LOS | |
| n=42 | n=38 | n=42 | n=21 | n=19 | n=21 | n=21 | n=20 | n=21 | |
| Corpus Callosum | −.433** | −.220 | .277* | −.518** | −.186 | .401* | −.192 | −.402* | −.012 |
| Cerebellum WM | −.040 | −.003 | .051 | .271 | −.079 | −.312 | −.371 | −.257 | .306 |
| Total Cerebral WM | −.193 | −.070 | −.096 | −.267 | −.015 | −.060 | −.192 | −.355 | −.120 |
| Frontal WM | −.128 | .048 | −.213 | −.234 | .100 | −.146 | −.456* | −.163 | −.129 |
| Cingulate WM | −.054 | −.096 | .038 | −.197 | −.108 | .215 | −.321 | −.127 | .089 |
| Temporal WM | −.198 | −.059 | −.112 | −.117 | .057 | −.080 | −.385* | −.260 | −.197 |
| Parietal WM | −.224 | −.174 | −.002 | −.221 | −.090 | .013 | −.409* | −.438* | .024 |
| Occipital WM | −.019 | −.111 | −.196 | −.058 | −.131 | −.180 | −.146 | −.272 | −.119 |
| Ventricles | −.061 | .195 | −.008 | −.047 | .000 | −.201 | −.108 | .551** | .125 |
DHD = Duration of Heavy Drinking, QFI = Quantity Frequency Index (a measure of alcohol consumption), and LOS = Length of Sobriety. Partial correlation p-values are one-tailed. Fisher r-to-z tests (two-tailed) of partial correlation coefficients of drinking variables with total/lobe-level white matter and ventricular volumes between alcoholic men and women were not significant.
(p ≤ .01),
(p ≤ .05).
Table 4.
Summary of All Significant Associations of Drinking History Variables with Subregional White Matter (WM) and Ventricular Volumes
| Duration of Heavy Drinking | All Alcoholics | Alcoholic Women | Alcoholic Men | Fisher r-to-z | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n=42 | n=21 | n=21 | ||||||||
|
| ||||||||||
| r | β | %/year | r | β | %/year | r | β | %/year | z | |
| Corpus Callosum | −.433** | −27.2** | −1.0 | −.192 | −6.3 | −0.2 | −.518** | −27.8* | −1.0 | 1.14 |
| Anterior | −.300* | −6.3* | −0.8 | −.176 | −4.3 | −0.5 | −.280 | −6.2 | −0.8 | 0.33 |
| Mid-anterior | −.423* | −4.1** | −1.0 | −.090 | −1.4 | −0.3 | −.546** | −4.2** | −1.1 | 1.57 |
| Central | − −.472*** | −3.8*** | −1.0 | −.119 | −1.4 | −0.3 | −.521** | −3.3* | −0.9 | 1.37 |
| Mid-posterior | −.477*** | −4.8*** | −1.4 | −.170 | −2.5 | −0.7 | −.571** | −4.5** | −1.4 | 1.43 |
| Posterior | −.319* | −8.1* | −1.0 | .098 | 3.3 | 0.4 | −.434* | −9.6* | −1.2 | 1.69 |
| Frontal WM | −.128 | −261.5 | −0.2 | −.456* | −1187.4* | −0.9 | −.234 | −476.1 | −0.3 | −0.76 |
| Left paracentral | −.162 | −13.4 | −0.4 | −.562** | −56.3** | −1.6 | −.279 | −21.9 | −0.5 | −1.05 |
| Left precentral | −.093 | −22.9 | −0.2 | −.655*** | −174.3** | −1.4 | −.014 | −3.83 | 0.0 | −2.31* |
| Right rostral middle | −.089 | −23.6 | −0.2 | −.459* | −167.9* | −1.5 | −.088 | −21.9 | −0.2 | −1.22 |
| Left superior | −.027 | −9.3 | −0.1 | −.397* | −191.4* | −1.2 | −.075 | −23.4 | −0.1 | −1.03 |
| Temporal WM | −.198 | −196.7 | −0.3 | −.385* | −537.5* | −0.9 | −.117 | −116.5 | −0.2 | −0.87 |
| Left parahippocampal | −.192 | −6.5 | −0.4 | −.459* | −19.8* | −1.3 | −.095 | −3.3 | −0.2 | −1.20 |
| Right parahippocampal | −.237 | −8.3 | −0.5 | −.435* | −20.1* | −1.3 | −.099 | −3.5 | −0.2 | −1.10 |
| Left fusiform | −.233 | −34.6 | −0.6 | −.433* | −83.8* | −1.4 | −.155 | −23.5 | −0.4 | −0.92 |
|
| ||||||||||
| Quantity Frequency Index | All Alcoholics | Alcoholic Women | Alcoholic Men | Fisher r-to-z | ||||||
|
| ||||||||||
| n=38 | n=20 | n=19 | ||||||||
|
| ||||||||||
| r | β | %/drink | r | β | %/drink | r | β | %/drink | z | |
|
| ||||||||||
| Corpus Callosum | −.220 | −22.5 | −0.8 | −.402* | −46.7* | −1.6 | −.186 | −9.1 | −0.3 | −0.68 |
| Mid-anterior | −.255 | −4.2 | −1.1 | −.463* | −9.2* | −2.3 | .218 | 1.7 | 0.4 | −2.07* |
| Central | −.199 | −2.8 | −0.7 | −.440* | −6.7* | −1.7 | −.040 | −0.3 | −0.1 | −1.24 |
| Ventricles | .195 | 754.8 | 2.5 | .551** | 2552.6** | 10.7 | .000 | −0.8 | 0.0 | 1.78 |
| Left lateral | .180 | 386.8 | 2.8 | .534** | 1302.1* | 11.9 | −.052 | −60.7 | −0.4 | 1.86 |
| Right lateral | .215 | 329.0 | 2.8 | .581** | 1055.3** | 11.2 | −.002 | −1.5 | 0.0 | 1.91 |
| Left inferior lateral | .079 | 9.9 | 1.7 | .513* | 61.0* | 13.7 | .249 | 17.6 | 2.4 | 0.90 |
| Right inferior lateral | −.093 | −9.8 | −2.0 | .425* | 33.4* | 8.2 | .210 | 13.4 | 2.4 | 0.69 |
|
| ||||||||||
| Length of Sobriety | All Alcoholics | Alcoholic Women | Alcoholic Men | Fisher r-to-z | ||||||
|
| ||||||||||
| n=42 | n=21 | n=21 | ||||||||
|
| ||||||||||
| r | β | %/year | r | β | %/year | r | β | %/year | z | |
|
| ||||||||||
| Corpus Callosum | .277* | 13.8* | 0.5 | −.012 | −0.6 | 0.0 | .401* | 20.5* | 0.8 | −1.31 |
| Anterior | .284* | 4.8* | 0.6 | .126 | 1.7 | 0.2 | .298 | 6.3 | 0.8 | −0.54 |
| Mid-anterior | .346* | 2.6* | 0.7 | .024 | 0.2 | 0.0 | .582** | 4.3* | 1.1 | −1.92* |
| Central | .368* | 2.4* | 0.6 | .155 | 1.0 | 0.2 | .472* | 2.9* | 0.8 | −1.07 |
| Mid-posterior | .313* | 2.5* | 0.7 | .179 | 1.4 | 0.4 | .403* | 3.0* | 1.0 | −0.74 |
r = Partial correlation of each drinking variable controlling for age and ICV.
β= Unstandardized regression coefficient for specified drinking variable. For DHD, this is expressed as mm3/year of heavy drinking. For QFI, this is expressed as ~mm3/drink. For LOS, this is expressed as mm3/year of abstinence.
%/year for DHD = β for DHD expressed as a percent of the total ROI volume for that group/region. Each year of additional heavy drinking is associated with the reported percentage volume loss (negative values) for that structure.
%/drink for QFI = β for QFI expressed as a percent of the total ROI volume for that group/region. Each additional drink per day is associated with the reported percentage volume loss (negative values) for that structure.
%/year for LOS = β for LOS expressed as a percent of the total ROI volume for that group/region. Each year of abstinence is associated with the reported percentage tissue gain (positive values) for that structure.
(p ≤ .05),
(p ≤ .01),
(p ≤ .001).
Figure 3.
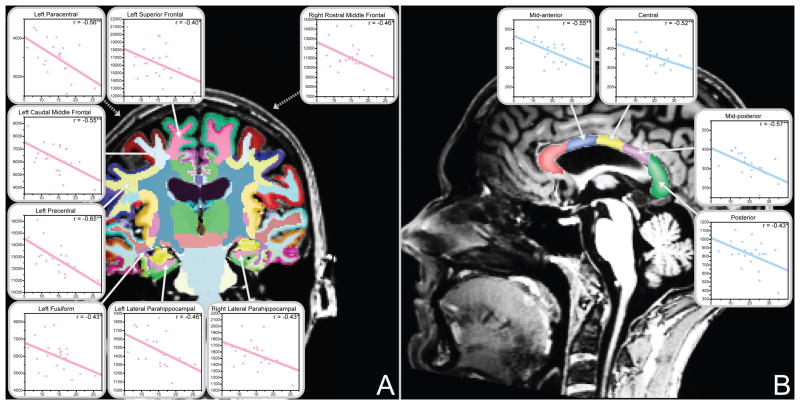
All significant subregional white matter volume correlations with duration of heavy drinking are shown. (A) Duration of heavy drinking was negatively associated with cortical white matter volumes among alcoholic women only. (B) Conversely, duration of heavy drinking was negatively associated with corpus callosum volumes among alcoholic men only (*p<0.05, **p<0.01). Plots display duration of heavy drinking (years) by ROI volume (cubic millimeters). Data for alcoholic women are shown in pink; data for alcoholic men are shown in blue. Correlations are adjusted for age and estimated total intracranial volume.
Figure 4.
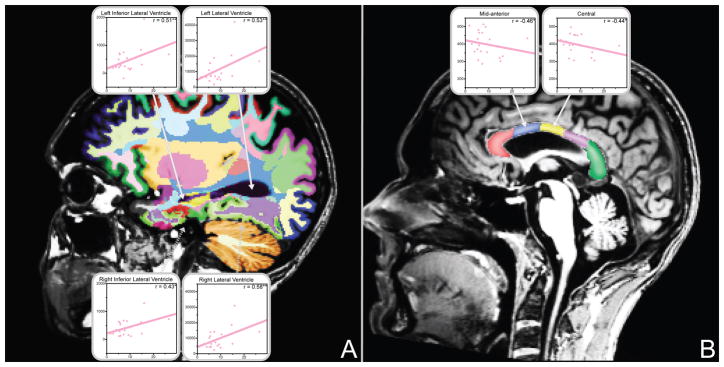
All significant subregional white matter and ventricular volume correlations with Quantity Frequency Index (QFI) scores are shown. (A) Among alcoholic women, QFI was positively correlated with ventricular volumes. (B) QFI was negatively correlated with corpus callosum volumes among alcoholic women (*p<0.05, **p<0.01). Plots display QFI score by ROI volume (cubic millimeters). Data for alcoholic women are shown in pink; no significant correlations with QFI were identified for alcoholic men. Correlations are adjusted for age and estimated total intracranial volume.
Figure 5.
All significant subregional white matter volume correlations with length of sobriety are shown. Length of sobriety was positively correlated with corpus callosum volumes among alcoholic men (*p<0.05, **p<0.01). Plots display length of sobriety (years) by ROI volume (cubic millimeters). Data for alcoholic men are shown in blue; no significant correlations with length of sobriety were identified for alcoholic women when those early and later in abstinence were considered as a single group. Correlations are adjusted for age and estimated total intracranial volume.
Additionally, to further quantify the drinking variable effects, three separate multiple linear regression models were constructed for all total/lobe-level areas and component subregions with significant partial correlation findings. In each regression model, the drinking variable (DHD, QFI, or LOS), age, and intracranial volume were included as regressors. Table 4 notes the slopes (as Beta coefficients), as well as slopes as percentage of mean volume of each region of interest, to provide estimates of the volumetric impact of drinking variables by percentage of estimated tissue gain or loss. These multiple regression models for examining drinking variables were then run with a gender interaction term.
Finally, a preliminary examination of how length of sobriety is associated with white matter and ventricular volumes in early and later abstinence among alcoholic men and women was conducted using partial correlations. For this investigation, alcoholic participants were divided into groups according to those who had been abstinent for less than one year (n=17, 7 women) and those who had been abstinent for a year or more (n=25, 14 women). The results of this analysis are shown in Table 5 and Figure 6.
Table 5.
Summary of All Significant Partial Correlations of Length of Sobriety with White Matter (WM) and Ventricular Volumes in Early and Later Abstinence
| LOS < 1 year | ALC Women | ALC Men | Fisher r-to-z | LOS ≥ 1 year | ALC Women | ALC Men | Fisher r-to-z |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n = 7 | n = 10 | n = 14 | n = 11 | ||||
|
|
|
||||||
| Partial Correlation | z | Partial Correlation | z | ||||
| Corpus Callosum | .793* | .030 | 1.67 | Corpus Callosum | −.248 | .648* | −2.21* |
| Anterior | .825* | −.053 | 1.95* | Anterior | .012 | .749** | −2.06* |
| Mid-posterior | .937** | .774 | 1.09 | Mid-anterior | −.090 | .697* | −2.05* |
| Total Cerebral WM | .794* | .186 | 1.43 | Total Cerebral WM | −.097 | .598* | −1.69 |
| Frontal WM | .924* | .430 | 1.84 | Central | −.082 | .694* | −2.02* |
| Left Caudal Middle | .849* | .655 | 0.75 | Mid-posterior | .048 | .718* | −1.84 |
| Left Frontal Pole | .845* | .111 | 1.80 | Frontal WM | −.126 | .693* | −2.11* |
| Right Frontal Pole | .849* | −.029 | 2.04* | Right Frontal Pole | −.312 | .601* | −2.19* |
| Left Lateral Orbitofrontal | .980** | .247 | 3.26** | Left Lateral Orbitofrontal | −.059 | .606* | −1.64 |
| Right Lateral Orbitofrontal | .803* | .031 | 1.72 | Right Lateral Orbitofrontal | −.292 | .696* | −2.50** |
| Left Medial Orbitofrontal | .866* | .418 | 1.39 | Right Precentral | −.122 | .616* | −1.81 |
| Left Pars Opercularis | .832* | .333 | 1.35 | ||||
| Left Precentral | .977** | .190 | 3.25** | ||||
| Right Precentral | .837* | .653 | 0.69 | ||||
| Left Superior | .943** | .606 | 1.69 | ||||
| Right Superior | .933** | .272 | 2.24* | ||||
| Cingulate WM | .824* | .301 | 1.37 | ||||
| Right Posterior | .871* | .547 | 1.15 | ||||
| Temporal WM | .813* | .468 | 1.00 | ||||
| Left Entorhinal | .851* | −.037 | 2.07* | ||||
| Left Inferior | .876* | .402 | 1.49 | ||||
| Right Inferior | .849* | .184 | 1.70 | ||||
| Left Superior | .959** | .345 | 2.51** | ||||
| Right Superior | .888* | .373 | 1.63 | ||||
| Left Temporal Pole | .891* | −.124 | 2.48** | ||||
| Right Temporal Pole | .870* | .525 | 1.20 | ||||
| Occipital WM | .812* | −.231 | 2.18* | ||||
| Ventricles | −.602 | .731* | −2.60** | ||||
| Left Lateral Ventricle | −.608 | .799** | −2.87** | ||||
| Right Lateral Ventricle | −.632 | .682* | −2.52** | ||||
| Fourth Ventricle | .514 | .616* | −0.24 | ||||
ALC = Alcoholic. LOS = Length of Sobriety. Partial correlation p-values are one-tailed. Fisher r-to-z p-values are two-tailed.
(p ≤ .05),
(p ≤ .01).
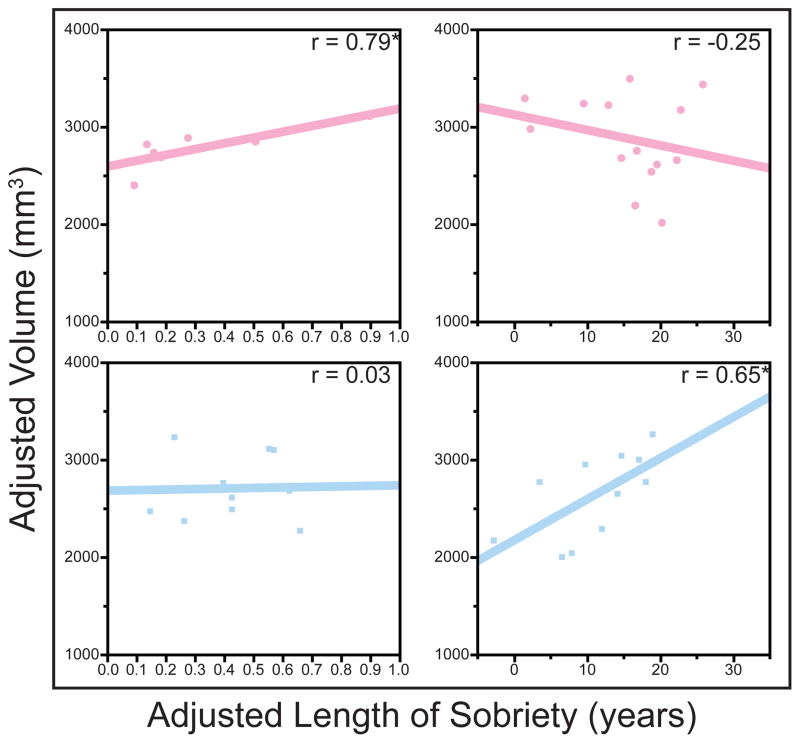
Figure 6.
In many ROIs, longer length of sobriety was associated with larger white matter volumes among alcoholic women sober for less than one year, but not among alcoholic men sober for less than one year. Conversely, in several ROIs, longer length of sobriety was associated with larger white matter volumes among alcoholic men with a year of sobriety or more, but not among alcoholic women sober for this long. Plots show the dissociation of this relationship for total corpus callosum volume (*p<0.05, **p<0.01). Data for alcoholic women are shown in pink; data for alcoholic men are shown in blue. Correlations are adjusted for age and estimated total intracranial volume.
RESULTS
Participants
Results from univariate ANOVA tests of group and gender effects on demographic, neuropsychological, and drinking history measures are reported in Table 1. As a whole, alcoholics did not vary significantly from controls by Age, Education, or IQ (WAIS-III Full Scale). Alcoholic men were slightly less educated than control men, and control women were slightly older than control men. As would be expected, years of heavy drinking were significantly longer and quantity of alcohol consumed was significantly higher for alcoholics than controls, and these effects were significant when genders were considered separately. As would be expected given that most control participants were social drinkers, duration of abstinence (i.e., time from last drink until date of the MRI scan) was significantly longer among alcoholics than controls, and again this was true when genders were considered separately. Alcoholic men, on average, drank heavily for a significantly greater number of years than did alcoholic women. Though alcoholic men tended to have consumed higher quantities of alcohol and have shorter durations of abstinence than alcoholic women, these differences were not statistically significant.
Group and Gender Effects on White Matter and Ventricular Volumes
Table 2 summarizes all Group and Group by Gender interaction effects observed for total/lobe-level white matter and ventricular regions. When considering just men, a significant effect of alcoholism was observed for total corpus callosum volume, which was smaller in alcoholic than control men. Following this significant finding, an exploration of the five corpus callosum subregions was conducted, showing significantly smaller volume in the mid-anterior, central, and mid-posterior sections of the corpus callosum among alcoholic men relative to control men. A trend existed for a similar effect among women, though this did not reach significance (see Figure 2). When considering genders combined, significant differences in white matter volume between alcoholics and controls were observed again only in the corpus callosum; this effect was present in all five component regions. Significant Group by Gender interactions effects for total/lobe-level white matter and ventricular volumes were not observed, nor for any subregions.
Drinking History Variable Associations with White Matter and Ventricular Volumes
Partial correlations of all total/lobe-level white matter and ventricular volumes with the three measures of drinking severity (duration of heavy drinking, quantity of alcohol consumption, length of sobriety) are reported in Table 3. Table 4 summarizes component subregions of the total/lobe-level areas in Table 3 that were significantly correlated with any of the three drinking variables, and provides results from the multiple regression analyses of these regions.
Duration of Heavy Drinking
Figure 3 highlights white matter subregions where partial correlations with duration of heavy drinking were significant for either alcoholic women or alcoholic men. Longer durations of alcohol abuse were associated with smaller corpus callosum volumes among alcoholic men and smaller frontal and temporal white matter volumes among alcoholic women. Multiple regression analyses of these effects showed that each additional year of heavy drinking was associated with approximately 1–1.5% volume loss in these structures. Among all subregions showing significant correlations with duration of heavy drinking in one gender but not the other, only in the left precentral white matter did the correlation values differ significantly by gender.
Quantity Frequency Index
Figure 4 summarizes subregions where partial correlations with Quantity Frequency Index scores were significant for either alcoholic women or alcoholic men. QFI scores were negatively associated with corpus callosum volumes and positively associated with ventricular volumes among alcoholic women. Multiple regression analyses of these effects showed that each additional daily drink for alcoholic women was associated with approximately 1.5–2.5% volume loss in the corpus callosum, and 8–12% volume increase in the ventricles. In the mid-anterior corpus callosum segment, QFI partial correlations differed significantly between alcoholic men and women, and the QFI by gender interaction in the multiple regression analysis for this region was also significant (β = −4.90, p < .05).
Length of Sobriety
Figure 5 displays subregions where partial correlations with length of sobriety were significant for either alcoholic women or alcoholic men. These existed only in the corpus callosum, where longer LOS was associated with larger corpus callosum volume among alcoholic men. Multiple regression analyses of these effects indicated that each additional year of abstinence among alcoholic men was associated with an approximately 1% increase in corpus callosum volume. In the mid-anterior corpus callosum segment, the correlation between LOS and volume was significantly different between alcoholic men and women.
Length of Sobriety in Early and Later Abstinence
Table 5 summarizes white matter and ventricular regions where volumes correlated significantly with length of sobriety when grouping alcoholic men and women into subgroups by early and later abstinence (i.e., less than 1 year of abstinence vs. 1 year or more). When considering just alcoholics sober for less than one year, a significant positive correlation between length of sobriety and white matter volumes was identified in many regions for alcoholic women, but none for alcoholic men. Conversely, when considering alcoholics sober for a year or more, volumes of several white matter regions correlated positively with length of sobriety among alcoholic men, but none among alcoholic women. Figure 6 demonstrates an example of this pattern of partial correlation outcomes for total corpus callosum volume. Ventricular volumes among alcoholic men sober for less than one year were positively correlated with length of sobriety, but this was not true among alcoholic women. Because small sample sizes were used for examining early and later abstinence, caution should be used in interpreting these results.
DISCUSSION
In the present study, the relationships among drinking history variables and white matter and ventricular volumes were assessed in alcoholic men and women. Our multilevel data analysis approach allowed us to confirm effects in total/lobe-level white matter and ventricular regions, and then to explore these effects on a more focal region of interest level than has been previously reported. Further, the interactions of alcoholism and drinking history with gender were studied. These analyses yielded stronger alcoholism group effects among men than women in corpus callosum volume (Figure 2). Years of drinking impacted alcoholic women primarily in frontal and temporal white matter (Figure 3a), whereas alcoholic men showed effects of prolonged alcoholism in the corpus callosum (Figure 3b). Quantity of alcohol consumed was associated with larger ventricles and smaller corpus callosum volumes among alcoholic women only (Figure 4), while length of sobriety was associated with larger corpus callosum volumes among alcoholic men only (Figure 5). Examining early and later abstinence separately, white matter increases with longer durations of sobriety were observed among alcoholic women in the first year of sobriety, but after more prolonged abstinence among alcoholic men (Figure 6).
Alcoholism Effects on White Matter and Ventricular Volumes
These analyses demonstrated a difference in corpus callosum volume between alcoholics and controls. In spite of evidence for larger corpus callosum volumes among alcoholic men with longer durations of sobriety, we found a stronger alcoholism group difference for men than for women, consistent with previous reports of alcoholism effects in the corpus callosum (Pfefferbaum et al., 1996;, Pfefferbaum et al., 2002). Alcoholic men also showed a stronger impact of years of heavy drinking in the corpus callosum, where negative associations between years of drinking and volume were larger than they were among alcoholic women. Taken together, these results suggest that alcoholic men may be more vulnerable to white matter damage in the corpus callosum than alcoholic women. The corpus callosum was the only region in our analyses where significant group differences were identified, perhaps due to positive associations between longer durations of sobriety and larger white matter volumes in men and women alike (mean length of sobriety among alcoholics was over eight years). Thus, white matter volume group differences that may have been detectable after shorter durations of abstinence were possibly ameliorated. Additionally, our results suggest that even when group-level differences cannot be detected in white matter volume, the impact of chronic alcoholism still can be detected by variability in volume on the basis of drinking history, in agreement with previous findings (Pfefferbaum et al., 2002).
Drinking History Variable Associations with White Matter and Ventricular Volumes
More years of heavy drinking was associated with smaller white matter volumes in different regions for alcoholic men and women. This association was present for alcoholic women in cortically-associated white matter regions in the frontal and temporal lobes. In contrast, among alcoholic men, duration of heavy drinking was not associated with smaller volumes in any cortical white matter regions, but was in the corpus callosum. Many of the frontal white matter regions we identified as being sensitive to years of heavy drinking in alcoholic women are those that play a role in emotional regulation and reward functioning, consistent with other reports of white matter disruption contributing to impairments in these functions (Schulte et al., 2010). While duration of heavy drinking was positively associated with age, multiple regression analyses showed that duration of heavy drinking was a stronger predictor of lower white matter volumes than was age in many regions.
The present study demonstrated a negative correlation between quantity of drinking and corpus callosum volume in alcoholic women, but not alcoholic men. Larger ventricular volumes also were observed among only alcoholic women in relationship to the quantity of alcohol they consumed. Research demonstrating associations between white matter damage and binge drinking behavior (McQueeny et al., 2009) suggests that high concentrations of blood alcohol could be related to severe white matter deficits. Because women generally have higher blood alcohol levels with the same amount of alcohol consumed (Graham et al., 1998), the impact of alcohol consumption quantity may be more easily detectable among women. This may explain in part why higher quantities of alcohol consumed were associated with smaller white matter volumes and larger ventricles among women, but not among men.
When considering alcoholic participants in early and later abstinence together, length of sobriety was positively associated with corpus callosum volume among alcoholic men, but not among alcoholic women. That this was effect was identified only among alcoholic men may be related to the finding that alcoholic men also showed stronger alcoholism group effects in the corpus callosum (suggesting that they had greater capacity for potential gains in corpus callosum volume relative to alcoholic women). The positive association between corpus callosum volume and sobriety duration among men only was consistent with the pattern found for participants later in abstinence (i.e., sober for more than one year), as described below.
Relationship of Length of Sobriety with White Matter and Ventricular Volume in Early and Later Abstinence
Throughout the brain, including corpus callosum, frontal, cingulate, temporal, and occipital regions, increases in white matter volume with longer abstinence were observed among alcoholic women with less than one year of abstinence. However, this relationship was not observed in any white matter region in alcoholic women with more than a year of sobriety. Conversely, no regional white matter volumes correlated with length of sobriety among alcoholic men with less than a year of abstinence, but length of sobriety was significantly correlated with corpus callosum and frontal white matter volumes among alcoholic men sober for a year or more. This preliminary double dissociation, though based on a small sample size, suggests that white matter recovery may occur following shorter durations of abstinence for alcoholic women than for alcoholic men. In agreement with our results, a positive correlation between cortical white matter and length of sobriety has been reported among alcoholic women with less than one year of sobriety (Pfefferbaum et al., 2002).
When alcoholic participants were grouped by early and later abstinence, we found positive associations between length of sobriety and white matter volumes particularly among several frontal areas, consistent with frontal white matter restoration reported from a longitudinal study of alcoholics after five weeks of recovery (Gazdzinski et al., 2010). While many longitudinal studies have reported increases in white matter following abstinence (Agartz et al., 2003; Cardenas et al., 2007; Demirakca et al., 2011; Gazdzinski et al., 2010), these effects were consistently observed following brief (<1 year) periods of abstinence. A study comparing recovering alcoholics to those still drinking included some participants with longer periods of abstinence and found larger frontal white matter volumes among recovering alcoholics; however, this study included both men and women and did not specifically address gender differences in white matter recovery (O’Neill et al., 2001).
Of note, in the present sample, alcoholic men sober for less than a year had larger ventricles with longer length of sobriety. Since positive white matter associations with sobriety length were never observed in alcoholic men with these shorter durations of abstinence, we would not expect a corresponding decrease in ventricular volume. However, an increase in ventricular volumes with length of sobriety is nonetheless unexpected, and may suggest that brain tissue shrinkage continues through the first year of abstinence in alcoholic men.
Limitations
In this study, our primary objective was to further localize effects within regions that are known to suffer white matter damage in chronic alcoholism, rather than to do an exploratory analysis of all regions in the brain. However, by only exploring subregions when a total/lobe-level region was significant, we may have failed to identify effects in isolated subregions (i.e., where similar effects did not exist elsewhere nearby).
In the present sample of alcoholics, men tended to have indicators of more severe alcoholism than women, consistent with typical drinking history patterns reported in the literature (Dawson and Archer, 1992). While this contributes to differential effects by gender, it lends ecological validity to the findings. We recognize that several factors known to contribute to the integrity of white matter were not included in these analyses, such as cigarette smoking, body mass index, hormone therapy, and comorbid mood disorders. How these factors interact with drinking variables to influence white matter and ventricular volumes are important avenues of further study.
While we included age as a covariate in our analyses, some studies have shown that aging effects on white matter volumes may be nonlinear (Westlye et al., 2010), and that the interaction of aging and alcoholism on brain volumes is dynamic (Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). Further, the effects of gender and alcoholism on volume may interact differently throughout the lifespan (Medina et al., 2008); thus, continued study of the interactions of aging effects with gender and drinking history is warranted.
Additionally, it has been suggested that microstructural changes in white matter precede macrostructural volumetric changes in alcoholism (Pfefferbaum and Sullivan, 2002). Future studies should closely address the relationship between microstructural white matter changes and white matter volume loss in alcoholism.
CONCLUSIONS
The present study confirms the effectiveness of utilizing gyrally-specific white matter volumes as a complementary tool for multimodal investigations of the impact of alcoholism on the human brain (Buhler and Mann, 2011). Our results support the understanding that that in order to have a complete picture of alcoholism-related brain and behavioral abnormalities, it is necessary to consider the contribution of gender, as mixing genders may mask effects. Further, this study has shown that exploring white matter and ventricular volumes at a focal level may be necessary to identify gender differences in the impact of chronic alcoholism, as changes in these volumes can be regionally specific.
Acknowledgments
This work was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01-AA07112 and K05-AA00219, and the Medical Research Service of the US Department of Veterans Affairs to Dr. Marlene Oscar Berman, as well as the Center for Functional Neuroimaging Technologies, P41RR14075. The authors thank Diane Merritt for recruitment assistance and neuropsychological testing.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol Alcohol. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the Human Brain: A Systematic Review of Different Neuroimaging Methods. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Monograph #6. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. American drinking practices: A national study of drinking behavior and attitudes. [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, Cointepas Y, Martelli C, Martinot JL. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Archer L. Gender differences in alcohol consumption: effects of measurement. Br J Addict. 1992;87:119–123. doi: 10.1111/j.1360-0443.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Filley CM. The Behavioral Neurology of White Matter. Oxford University Press; New York: 2001. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Graham K, Wilsnack R, Dawson D, Vogeltanz N. Should alcohol consumption measures be adjusted for gender differences? Addiction. 1998;93:1137–1147. doi: 10.1046/j.1360-0443.1998.93811372.x. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- MacVane J, Butters N, Montgomery K, Farber J. Cognitive functioning in men social drinkers; a replication study. J Stud Alcohol. 1982;43:81–95. doi: 10.15288/jsa.1982.43.81. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Delucchi K, Durazzo T, Gazdzinski S, Meyerhoff D. A mathematical formula for prediction of gray and white matter volume recovery in abstinent alcohol dependent individuals. Psychiatry Res. 2011;194:198–204. doi: 10.1016/j.pscychresns.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, Sullivan EV. Global-local interference is related to callosal compromise in alcoholism: a behavior-DTI association study. Alcohol Clin Exp Res. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. Function and dysfunction of prefrontal brain circuitry in alcoholic Korsakoff’s syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9198-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV. Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res. 2002;26:400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol Clin Exp Res. 2010;34:1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Helzer J, Cottler L, Goldring E. NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R) 1989. [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues Clin Neurosci. 2010;12:554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]