Abstract
Purpose
To determine whether 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) affect the risk of developing open-angle glaucoma (OAG) in persons with hyperlipidemia.
Design
Retrospective longitudinal cohort analysis.
Participants
Individuals age ≥60 with hyperlipidemia enrolled in a national United States managed care network between 2001 and 2009.
Methods
Multivariable Cox regression analyses were performed to assess the relationship between statin use and the development of OAG (from no prior OAG diagnosis), progression from a prior diagnosis of suspected glaucoma to a diagnosis of OAG, and need for medical or surgical interventions for OAG. Regression models were adjusted for sociodemographic factors, medical and ocular comorbidities.
Main Outcome Measures
Hazard ratios (HR) with 95% confidence intervals (CI).
Results
Of the 524,109 individuals with hyperlipidemia, 316,182 (60%) had at least 1 outpatient prescription for statins. The hazard of developing OAG decreased 0.3% (adjusted HR = 0.997, CI (0.994–0.999)) for every additional month of statin consumption. Individuals with hyperlipidemia who took statins continuously for 2 years had an 8% (adjusted HR = 0.922, CI (0.870–0.976) decreased OAG risk relative to those who received no statin therapy. The hazard of progressing from a diagnosis of suspected glaucoma to OAG decreased 0.4% (adjusted HR = 0.996, CI (0.993–0.999)) for every additional month of statin exposure. Individuals who took statins continuously for 2 years had a 9% (adjusted HR = 0.907, CI (0.846–0.973) decreased risk of progressing from suspected glaucoma to OAG relative to those who received no statin therapy. The hazard of requiring medical treatment for OAG decreased 0.4% (adjusted HR = 0.996, CI (0.993–0.998)) for every additional month of statin exposure. No significant differences in need for glaucoma surgery were noted among those with OAG who were and were not taking statins (adjusted HR = 1.002, CI (0.994–1.010)).
Conclusion
Statin use was associated with a significant reduction in the risk of OAG in persons with hyperlipidemia. Given the mounting evidence of statin protection against OAG including both basic science and observational clinical studies, an interventional prospective study might provide additional insights into the role of statins in the prevention of early OAG.
Introduction
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) are a class of medications that are widely used to lower cholesterol in patients with hyperlipidemia. Recent evidence suggests that statins are capable of reducing the risk of cerebrovascular and cardiovascular events, independent of their effect on cholesterol levels, which has resulted in an expansion of the indications for their usage.1,2 There has been growing evidence in the basic science and clinical literature supporting the notion that statins may also be useful in patients with several diseases of the central nervous system including ischemic stroke, Alzheimer disease, and multiple sclerosis.3 Since the optic nerve and retinal nerve fiber layer are the primary structures which are damaged in patients with open-angle glaucoma (OAG), evidence that statins play a protective role in patients with other diseases of the central nervous system leads us to hypothesize that these agents may also be beneficial in preventing the development of OAG in persons who are at high risk for this visually disabling condition.
There are conflicting findings in the literature as to whether statins may be beneficial in patients with OAG. Some studies suggest that statins may be protective against OAG 4–6 while other studies do not support this effect.7,8 In a recent study assessing the relationship between components of metabolic syndrome and OAG, we found that while adults with diabetes mellitus or hypertension demonstrated an increased risk of developing OAG, enrollees with hyperlipidemia actually had a 5% decreased risk of developing OAG.9 Our earlier work also demonstrated that comorbid hyperlipidemia attenuated the increased risk of OAG associated with diabetes or hypertension. In the present study, we seek to determine whether it is the hyperlipidemia or the medications used to treat hyperlipidemia (statins) which may be responsible for the reduced risk of OAG that was observed in the earlier study.
Using a large national health care claims database containing detailed medical records for over 500,000 older Americans with hyperlipidemia, we sought to determine whether an association exists between statin use and the development of OAG, the conversion from suspected glaucoma to OAG, or the need for medical or surgical therapy for OAG. Should statins be found to be protective against OAG, this may lead to novel prevention strategies for this visually disabling condition.
Methods
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains detailed records of all beneficiaries in a managed care network with members throughout the United States. The dataset contains all individuals with one or more International Classification of Diseases, Ninth Revision (ICD-9-CM)10 codes for eye-related diagnoses (360–379.9), one or more Current Procedural Terminology (CPT)11 codes for any eye-related visits, diagnostic, or therapeutic procedures (65091–68899 or 92002–92499), or any other claim submitted by an ophthalmologist or optometrist from January 1, 2001 through December 31, 2009. For each enrollee, we had access to all medical claims for ocular and non-ocular conditions and sociodemographic information including age, sex, race, education level and household net worth. The database also contained records of all outpatient pharmacy prescriptions that were filled. During the time period when a beneficiary was enrolled in the medical plan, they were also fully enrolled in the pharmacy plan. This database has been used in the past to study patients with OAG.9,12,13
Since all the data were de-identified, the University of Michigan’s Institutional Review Board determined that this study was exempt from requiring their approval.
Participants and Sample Selection
Individuals were included in the analysis if they met the following criteria: age ≥ 60 years, continuous enrollment in the medical plan for at least two years, two or more visits to an eye care provider (ophthalmologist or optometrist) and at least one diagnosis of hyperlipidemia (ICD-9-CM codes 272.0–272.9).
Statin Use
Individuals were classified as receiving statins if they had ≥ 1 outpatient pharmacy prescription for any of the following medications: Altocor, Altoprev, Baycol, Cauduet, Crestor, Lescol, Lescol XL, Lipitor, Lovastatin, Mevacor, Pravachol, Simvastatin, Pravastatin Sodium, Pravigard PAC, Zocor, Advicor, or Vytorin. The database contains information on the number of days for which an enrollee was supplied a given medication and this information was included in the regression model (see below). In addition to identifying and quantifying statin use, we also assessed exposure to the following non-statin cholesterol lowering medications: Advicor, Antara, Atromid-s, Cholestyramine, Cholestyramine light, Cholestyramine resin, Clofibrate, Colestid, Colestipol HCL, Fenofibrate, Fenoglide, Fibricor, Gemfibrozil, Lipofen, Locholest, Locholest light, Lofibra, Lopid, Niacin, Niacor, Niaspan, Prevalite, Questran, Questran light, Simcor, Slo-niacin, Tricor, Triglide, Trilipix, Vytorin, Welchol, and Zetia. Individuals who were prescribed Advicor or Vytorin were counted in the statin and other cholesterol-lowering medication groups, since these products contain both statin and a non-statin cholesterol lowering medications.
Dependent Variables
The dependent variables for these analyses included the development of OAG (from no prior OAG diagnosis), the progression from a diagnosis of suspected glaucoma to one of OAG, the need for medical therapy for OAG, and the need for laser or incisional surgery for OAG. Beneficiaries were identified as having OAG if they had one or more of the following ICD-9-CM codes: 365.1, 365.10, 365.11, 365.12, and 365.15 during their time in the plan. Those coded with suspected glaucoma only (ICD-9-CM codes 365.0, 365.00, 365.01, 365.04) who never received a diagnosis of OAG were considered to have suspected glaucoma only, not OAG. (Table 1, available at http://aaojournal.org).
Table 1.
International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) Codes Used in the Analysis
| Condition | ICD-9-CM Codes |
|---|---|
| Age-related macular degeneration | 362.50, 362.51, 362.52, 362.57 |
| Cataract | 366, 366.0, 366.00, 366.01, 366.02, 366.03, 366.04, 366.09, 366.1, 366.10, 366.12, 366.13, 366.14, 366.15, 366.16, 366.17, 366.18, 366.19, 366.41, 366.45 |
| Diabetes mellitus | 250.0, 250.00, 250.01, 250.02, 250.03, 250.1, 250.10, 250.11, 250.12, 250.13, 250.2, 250.20, 250.21, 250.22, 250.23, 250.3, 250.30, 250.31, 250.32, 250.33, 250.4, 250.40, 250.41, 250.42, 250.43, 250.5, 250.50, 250.51, 250.52, 250.53, 250.5, 250.50, 250.51, 250.52, 250.53, 250.6, 250.60, 250.61, 250.62, 250.63, 250.7, 250.70, 250.71, 250.72, 250.73, 250.8, 250.80, 250.81, 250.82, 250.83, 250.9, 250.90, 250.91, 250.92, 250.93, 362.01, 362.02, 362.03, 362.04, 362.05, 362.06, 362.07 |
| Diabetic retinopathy | 362.01, 362.02, 362.03, 362.04, 362.05, 362.06, 362.07 |
| Glaucoma Suspect | 365.00, 365.01, 365.04 |
| Hyperlipidemia | 272, 272.0, 272.1, 272.2, 272.3, 272.4, 272.5, 272.6, 272.7, 272.8, 272.9 |
| Hypertension | 401, 401.0, 401.1, 401.9, 405, 405.0, 405.1, 405.01, 405.09, 405.11, 405.19, 405.9, 405.91, 405.99, 362.11, 402, 402.0, 402.00, 402.01, 402.1, 402.10, 402.11, 402.9, 402.90, 402.91, 403, 403.0, 403.00, 403.01, 403.1, 403.10, 403.11, 403.9, 403.90, 403.91, 404.0, 404.00, 404.01, 404.02, 404.03, 404.1, 404.10, 404.11, 404.12, 404.13, 404.9, 404.90, 404.91, 404.92, 404.93 |
| Migraine | 346, 346.0, 346.00, 346.01, 346.1, 346.10, 346.11, 346.2, 346.20, 346.21, 346.8, 346.80, 346.81, 346.9, 346.90, 346.91 |
| Hypotension | 458, 458.0, 458.1, 458.2, 458.21, 458.29, 458.8, 458.9 |
| Obesity | 278.0, 278.00, 278.01, 278.02 |
| Open-angle glaucoma | 365.1, 365.10, 365.11, 365.12, 365.15 |
| Pseudophakia or aphakia | V43.1, 379.3, 379.31 |
| Sleep apnea syndrome | 327.2, 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, 780.57 |
Analyses
Statistical analyses were performed by using SAS software, version 9.2 (SAS Institute, Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations for continuous variables and frequencies and percentages for categorical variables.
Cox regression with delayed entry was used to estimate the hazard of developing OAG (going from no record of ICD-9-CM codes 365.1, 365.10, 365.11, 365.12, and 365.15 to a diagnosis of one of these codes) associated with statin use. We used the first two years each beneficiary was enrolled in the medical plan as a look back period. Individuals who received one or more OAG diagnoses during this look back period were excluded from the analysis since we were unable to determine with any certainty whether they had the condition before enrollment in the medical plan or whether they were first diagnosed with this condition during the look back period. To be even more certain that enrollees did not have the outcome of interest (ex: OAG), we also required one or more visits to an eye care provider during the look back period with no record of this condition. All beneficiaries were followed in the model from the index date (two years after entry into the plan) until they either developed the outcome of interest, or were censored. Censoring occurred at the time of a participant’s last visit to an eye care provider during the follow-up period as this was their last opportunity to receive the event of interest.
Our key predictor variable was the use of statins which was treated as a time dependent covariate in the models. The number of days each beneficiary had covered by a prescription for statins was totaled over a 24 month period for every day they were followed in the plan prior to the event or censoring. This amount of use, in days, was then converted to months for ease of interpretation. In addition, we also estimated the change in hazard for each outcome if statins were taken for a year in the last two years or for the entire two year period. Multivariable models were adjusted for age (the time axis), sex, race, education level, household net worth, region of residence at the time of medical plan enrollment, time-dependent use of non-statin cholesterol-lowering medications, the following ocular comorbidities (cataract, pseudophakia or aphakia, macular degeneration, diabetic retinopathy), the following medical comorbidities (diabetes mellitus, systemic hypertension, obesity, systemic hypotension, sleep apnea, migraine headache), and the Charlson comorbidity index (a measure of overall health).14
Additional Cox regression models were performed to assess whether statin use was associated with progression from suspected glaucoma to OAG (going from an ICD-9-CM diagnosis of glaucoma suspect [365.00, 365.01, or 365.04] to and the receipt of an ICD-9-CM code of OAG [365.1, 365.10, 365.11, 365.12, and 365.15]), the need for a topical glaucoma medication (prostaglandin analogues, beta-adrenergic receptor blockers, alpha-2 adrenergic agonists, carbonic anhydrase inhibitors, sympathomimetics, and parasympathomimetics), and among individuals who were known to have OAG, the need for laser or incisional glaucoma surgery.
Finally, a sensitivity analysis was performed to determine whether the association between the main predictor variables and OAG was affected if we changed our definition of OAG from requiring at least one ICD-9-CM code for OAG to requiring at least two codes for OAG. We also tested an interaction between age and statin use on risk of developing OAG. For all analyses, p-values of < 0.05 were considered statistically significant.
RESULTS
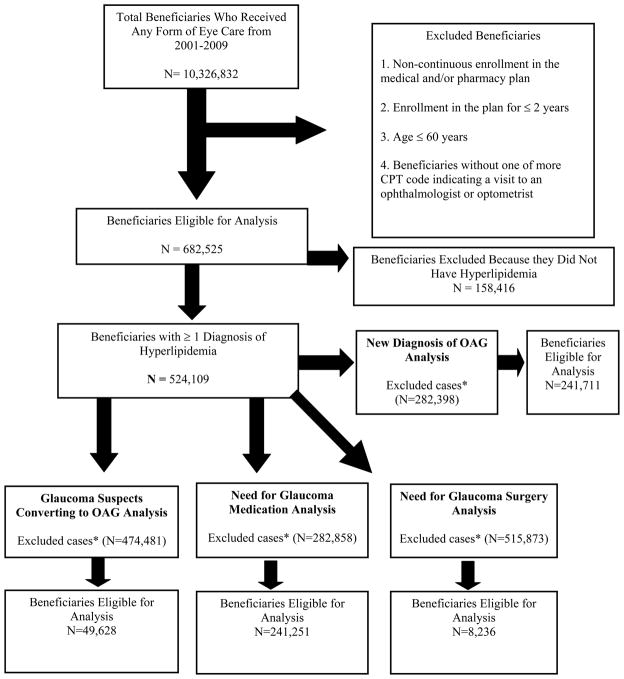
There were 524,109 persons with hyperlipidemia who met the study inclusion criteria. (Figure 1). The cohort of beneficiaries who met inclusion criteria were enrolled in the plan for a mean (± standard deviation [SD]) of 4.3 (±1.8) years. Among those enrolled in the study, the mean (± SD) age was 68.1 (± 6.7) years. There were 280,543 females (53.5%) and the racial distribution included 413,066 whites (87.4%), 28,004 blacks (5.9%), 19,651 Latinos (4.2%), 8,644 Asian Americans (1.8%), and 3,497 individuals of other races (0.7%) (Table 2).
Figure 1.
Selection of Beneficiaries for Analysis.
OAG=open-angle glaucoma; IOP=intraocular pressure
* Excluded due to a non-incident case of the outcome, no visit to an eye care provider either in the look-back period or in the follow-up period, (for glaucoma suspect converting to OAG analysis) no evidence of glaucoma suspect and (for glaucoma surgery analysis) no evidence of incident OAG to begin with.
Table 2.
Demographic Characteristics of Beneficiary Population
| Development of Open-Angle Glaucoma Analysis | Conversion from Suspected Glaucoma to Open-Angle Glaucoma Analysis | Need for Glaucoma Medications Analysis | Need for Glaucoma Surgery Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Beneficiaries Who Did Not Develop Open-Angle Glaucoma | Beneficiaries Who Developed Open-Angle Glaucoma | Beneficiaries Who Did Not Convert from Glaucoma Suspect to Open-Angle Glaucoma | Beneficiaries Who Converted from being a Glaucoma Suspect to Open-Angle Glaucoma | Beneficiaries Who Were Not Newly Prescribed a Glaucoma Medication | Beneficiaries Who Were Newly Prescribed a Glaucoma Medication | Beneficiaries Who Did Not Need Glaucoma Surgery | Beneficiaries Who Needed Glaucoma Surgery | |
| Number of Beneficiaries, n | 231,445 | 10,266 | 42,694 | 6,934 | 229,831 | 11,420 | 7,227 | 1,009 |
| Age | ||||||||
| Mean, years [± Standard Deviation] | 68.9 [± 6.7] | 69.7 [±6.7] | 68.0 [±6.6] | 69.3 [±6.6] | 69.0 [±6.8] | 69.7 [±6.7] | 69.8[±6.7] | 69.5 [±6.3] |
| Range, years | 60–86.8 | 60–86 | 60–87 | 60–86 | 60–86.8 | 60–86 | 60–86 | 60–84.5 |
| Sex | ||||||||
| Male, n(%) | 100,576 (43.5%) | 4,514 (44.0%) | 18,115 (42.4%) | 3,018 (43.5%) | 99,535 (43.3%) | 4,969 (43.5%) | 3,184 (44.1%) | 431 (42.7%) |
| Female, n(%) | 130,869 (56.5%) | 5,752 (56.0%) | 24,579 (57.6%) | 3,916 (56.5%) | 130,296 (56.7%) | 6,451 (56.5%) | 4,043 (55.9%) | 578 (57.3%) |
| Race | ||||||||
| White, n(%) | 187,456 (89.3%) | 7,875 (84.5%) | 33,457 (86.5%) | 5,335 (84.8%) | 185,306 (88.9%) | 8,873 (85.0%) | 5,566 (85.0%) | 799 (85.3%) |
| Black, n(%) | 10,240 (4.9%) | 740 (8.0%) | 2,414 (6.2%) | 481 (7.6%) | 10,986 (5.3%) | 761 (7.3%) | 526 (8.0%) | 66 (7.0%) |
| Latino, n(%) | 7,305 (3.5%) | 437 (4.7%) | 1,657 (4.3%) | 306 (4.9%) | 7,265 (3.5%) | 489 (4.7%) | 282 (4.3%) | 45 (4.8%) |
| Asian-American, n (%) | 3,353 (1.6%) | 201 (2.2%) | 836 (2.2%) | 133 (2.1%) | 3,378 (1.6%) | 239 (2.3%) | 136 (2.1%) | 18 (1.9%) |
| Other race, n (%) | 1,491 (0.7%) | 61 (0.6%) | 309 (0.8%) | 37 (0.6%) | 1,460 (0.7%) | 79 (0.7%) | 41 (0.6%) | 9 (1.0%) |
A total of 316,182 out of the 524,109 enrollees with hyperlipidemia (60.3%) had at least one prescription for a statin during their time in the plan, 20,776 (4%) enrollees were prescribed only non-statin cholesterol lowering medications, and 187,151 (35.7%) beneficiaries were not prescribed any cholesterol lowering medication during the follow-up period. Among the 316,182 statin users, 92,955 (29.4%) also filled at least one prescription for a non-statin cholesterol lowering medication. For those who were receiving statins, the mean length of time they were taking these medications was 800 days ± 621 days (range 1–3266 days) and 96% used these medications for at least 30 days duration. The most commonly prescribed statins were Lipitor, Zocor and Simvastatin. The median number of visits to eye care providers for users and non-users of statins was 3.0.
Development of OAG
Overall, 10,266 individuals (4.3%) received at least one incident OAG diagnosis during their time in the medical plan, out of the 241,711 that met the eligibility criteria. The mean age of those who developed incident OAG was 69.7 (±6.7) years compared with 68.9 (±6.7) years for those who did not develop OAG. Relative to whites, blacks, Latinos, and Asian Americans had an increased hazard of developing OAG (p<0.05).
After adjustment for confounding factors, the hazard of developing OAG decreased 0.3% (adjusted hazard ratio (HR) 0.997, 95% confidence interval (CI) 0.994–0.999) (p=0.0056) for every additional month of statin consumption. Persons with hyperlipidemia who took statins for 1 year duration over the prior two years had a 4% (adjusted HR = 0.960, 95% CI 0.933–0.988) decreased hazard of OAG relative to individuals with hyperlipidemia who never received statins in the last 2 years, assuming all other characteristics being the same. Likewise, persons who took statins continuously for 2 years had an 8% (adjusted HR = 0.922, 95% CI 0.870–0.976) decreased hazard of OAG relative to those who did not receive any statins in the last 2 years. (Table 3) There was no significant association between consumption of non-statin cholesterol lowering medications and development of OAG (p=0.12).
Table 3.
Protective Effects of Statins
| Eventa | Number of Beneficiaries Included in the Analysis (N) | Number of Beneficiaries with Incident Event (N) | Hazard Ratio [95% Confidence Interval] per Month of Statin Use p-Value | Percentage Decreased Hazard of Developing Event per Month of Medication Use | Hazard Ratio [95% Confidence Interval] per Year of Statin Use p-Value | Decreased Risk of Event With One Year of Continuous Statin Useb | Hazard Ratio [95% Confidence Interval] per Two-Years of Statin Use p-Value | Decreased Risk of Event With Two Years of Continuous Statin Usec |
|---|---|---|---|---|---|---|---|---|
| New Diagnosis of OAG | 241,711 | 10,266 | 0.997 [0.994–0.999]d p =0.0056 |
0.3% | 0.960 [0.933–0.988]d p =0.0056 |
4% | 0.922 [0.870–0.976]d p =0.0056 |
8% |
| Conversion from Suspected Glaucoma to OAG | 49,628 | 6.934 | 0.996 [0.993–0.999] p = 0.0062 |
0.4% | 0.952 [0.920–0.986] p = 0.0062 |
5% | 0.907 [0.846–0.973] p = 0.0062 |
9% |
| New Prescription of IOP Lowering Medication | 241,251 | 11,420 | 0.996 [0.993–0.998] p = 0.0002 |
0.4% | 0.950 [0.924–0.976] p = 0.0002 |
5% | 0.902 [0.854–0.953] p = 0.0002 |
10% |
| Need for Glaucoma Laser or Incisional Surgery | 8,236 | 1,009 | 1.002 [0.994–1.010] p = 0.6811, NS |
1.021 [0.925–1.127] p = 0.6811, NS |
1.042 [0.856–1.269] p = 0.6811, NS |
Those diagnosed with the event during the two-year look-back period were excluded to exclude non-incident cases.
Reference group: Patients not on statins at all in the past year.
Reference group: Patients not on statins at all in the past two years.
For example, a Hazard Ratio of 0.996 means a 0.4% decreased risk of developing OAG with every additional month of statin use.
NS = non-significant, IOP = intraocular pressure, OAG = open-angle glaucoma
Conversion from Suspected Glaucoma to OAG
After excluding individuals who were diagnosed with OAG during their first two years in the health plan, there were 6,934 (14.0%) beneficiaries who developed OAG among the 49,628 who had been diagnosed in the look back period with suspected glaucoma. After adjustment for confounding factors, the hazard of progressing from suspected glaucoma to OAG decreased 0.4% (adjusted HR = 0.996 (0.993–0.999), p= 0.0062) for every additional month of statin exposure. Persons who took statins for 1 year over the past 2 years had a 5% (adjusted HR = 0.952, 95% CI 0.920–0.986) decreased hazard of progressing from suspected glaucoma to OAG relative to glaucoma suspects who never received statins in the last 2 years. Those who took statins continuously for 2 years had a 9% (adjusted HR = 0.907, 95% CI0.846–0.973) decreased hazard of OAG relative to the group who did not receive any statins in the last 2 years. (Table 3) There was no statistically significant difference in the hazard of progressing from suspected glaucoma to OAG for those who used non-statin cholesterol lowering medications (p=0.077).
Need for Medical Therapy for OAG
There were 47,511 enrollees (17.0%) who were prescribed one or more glaucoma medications during their time in the plan. After excluding any beneficiary who was treated with glaucoma medications during their first two years in the plan, there were 11,420 enrollees who were newly prescribed a glaucoma medication. After adjustment for confounders, the hazard for being prescribed a glaucoma medication decreased 0.4% (adjusted HR = 0.996 [0.993–0.998], p= 0.0002) for every additional month of statin exposure. Plan participants who took statins for 1 year over the past 2 years had a 5% (adjusted HR = 0.950, 95% CI 0.924–0.976) decreased hazard of requiring an intraocular pressure lowering medication relative to those who never received statins in the last 2 years. Taking statins continuously for 2 years resulted in a 10% (adjusted HR = 0.902, 95% CI 0.854–0.953) decreased hazard of requiring a pressure-lowering medication relative to never taking any statins in the last 2 years. (Table 3) The hazard for being prescribed a glaucoma medication decreased 0.6% (adjusted HR = 0.994 [0.990–0.998], p=0.0017) per month for those individuals treated with non-statin cholesterol lowering medications. Persons who took non-statin cholesterol lowering medications for one year over the past 2 years had a 7% (adjusted HR = 0.928, 95% CI 0.886–0.972) decreased risk of being prescribed glaucoma medications and if a person took a non-statin cholesterol lowering medication for two years, they had a 14% (adjusted HR = 0.862, 95% CI 0.785–0.946) decreased risk of being prescribed a glaucoma medication.
Need for Surgical Interventions for OAG
Among the 8,236 persons with incident OAG who had no glaucoma surgical intervention coded prior to their incident diagnosis of OAG, 1,009 (12.3%) went on to require laser or incisional glaucoma surgery during their time in the plan. The average length of time from first diagnosis of OAG to laser or incisional glaucoma surgery was 268 ± 387 days. After adjustment for confounders, the hazard of an individual with OAG later requiring laser or incisional glaucoma surgery was not significantly different (adjusted HR = 1.002 (0.994–1.010) with each additional month of statin exposure. (p=0.68). (Table 3) We found a similar result for non-statin cholesterol lowering medications (adjusted HR = 0.992 [0.978–1.006], p=0.27).
Sensitivity Analyses
Sensitivity analyses were performed to determine whether the results changed if we required a confirmatory diagnosis of OAG, and the findings did not materially change from those presented above (results not shown). In addition, we performed descriptive analyses (Table 4, available at http://aaojournal.org) and tested an interaction between age and statin use in the multivariable regression model to determine whether statin use by older individuals (individuals > 67 years old) affects the risk of developing OAG differently from statin use among younger individuals (those ≤67 years old). This interaction was not significant. (p=0.43)
Table 4.
Relationship Between Statin Use and Open-Angle Glaucoma, Stratified by Age*
| Non-statin users | Statin users | ||||
|---|---|---|---|---|---|
| Age (years) | No OAG | OAG | No OAG | OAG | P-value |
| 60–69 | 116190 (97.4%) | 3141 (2.6%) | 185518 (97.3%) | 5158 (2.7%) | 0.22 |
| 70–79 | 54763 (96.0%) | 2305 (4.0%) | 78092 (95.9%) | 3362 (4.1%) | 0.41 |
| 80–89 | 13532 (96.0%) | 567 (4.0%) | 17571 (96.1%) | 712 (3.9%) | 0.56 |
Table shows the relationship between statin use and development of open-angle glaucoma, stratified by age, without adjustment for potential confounders
OAG = open-angle glaucoma
Discussion
In this longitudinal analysis of a large group of older Americans with hyperlipidemia, after adjustment for a number of important confounding factors, we found that those who were prescribed statins experienced a decreased risk of developing OAG from no prior OAG diagnosis, a decreased risk of converting from a diagnosis of suspected glaucoma to one of OAG, and a decreased risk of receiving one or more prescriptions for intraocular pressure lowering medications. Furthermore, a dose response effect was observed, whereby the greater the number of months of exposure to statins over the past 2 year duration, the greater the reduction in risk of developing OAG or requiring medical treatment for glaucoma. Individuals who were prescribed statins for 1 year had a 5% decreased hazard of developing OAG while those who were prescribed statins for 2 years had a 9% decreased hazard of OAG relative to those who were not taking statins. For those taking non-statin cholesterol-lowering medications, we found a reduction in the use of medical therapy for glaucoma, but we did not find that these medications conferred a significant reduction in risk of receiving a diagnosis of OAG, of converting from a glaucoma suspect to OAG or requiring glaucoma surgery. While one of the mechanisms through which statins and non-statin cholesterol lowering medications may exert their protective effect is through lowering cholesterol, the fact that statins significantly attenuated the hazard of developing OAG and of converting from suspected OAG to OAG while other cholesterol-lowering medications did not suggests that statins may have an effect on the development of glaucoma that is independent of their cholesterol-lowering properties.
Several other population-based studies and studies using administrative claims data have investigated the relationship between statin use and glaucoma. In a case-control study of male patients at a Veterans Administration Medical Center (VAMC), McGwin and coworkers found that individuals prescribed statins for at least 24 months had a 40% reduced odds of developing OAG. This group also showed a protective effect against OAG among those prescribed other cholesterol-lowering agents.4 A prospective study by Leung and colleagues demonstrated that statin use was associated with visual field stabilization over three years in patients with normal tension glaucoma.5 DeCastro and coworkers showed that statin use slowed glaucomatous changes to the optic nerve and nerve fiber layer on confocal scanning laser ophthalmoscopy.6 While these studies, like ours, suggest that statins may be protective against OAG, not all do. A large case-control study by Owen and colleagues using information from a primary care database in the United Kingdom found no relationship between statin use and OAG.7 Using administrative data from Canada, Iskedjain and colleagues showed no difference in the need for additional pressure lowering medications among persons taking prostaglandin analogs with or without statins.8 Direct comparisons of these studies with one another and with the findings of the present analysis are challenging due to differences in study design, differences in the extent to which potential confounding factors were accounted for, and differences in the sociodemographic profiles of the study patients. Since hyperlipidemia is associated with other components of metabolic syndrome, including hypertension and diabetes mellitus, both of which have been found to be associated with OAG,15–27 when studying the relationship between statin use and OAG, it is important to adjust for these medical co-morbidities. Some of the prior studies did not consider these potential confounders unlike the current study which did.
There are several proposed mechanisms that could explain why individuals who are taking statins have a reduced risk of OAG. Statins have been shown to upregulate endothelial nitric oxide synthase. Nitric oxide synthase causes vasodilation and an increase in retinal and choroidal blood flow.28 Improved retinal and choroidal perfusion may be important in maintenance of the health of the optic nerve and retinal nerve fiber layer. Additionally, statins are known to exert a neuro-protective effect in the setting of ischemia and may protect retinal ganglion cells in a similar way, as was demonstrated in an ischemia-reperfusion model of the rat retina.29 Proposed mechanisms for the neuro-protective effect of statins include decreasing gluatamate-mediated cytotoxicity30,31 and protecting against apoptosis in the central nervous system.3 There is some evidence that increased plasma nitric oxide lowers intraocular pressure.32,33 Statins may lower intraocular pressure via other mechanisms as well; research has shown that statins affect various molecular intermediaries in the aqueous outflow pathways, including Rho kinase activity and Myosin II ATPase activity, which all increase aqueous outflow facility through the trabecular meshwork and result in a reduction of intraocular pressure, a known risk factor for glaucoma34,35
A secondary aim of this analysis was to identify, to the best of our abilities and within the limitations of a retrospective review of claims data, whether statins are protective against OAG early or later in the disease course. We found that statins conferred a protective effect against the development of OAG from no prior OAG diagnosis, the conversion from a diagnosis of suspected glaucoma to OAG, and the need for a medical therapy for OAG suggesting these medications may be beneficial early in the disease process. In order to evaluate whether statins are beneficial in more advanced glaucoma, we also assessed differences in the need for glaucoma surgery among persons with OAG who were and were not taking statins and found no significant reduction in the need for surgery among statin users. (p=0.68).
Study Strengths and Limitations
The strengths of this study include its large sample size and the ability for us to follow patients over time to assess the relationship between cholesterol lowering medication exposure and subsequent development of OAG. Unlike other studies on this subject that were conducted at specific medical centers, the patients in this study come from communities throughout the United States. In addition, instead of relying on patient self-report to identify medical conditions and exposure to medications, the data from this study were obtained from providers and pharmacy records, and thus may be more accurate. (Patty LE, Wu S, Torres M, Varma R. LALES Group, Doheny Eye Institute/Keck- USC School of Medicine. Validity of self-reported diagnosis and treatment among Latinos in the Los Angeles Latino Eye Study. Poster session 516. Correlates and Outcomes of Eye Diseases. Association of Research and Vision in Ophthalmology, May 6, 2010, Fort Lauderdale, Florida.) Finally, in this analysis we were able to adjust for a variety of potential confounding factors, including other components of metabolic syndrome, which have previously been documented to be associated with OAG.
There are several limitations that need to be acknowledged. The data source used in this analysis does not contain clinical information such as intraocular pressure, central corneal thickness, and findings from visual field and optic nerve evaluations. Therefore, we cannot tell with certainty whether all of the beneficiaries who were diagnosed with OAG indeed had this condition, nor could we fully capture disease severity to incorporate into our analyses. Second, our analysis did not consider results of laboratory testing such as levels of different types of cholesterol and triglycerides. Third, since all of the enrollees in this plan had insurance, one must be cautious in generalizing our study findings to other populations such as uninsured individuals and those residing outside the United States. The fact that our study findings are consistent with findings from the research done by McGwin and colleagues4, which was conducted at a VAMC in the United States, and the study by Leung and colleagues5, which was conducted in China, suggest that the relationship between statin use and OAG may be applicable to other groups. Fourth, it is possible that enrollees who take statins are more health conscious than others with hyperlipidemia who are not prescribed these medications and this may impact their use of eye care services. If these individuals have greater use of eye care services, we would expect this to increase, not decrease, their risk of receiving an OAG diagnosis. On the other hand, those who have untreated hyperlipidemia may have more severe ocular co-morbidities, such as diabetic retinopathy, which would necessitate more eye care services, and increase their chance of being diagnosed with OAG. In our analysis we attempted to deal with this issue by controlling for other ocular co-morbidities, though there may be additional confounders not adequately captured in claims data. Fifth, we have no way of knowing with certainty whether enrollees actually consumed all of the prescribed medications. The presumption in this type of analysis is that a patient will not keep filling prescriptions if they are not taking the drug. Thus while these data cannot provide assurance that a specific patient took a prescribed statin drug on a specific date, the expectation is that those who repeatedly filled prescriptions were actually taking the prescribed statin medication as opposed to those who were never prescribed the statin medication, and that a tally of the number of days for which prescriptions were filled is a reasonable surrogate measure of drug consumption. In addition, poor adherence with medications, if anything, should bias our findings to the null, and yet we are finding statins to be protective against OAG.
Finally, it is important to also acknowledge that our findings may not be generalizable to individuals who do not have hyperlipidemia. Statins may not exert a protective effect against developing OAG if the person does not have a derangement in their lipid metabolism. However, in McGwin’s study of glaucoma patients at a VAMC, statins had a protective effect against developing OAG in patients with hyperlipidemia as well as for the subset of persons who were taking statins for other reasons besides treatment of hyperlipidemia.4
The findings of the present analysis support earlier studies demonstrating that treating hyperlipidemia with a statin plays a protective role in patients with OAG, especially in early stages of the disease. After adjustment for important confounding influences, we found that enrollees taking statins had a decreased hazard of developing OAG, of progressing from suspected glaucoma to OAG, and of requiring pressure-lowering medications. Moreover, our study shows a dose-response effect of statin exposure whereby the longer an enrollee was prescribed these medications, the greater the protective effect. Given the mounting evidence of statin protection against OAG including both basic science and observational clinical studies, an interventional prospective study might provide additional insights into the role of statins in the prevention of early OAG.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS:1K23EY019511-01); American Glaucoma Society Clinician Scientist Grant (JDS), Blue Cross Blue Shield of Michigan Foundation (JDS), Research to Prevent Blindness (DCM and JER); National Eye Institute Core Grant EY00703.
Footnotes
The authors have no proprietary interest in any material discussed in this manuscript
Presented, in part, at: American Glaucoma Society Annual Meeting March 4, 2011
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sillesen H, Amarenco P, Hennerici MG, et al. SPARCL Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–302. doi: 10.1161/STROKEAHA.108.516450. [DOI] [PubMed] [Google Scholar]
- 2.Downs JR, Clearfield M, Weiss S, et al. AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS//TexCAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 3.Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: general mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24:79–95. [PubMed] [Google Scholar]
- 4.McGwin G, Jr, McNeal S, Owsley C, et al. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122:822–6. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]
- 5.Leung DY, Li FC, Kwong YY, et al. Simvastatin and disease stabilization in normal tension glaucoma: a cohort study. Ophthalmology. 2010;117:471–6. doi: 10.1016/j.ophtha.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 6.De Castro DK, Punjabi OS, Bostrom AG, et al. Effect of statin drugs and aspirin on progression in open-angle glaucoma suspects using confocal scanning laser ophthalmoscopy. Clin Experiment Ophthalmol. 2007;35:506–13. doi: 10.1111/j.1442-9071.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 7.Owen CG, Carey IM, Shah S, et al. Hypotensive medication, statins, and the risk of glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3524–30. doi: 10.1167/iovs.09-4821. [DOI] [PubMed] [Google Scholar]
- 8.Iskedjian M, Walker JH, Desjardins O, et al. Effect of selected antihypertensives, antidiabetics, statins, and diuretics on adjunctive medical treatment of glaucoma: a population based study. Curr Med Res Opin. 2009;25:1879–88. doi: 10.1185/03007990903035083. [DOI] [PubMed] [Google Scholar]
- 9.Newman-Casey PA, Talwar N, Nan B, et al. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–26. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Classification of Diseases. Physician ICD-9-CM 2006. Vol. 1. Chicago, IL: AMA Press; 2006. 9th revision, Clinical Modification. [Google Scholar]
- 11.CPT 2006: Current Procedural Terminology. Chicago, IL: AMA Press; 2006. Professional ed. [Google Scholar]
- 12.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein JD, Kim DS, Mundy KM, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152:989–98. doi: 10.1016/j.ajo.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Kahn HA, Milton RC. Alternative definitions of open-angle glaucoma: effect on prevalence and associations in the Framingham Eye Study. Arch Ophthalmol. 1980;98:2172–7. doi: 10.1001/archopht.1980.01020041024003. [DOI] [PubMed] [Google Scholar]
- 16.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes: the Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–7. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountain Eye Study, Australia. Ophthalmology. 1997;104:712–8. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 18.Chopra V, Varma R, Francis BA, et al. Los Angeles Latino Eye Study Group. Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–32. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz J, Sommer A. Risk factors for primary open-angle glaucoma. Am J Prev Med. 1988;4:110–4. [PubMed] [Google Scholar]
- 20.Pasquale LR, Kang JH, Manson JE, et al. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113:1081–6. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Bonovas S, Peponis V, Filioussi K. Diabetes mellitus as a risk factor for primary open-angle glaucoma: a meta-analysis. Diabet Med. 2004;21:609–14. doi: 10.1111/j.1464-5491.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 22.Zafra Perez JJ, Vellegas Perez MP, Canteras Jordana M, Miralles De Imperial J. Intraocular pressure and prevalence of occult glaucoma in a village of Murcia [in Spanish] Arch Soc Esp Oftalmol. 2000;75:171–8. [PubMed] [Google Scholar]
- 23.Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975;59:717–20. doi: 10.1136/bjo.59.12.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population: the Rotterdam Study. Ophthalmology. 1995;102:54–60. doi: 10.1016/s0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 25.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Arch Ophthalmol. 1995;113:216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 26.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open-angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson MR, Hertzmark E, Walker AM, et al. A case-control study of risk factors in open-angle glaucoma. Arch Ophthalmol. 1987;105:1066–71. doi: 10.1001/archopht.1987.01060080068030. [DOI] [PubMed] [Google Scholar]
- 28.Nagaoka T, Takahashi A, Sato E, et al. Effect of systemic administration of simvastatin on retinal circulation. Arch Ophthalmol. 2006;124:665–70. doi: 10.1001/archopht.124.5.665. [DOI] [PubMed] [Google Scholar]
- 29.Honjo M, Tanihara H, Nishijima K, et al. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol. 2002;120:1707–13. doi: 10.1001/archopht.120.12.1707. [DOI] [PubMed] [Google Scholar]
- 30.Zacco A, Togo J, Spence K, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosel J, Gandor F, Harms C, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–98. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- 32.Kotikoski H, Oksala O, Vapaatalo H, Aine E. Aqueous humour flow after a single oral dose of isosorbide-5-mononitrate in healthy volunteers. Acta Ophthalmol Scand. 2003;81:355–60. doi: 10.1034/j.1600-0420.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 33.Kotikoski H, Vapaatalo H, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003;26:119–23. doi: 10.1076/ceyr.26.2.119.14511. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46:4130–8. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46:2424–32. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.