Abstract
Objective
To investigate the effect of an exercise intervention on flow-mediated dilation (FMD) and circulating endothelial biomarkers in adults with type 2 diabetes (T2DM)
Methods
Sedentary adults (n=140), aged 40–65, with T2DM and untreated pre- or Stage I hypertension or treated hypertension were randomized to a 6-month, supervised, exercise program (3×week) or a sedentary control. Assessments included BMI, body and visceral fat, blood pressure, lipids, HbA1c, insulin sensitivity (QUICKI), fitness, FMD, E-selectin, P-selectin, intracellular and vascular cellular adhesion molecules (ICAM, VCAM), and tissue plasminogen activator (tPA). Intervention effects were compared by t-tests. Pearson’s correlations were calculated between changes in cardiovascular risk factors and endothelial outcomes.
Results
Exercisers significantly improved BMI (−0.6 kg/m2), body fat % (−1.4%), HbA1c (−0.5%), and fitness (2.9 mL/kg·min) vs. controls (p<0.05). However, there were no differences between groups in changes in FMD, E-selectin, P-selectin, ICAM, VCAM, or tPA. Among exercisers, changes in cardiovascular risk factors correlated with several biomarkers. Decreased P-selectin correlated with decreased BMI (r=0.29, p=0.04) and increased HDL cholesterol (r=−0.36, p=0.01). Decreased ICAM correlated with decreased triglycerides and HbA1c (r=0.30, p=0.04; r=0.31, p=0.03) and increased QUICKI (r=−0.28, p=0.05). Decreased tPA correlated with decreased total body and visceral fat (r=0.28, p=0.05; r=0.38, p=0.008) and increased QUICKI (r=−0.38, p=0.007).
Conclusions
While exercise resulted in improved fitness, body composition, and glycemic control, there were no changes in FMD or circulating endothelial biomarkers. The associations of changes in cardiovascular risk factors and endothelial biomarkers suggest that improvement in risk factors could mediate the exercise-induced improvements in endothelial function seen in prior studies.
Keywords: Endothelial function, adhesion molecules, exercise, type 2 diabetes, vasodilation
Introduction
Type 2 diabetes (T2DM) increases the risk of cardiovascular disease (CVD) 2- to 4-fold.1, 2 In contrast, physical activity reduces CVD risk and has been recommended by the American Diabetes Association (ADA) as a treatment strategy for T2DM with the highest level of evidence.3 Physical activity is believed to confer cardioprotection through improvements in traditional CVD risk factors, such as obesity, glycemic control, hypertension, and dyslipidemia.4 Yet, risk factor improvements do not fully account for the reduction in mortality risk seen in observation studies, leading some researchers to suggest that this unexplained benefit may be attributable, in part, to a direct effect of exercise on vascular health.5, 6 One theory is that the repetitive changes in shear stress associated with regular exercise provides the stimulus for beneficial vascular adaptations, most notably improved endothelial function.7 Specifically, increased shear stress during exercise improves the bioavailability of nitric oxide, which is thought to increase the liberation of endothelial progenitor cells from the bone marrow which could help repair a damaged endothelium8 and decrease oxidative stress and expression of atherogenic molecules (e.g. adhesion molecules).9 The endothelium is a key regulator of vascular homeostasis,10 and endothelial dysfunction is an independent predictor of cardiovascular events,11 is considered an early marker of atherosclerosis,12 and is common in T2DM.13
Several methodologies are available to assess endothelial function.14 Among them, endothelium-dependent flow-mediated dilation (FMD) describes the vasodilatory response of conduit and resistance vessels to hyperemic blood flow.15 FMD predicts cardiovascular events11 and generally improves with exercise training in populations with CVD risk factors or established CVD.16 Biomarkers of endothelial origin circulating in the blood also reflect endothelial function. Adhesion molecules, including E-selectin, P-selectin, intracellular adhesion molecule 1 (ICAM), and vascular cell adhesion molecule 1 (VCAM), are expressed on the endothelium and leukocytes, and play a role in the recruitment of circulating inflammatory molecules during the initial phases of atherosclerosis. Higher serum levels of these markers are thought to reflect endothelial activation or damage14, 17 and associate with CVD risk factors and events.17 Tissue plasminogen activator (tPA), a molecule involved in fibrinolysis, is a later consequence of endothelial activation and higher serum levels are also linked to CVD outcomes.18
Though exercise usually improves FMD in adults with pre-existing vascular dysfunction,19 fewer studies have investigated the effect of exercise on FMD or endothelial biomarkers in persons with T2DM.20–25 Further, since lower FMD and higher levels of endothelial biomarkers are associated with CVD risk factors that improve with exercise training (i.e. obesity, hypertension, glycemia, and hyperlipidemia), it is uncertain whether exercise-induced changes in FMD and endothelial biomarkers are due to concomitant changes in these CVD risk factors or as a direct effect of exercise.
We report ancillary outcomes from the clinical trial, Sugar, Hypertension, and Physical Exercise (SHAPE2),26 which randomized participants with T2DM and milder forms of hypertension to either a 6- month exercise intervention or a control group with resting blood pressure (BP) as the primary outcome. The primary aim of the current investigation was to measure the effect of exercise training in this population on FMD and biomarkers of endothelial function. Our secondary aim was to describe associations of exercise-induced changes in FMD and endothelial biomarkers with changes in CVD risk factors.
Methods
Participants
Subjects were recruited primarily through newspaper advertisements from the greater Baltimore area. Sedentary participants, aged 40–65, with T2DM and with untreated pre- or Stage I hypertension, or treated hypertension, were randomized to a supervised exercise program or a usual care control group. Pre- or Stage I hypertension, as defined by the JNC VII Guidelines,27 was a systolic BP 120–159 mmHg or a diastolic BP 80–99 mmHg. Participants who were being treated for hypertension were also eligible if their SBP/DBP was less than 160/100 mmHg, with no lower limit criteria. Eligible participants had to have T2DM confirmed by a primary care provider and based on the 2003 ADA diagnostic criteria. T2DM could be controlled by diet or oral medications only; subjects using insulin were excluded. Other exclusions included known CVD or other serious illnesses, a positive graded exercise stress test, smoking, or self-report of moderate-to-vigorous intensity exercise >90 minutes per week.
Intervention
The exercise intervention followed guidelines for diabetes and hypertension and included 3 supervised sessions per week.28 Each session included a 10–15 minute warm-up, 45 minutes of aerobic exercise at a target heart rate between 60–90% of maximum heart rate, and a cool down. Each session also included 7 weight training exercises (latisissimus dorsi pull down, leg extension, leg curl, bench press, leg press, shoulder press, and seated mid-rowing) performed as 2 sets of 12–15 repetitions at 50% of 1-repetition maximum. Participants were expected to attend at least 62 sessions in the 26-week period (80%).
All participants, including controls, were given written exercise guidelines from the National Institute of Aging during screening (http://www.nia.nih.gov/HealthInformation/Publications/ExerciseGuide) and information about the American Heart Association Diet but given no further dietary advice. Controls received no further intervention.
Assessments
Assessments occurred at baseline and 6 months. Participants completed a graded exercise test on a treadmill to measure aerobic fitness by peak oxygen uptake (VO2peak). A modified Balke protocol was used: walking began at 4.8 km/h (3 mph) and 0% grade, grade was increased 2.5% every 3 minutes, and participants were pushed to volitional fatigue. All participants underwent a practice treadmill test during screening, before baseline measurement, to confirm eligibility and reduce the potential effects of habituation.
Resting BP was assessed after 5 minutes of rest by an automated device (Dinamap MPS Select; Johnson & Johnson, New Brunswick, NJ). Three readings taken 1 minute apart were averaged and additional readings were taken until 3 readings within 5 mmHg were obtained. General fatness (%) was assessed by dual x-ray absorptiometry (GE Lunar Prodigy; General Electric Medical Systems, Milwaukee, Wisconsin, USA). Abdominal fat was measured with magnetic resonance imaging (Siemans Vision 1.5T, Siemans Medical Systems, Iselin, NJ) by a 3-slice axial spin-echo series with the center slice at the level of the umbilicus. An experienced reader measured fat volume from these images using NIH Image (http://rsb.info.nih.gov/nih-image/). The Johns Hopkins General Clinical Research Center (GCRC) Core Laboratory analyzed fasting blood samples using standard methods for lipids (Cholestech Corp.), insulin (Linco Research Inc.), glucose (Beckman Diagnostics), and HbA1c (Med. Computer Systems). We calculated the quantitative insulin sensitivity check index (QUICKI) to estimate insulin sensitivity using the formula QUICKI = 1/[log fasting insulin x log fasting glucose]. Three-day food records were collected and analyzed using Nutritionist V (First DataBank, San Bruno, CA).
Measurement of FMD and Biomarkers of Endothelial Function
As previously described,29 participants in SHAPE2 underwent FMD testing in a quiet, climate controlled laboratory between 8:00 AM and 10:00 AM. They were required to have fasted and to have abstained from vasoactive medications, exercise, foods high in nitrates, and caffeine for 24 hours and alcohol for 48 hours before testing.15 The left arm was extended laterally and stabilized by a foam support. A rapid inflation/deflation pneumatic cuff was placed just proximal to a 10 MHz multi-frequency linear array transducer. The ultrasound transducer was stabilized by a mechanical clamp and attached to a high-resolution Toshiba Aplio Ultrasound system. Blood velocity was acquired simultaneously using pulsed wave Doppler at an angle of insonation <60° and the sample gate extending from the near to far wall of the vessel. After 20 minutes of lying quietly, baseline images and velocity were assessed for 30 seconds. Directly following baseline image acquisition, the cuff was inflated to >200 mmHg to occlude blood flow to the lower arm for 5 minutes. The cuff was released and brachial diameter images and velocities were recorded continuously from the last 30 seconds of occlusion through 180 seconds of hyperemia. After a 20 minute waiting period, brachial artery diameters were again measured at rest and for 4 minutes following the administration of 2.5 mg of sublingual nitroglycerin.
Brachial images were measured using the Brachial Tools edge detection software package (Medical Imaging applications, LLC., Coralville, IA). A region of interest was identified on a clear portion of the vessel and diameters were calculated as the mean distance between the anterior and posterior wall at the blood vessel interface. Brachial diameters were collected at a frequency of eight frames per second and measures at diastole were identified as local minima with at least two measures before and after of greater or equal value. Resting diameter was the average of measures at diastole over the 30 second baseline acquisition period. Peak diameter during hyperemia was calculated as the maximal value of a third order polynomial fit to the brachial diameters at diastole, to reduce the influence of outliers. FMD was calculated as the absolute and % difference between maximal hyperemic and baseline diameter. Peak shear rate (SRpeak) was calculated as 4(maximal velocity)/diameter at the time of peak velocity and has been shown to be a useful metric of shear stress in this population.29 Nitroglycerin-mediated dilation (NMD) was calculated using the same methods described for FMD. Rereadings of 5 FMD images in our laboratory had an intraclass correlation coefficient of 90%.
Biomarkers of endothelial function included adhesion molecules (E-selectin, P-selectin, ICAM-1, VCAM-1) and the fibrinolytic molecule, tPA. These were measured from frozen serum samples stored at −80°C. Enzyme-linked immunosorbent assays (ELISAs) were used to measure soluble levels of E-selectin, P-selectin, ICAM-1 and VCAM-1 (R&D Systems), and tPA (Bender Medsystems). Intra-assay coefficients of variation calculated by the Core Laboratory ranged from 1.9%–6.3% and inter-assay coefficients of variation ranged from 1.8%–9.9%.
Statistical Analysis
Our primary aim was to measure the impact of exercise training on FMD and endothelial biomarkers, and we compared average 6-month changes across groups for each outcome using independent t-tests or Wilcoxon rank sum tests. The primary analysis reports results from participants completing baseline and follow-up testing, though we repeated the analysis using imputed values for missing data in an intention-to-treat analysis. Missing data was imputed by chained equations (STATA ice command), generating 10 imputations for each missing measurement from regression equations to predict these outcomes. Further sensitivity analyses investigated between-group differences in the 6-month change for outcomes using linear regression to adjust for 1) baseline value of the outcome, 2) group differences in baseline characteristics among completers, and 3) peak shear rate (FMD only). We also repeated analyses excluding individuals who stopped or started relevant medications during the study. To address our secondary aim, we calculated Pearson’s correlations (r) between 6-month changes in measures of adiposity, BP, lipids, glycemia, and fitness with FMD and endothelial biomarkers. Correlations were calculated separately within intervention groups to understand relationships with and without an exercise intervention. The type 1 error rate was set at α=0.05, though a Bonferroni adjusted α-level was also considered post hoc. Sample size (n=140) was based on the primary outcome of the SHAPE2 trial, BP.26 However, assuming a drop-out rate of 15%, a clinically significant increase in FMD of 3%, and a standard deviation of 5%, we had 90% power to detect a significant difference in FMD change between groups. This corresponds to a moderate effect size of 0.6 for the other outcomes.
Results
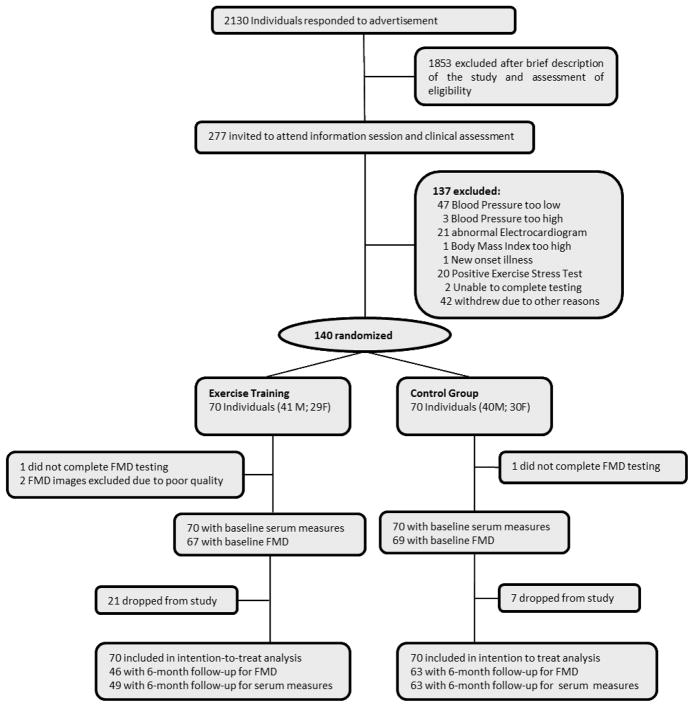
A total of 140 participants were randomized to either the exercise (n=70) or the control (n=70) condition. Of these, 80% (49 exercisers and 63 controls) completed the study, with 46 subjects having complete data for FMD (See Figure 1). Participants who completed or did not complete the study were similar with respect to baseline variables, except that those who completed the study were more likely to be Caucasian (64% vs. 36%, p=0.03) and male (62% vs. 38%, p=0.03), have less body fat (37% vs. 42%, p=0.006), higher fitness (23 mL/kg·min vs. 19 mL/kg·min, p=0.002), and greater baseline brachial diameter (4.6mm vs. 4.2 mm, p=0.03). Participants randomized to exercise training who completed the study protocol attended 94% of prescribed sessions.
Figure 1.
Flow chart of participant recruitment, screening, and study participation
At baseline among completers, groups were balanced by randomization except for slightly older age, more frequent use of ace-inhibitors and antilipid medications (Table 1), and lower triglycerides (Table 2) in the exercise group. Baseline FMD and endothelial biomarkers were also similar across groups, with the exception of higher E-selectin levels among controls (Table 2).
Table 1.
Demographics and Baseline Clinical Variables
| Exercise (n=49) | Controls (n=63) | p-value | |
|---|---|---|---|
| Age, years | 58 ± 5 | 56 ± 6 | 0.033 |
|
| |||
| Gender | |||
| Male | 32 (65%) | 37 (59%) | 0.478 |
| Female | 17 (34%) | 26 (41%) | |
|
| |||
| Race | |||
| Caucasian | 31 (63%) | 40 (63%) | |
| African American | 17 (35%) | 21 (33%) | |
| Other | 1 (2%) | 2 (3%) | 0.929 |
|
| |||
| Ace-inhibitor use | 24 (49%) | 18 (29%) | 0.027 |
|
| |||
| Biguanide use | 31 (63%) | 36 (57%) | 0.512 |
|
| |||
| Thiazolidine use | 12 (24%) | 16 (25%) | 0.912 |
|
| |||
| Antilipid medication use | 31 (63%) | 26 (41%) | 0.021 |
|
| |||
| HRT use | 2 (4%) | 4 (6%) | 0.597 |
Data are presented as mean ± standard deviation or n (%)
Abbreviations: HRT, hormone replacement therapy
Table 2.
Baseline, 6-month, and Changes in Vascular and Endothelial Measures in Exercise and Control Subjects in the SHAPE2 Study
| Exercisers (n=49) | Controls (n=63) | p-valuea | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline | 6-months | Absolute Change | Baseline | 6-months | Absolute Change | ||
|
|
|||||||
| Cardiometabolic Risk Factors | |||||||
| Body fat, % | 36.6 ± 7.6 | 35.2 ± 7.0 | −1.4 ± 1.9 | 38.0 ± 7.7 | 37.9 ± 7.7 | 0.0 ± 2.2 | <0.001 |
| BMI, kg/m2 | 32.3 ± 5.3 | 31.6 ± 4.9 | −0.7 ± 1.6 | 33.5 ± 4.3 | 33.4 ± 4.4 | −0.1 ± 1.3 | 0.022 |
| Visceral abdominal fat, cm2 | 155 ± 70 | 149 ± 68 | −6 ± 33 | 169 ± 75 | 167 ± 71 | −2 ± 36 | 0.353 |
| SBP, mmHg | 127 ± 13 | 125 ± 14 | −2 ± 10 | 126 ± 13 | 125 ± 13 | −1 ± 10 | 0.685 |
| DBP, mmHg | 72 ± 8 | 69 ± 9 | −2 ± 6 | 70 ± 9 | 70 ± 8 | 0 ± 6 | 0.105 |
| LDL cholesterol, mg/dL | 102 ± 26 | 98 ± 26 | −4 ± 28 | 110 ± 32 | 110 ± 35 | 0 ± 27 | 0.500 |
| HDL cholesterol, mg/dL | 49 ± 14 | 50 ± 15 | 1 ± 14 | 48 ± 13 | 49 ± 14 | 1 ± 9 | 0.808 |
| Triglycerides, mg/dL | 98 (80, 134)* | 92 (67, 129) | −9 (−27, 14) | 123 (95, 190)* | 134 (106, 187) | 2 (−26, 30) | 0.48 |
| HbA1c, % | 6.6 ± 1.5 | 6.4 ± 1.2 | −0.2 ± 1.2 | 6.6 ± 1.4 | 6.9 ± 1.3 | 0.3 ± 1.1 | 0.025 |
| QUICKI | 0.298 ± 0.026 | 0.300 ± 0.022 | 0.002 ± 0.021 | 0.286 ± 0.018 | 0.290 ± 0.021 | 0.002 ± 0.020 | 0.939 |
| VO2peak, mL/kg·min | 22.7 ± 5.9 | 26.2 ± 6.2 | 3.4 ± 2.9 | 22.4 ± 5.3 | 22.9 ± 6.3 | 0.5 ± 3.0 | <0.001 |
| Vascular Measures | |||||||
| Baseline diameter, mm | 4.7 ± 0.8 | 4.7 ± 0.8 | 0.0 ± 0.3 | 4.5 ± 0.7 | 4.6 ± 0.7 | 0.1 ± 0.3 | 0.217 |
| Peak FMD diameter, mm | 4.9 ± 0.8 | 5.0 ± 0.7 | 0.0 ± 0.3 | 4.7 ± 0.7 | 4.8 ± 0.7 | 0.1 ± 0.3 | 0.487 |
| FMD, mm change from baselineb | 0.26 ± 0.15 | 0.29 ± 0.19 | 0.03 ± 0.20 | 0.27 ± 0.17 | 0.26 ± 0.16 | 0.0 ± 0.20 | 0.391 |
| FMD, % change from baselineb | 6.0 ± 4.0 | 6.6 ± 5.0 | 0.7 ± 4.6 | 6.2 ± 4.3 | 6.0 ± 4.2 | −0.2 ± 5.3 | 0.373 |
| Peak shear ratec, s−1 × 103 | 1.01 ± 0.41 | 1.13 ± 0.40 | 0.11 ± 0.40 | 1.02 ± 0.37 | 1.14 ± 0.41 | 0.12 ± 0.42 | 0.935 |
| NMD, % change from baselined | 10.0 ± 5.0 | 9.9 ± 5.1 | −0.2 ± 5.6 | 9.0 ± 4.7 | 10.7 ± 5.6 | 1.7 ± 5.6 | 0.097 |
| Endothelial Biomarkers | |||||||
| E-selectin, ng/mL | 44 ± 17* | 41 ± 16 | −3 ± 10 | 56 ± 25* | 55 ± 21 | −2 ± 12 | 0.502 |
| P-selectin, ng/mL | 69 ± 22 | 68 ± 24 | 0 ± 16 | 75 ± 20 | 75 ± 22 | 0 ± 18 | 0.897 |
| ICAM-1, ng/mL | 203 ± 63 | 201 ± 56 | −3 ± 37 | 228 ± 90 | 228 ± 80 | 0 ± 55 | 0.215 |
| VCAM-1, ng/mL | 692 ± 196 | 713 ± 220 | 21 ± 141 | 692 ± 184 | 675 ± 217 | −17 ± 142 | 0.623 |
| tPA, ng/mL | 5.2 ± 2.4 | 4.9 ± 2.5 | −0.3 ± 2.2 | 5.6 ± 3.0 | 5.5 ± 2.9 | −0.1 ± 1.8 | 0.622 |
Data are presented as mean ± standard deviation or median (IQR)
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FMD, flow-mediated dilation; HDL, high density lipoprotein; ICAM, intracellular adhesion molecule; LDL, low density lipoprotein, NMD, nitroglycerin-mediated dilation; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure; tPA, tissue plasminogen activator; VCAM, vascular cellular adhesion molecule; VO2peak, peak oxygen uptake
p<0.05 comparing exercisers and controls at baseline by independent t test or rank sum test, as appropriate
Comparing absolute change in exercisers vs. controls by independent t test or rank sum test, as appropriate
3 baseline or follow-up FMD images not collected or unreadable
8 baseline and 9 follow-up blood velocity images not collected or unreadable for assessment of peak shear rate
8 baseline and 10 follow-up NMD images not collected or unreadable
Intervention Effects
At 6-months, exercisers decreased BMI, body fat, and HbA1c and increased peak oxygen consumption compared to controls (Table 2). At baseline and at 6 months, exercisers and controls reached similar levels of respiratory exchange ratio (baseline 1.08 vs. 1.08, p=0.80; 6 months 1.08 vs. 1.07, p=0.76), maximal heart rate (baseline 159 vs. 156, p=0.29; 6 months 159 vs. 155, p=0.25), rating of perceived exertion (baseline 17.4 vs. 17.3, p=0.69; 6 months 17.9 vs. 17.7, p=0.58), and peak respiratory rate (baseline 36.3 vs. 36.1 breaths/min, p=0.92; 6 months 36.9 vs. 36.3, p=0.57), indicating that subjects were pushed to a maximal effort at both time points. These data suggest a true training effect for cardiorespiratory fitness. However, exercisers did not improve BP, the primary endpoint of SHAPE2 (Table 2).26 Dietary intake with respect to total kilocalories, macronutrient composition, sodium, and potassium was unchanged in both groups.
The exercise intervention did not have a significant effect on FMD or biomarkers of endothelial function (Table 2). Despite the differential rates of follow-up by group, results were consistent using an intention-to-treat approach with multiply imputed outcomes and when adjusting for baseline imbalances in the exercise vs. control group (Appendix Table). Adjustment of FMD outcomes for peak shear rate produced similar results (Appendix Table). When adjusting for baseline value, exercisers had a nearly significant decrease in E-selectin vs. control participants (−4 ng/mL, p=0.06) which is relevant since E-selectin was significantly lower in exercisers at baseline.
Correlations of Changes in CVD risk factors with FMD and Biomarkers of Endothelial Function
For our secondary aim, we observed that changes in FMD and endothelial biomarkers were associated with changes in various CVD risk factors. Specifically in exercisers, decreased P-selectin was associated with decreased BMI (r=0.29, p=0.04) and increased HDL cholesterol (r=−0.36, p=0.01). Decreased ICAM was associated with decreased triglycerides (r=0.30, p=0.04), decreased HbA1c (r=0.31, p=0.03), increased insulin sensitivity (QUICKI) (r=−0.28, p=0.05), and increased fitness (r=−0.32, p=0.03). Decreased tPa was associated with decreased body fat (r=0.28, p=0.05), decreased visceral fat (r=0.38, p=0.008), and increased QUICKI (r=−0.38, p=0.008). Improvements in FMD, E-selectin, and VCAM were not associated with improved CVD risk factors among exercisers, though decreased E-selectin was unexpectedly associated with an increase in HbA1c (r=−0.30, p=0.04).
In the control group, fewer and less consistent associations were observed. Increased FMD (r=− 0.39, p=0.003) was associated with decreased LDL cholesterol. Decreased E-selectin was associated with decreased triglycerides (r=0.25, p=0.05) and increased QUICKI (r=−0.28, p=0.03). Decreased VCAM was associated with increased body fat (r=−0.27, p=0.03) and LDL cholesterol (r=−0.35, p=0.007).
Though adjustment for multiple comparisons may be overly conservative with correlated outcomes, associations that continued to be significant after Bonferroni adjustment for 6 outcomes were changes in visceral fat and QUICKI with changes in tPA (intervention group) and changes in LDL cholesterol with changes in FMD and VCAM (control group).
Discussion
We found that 6-months of exercise training produced the expected training effect as evidenced by decreased BMI and body fat percentage, improved HbA1c, and improved VO2peak compared to controls in participants with T2DM and mild forms of hypertension. Nevertheless, we did not find a change in FMD or endothelial biomarkers. In further analysis, we found that improvements in CVD risk factors were associated with improvements in endothelial biomarkers among exercisers, suggesting that individual changes in these biomarkers may be mediated, at least in part, by concomitant changes in CVD risk factors.
Our findings of no intervention effects on vascular outcomes add to the current body of literature investigating the benefit of exercise on vascular function as measured by FMD in T2DM. At least three smaller studies have reported that exercise improved FMD: an 8-week cross-over study in 16 middle-aged subjects with an aerobic and resistance intervention performed 3×week for 60 minutes,22 a 3-month study in 38 middle-aged subjects with an aerobic and resistance training intervention 3–5×week for 60 minutes,23 and a 12-week, 3-arm study (aerobic, resistance, control) in 40 overweight women with a training frequency of 60 minutes, 5×week (the aerobic group improved vs. the other two groups).25 In contrast, an in-hospital study in 20 adults with T2DM and chronic heart failure observed no improvement in FMD following 4 weeks of aerobic and resistance training 3×week. 24
Though our investigation was at least twice the size of the above-mentioned studies and used a randomized design, several factors could explain our observed lack of effect on FMD. First, our study was longer than previously conducted in this population. The time course of FMD adaptation to exercise training has been described in a healthy population, such that FMD improves then returns to baseline levels following 8 weeks as a result of vascular remodeling.30 Because FMD was measured 6 months after exercise training began, it is possible that FMD may have increased during the course of the study but returned to baseline levels by 6 months. We did not measure FMD at interim time points during our study or assess vasodilator capacity, a measure which may reflect vascular improvements after beneficial arterial remodeling and return of FMD to baseline levels.30 We acknowledge these as limitations and areas for future research. However, vessel diameter did not change in exercisers from baseline which supports a lack of vascular remodeling. Another possibility is that the intensity or volume of our exercise training program was inadequate to elicit vascular changes. This seems unlikely however, since we observed gains in fitness22, 23 and CVD risk factors22, 23, 25 that were comparable to previously mentioned studies. Lastly, inclusion of resistance training may have attenuated expected FMD improvements because resistance training may increase vascular stiffness and in combination with aerobic training may produce a null effect.31 Consistent with this, pulse wave velocity did not improve among exercisers during our intervention (922 vs. 930 cm/s, p=0.87).26 Two of the previously mentioned studies reporting improved FMD with combined aerobic and resistance training using either a circuit-training protocol that alternated 45-second aerobic and resistance exercises22 or aerobic followed by resistance training.23 Sequencing of aerobic and resistance training was not systematic in our study and all subjects received both aerobic and resistance training, thus limiting our ability to evaluate the impact of combined resistance and aerobic training vs. aerobic-only training or the effect of alternate sequencing of aerobic and resistance training on endothelial function.
With respect to biomarkers of endothelial function, exercise training also produced no change. Though consistent with our FMD findings, these results contrast with previous reports which found: improved levels of E-selectin, ICAM, and VCAM in 20 subjects completing aerobic and resistance training 4×week for 4 weeks;20 decreased circulating tPA comparing exercise-only vs. controls in a factorial study with an 8-week exercise and dietary fish intervention (n=49);32 and significantly decreased P-selection and ICAM in 16 older subjects completing aerobic training 2×week week for 6 months.21 A study in young subjects with early-onset T2DM found that 3 months of aerobic training 4×week did not affect E-selectin, P-selectin, ICAM, or VCAM,33 though the investigators postulated that the lack of improvement may have resulted from differences in underlying cellular abnormalities between early-onset and adult-onset T2DM. While the reasons we observed a lack of change are not immediately clear, we found that increased VO2peak correlated with decreased ICAM among exercisers, suggesting that a more intense intervention could yield improvements detectible on the group level. Another possibility is that average endothelial biomarker levels in our participants were lower or ‘healthier’ with less room to improve compared to other studies. The one prior 6-month study that observed improvement in older adults with T2DM at a lesser intensity had higher baseline levels of these markers, thus allowing for greater change.21
Although exercise is purported to have a direct effect on endothelial function due to repeated shear stress during exercise training,19 it is still unclear to what extent, if any, exercise-induced changes in other CVD risk factors mediate the improvement in persons with T2DM. Since we did not observe an overall effect of exercise on endothelial function, we were unable to address this question of whether an effect of exercise exists independent of changes in CVD risks factors. To our knowledge, two small studies have reported an independent effect of exercise training on circulating markers of endothelial function after adjustment for concomitant changes in other CVD risk factors.20, 21 For FMD, an independent effect has been suggested when no statistical improvement is observed in the CVD risk factors or when the correlation between changes in the risk factor and FMD is nonsignificant.16, 23 However, significance of group mean changes and correlations are highly dependent on sample sizes and most training studies measuring these markers are small. A planned study with adequate power to detect an independent effect of exercise training on endothelial function is needed to address this question definitively.
Though we were not able to establish whether an effect of exercise on endothelial function persisted after accounting for changes in CVD risk factors using multivariate adjustment, we found that favorable changes in adiposity, lipids, and glycemic control correlated with improvements ICAM, tPA, and P-selectin in secondary analyses among exercisers. These data agree with other studies in subjects with T2DM that have found associations between exercise-induced changes in endothelial biomarkers with BMI,20 waist circumference,32 and fasting insulin and glucose.20 The lack of associations between changes in FMD and CVD risk factors in our study agrees with a study of 40 women with T2DM training for 12 weeks (nonsignificant correlations with HbA1c or weight)25 and a study in 47 subjects with mixed CVD risk factors,16 though nonsignificant associations in these studies could reflect inadequate power. Our results suggest that despite a lack of effect in exercisers vs. controls, decreased adiposity, improved lipids, and improved insulin sensitivity may be important pathways through which exercise improves circulating biomarkers of endothelial health in patients with T2DM.
Low-grade inflammation is also a risk factor for CVD, is more prevalent in T2DM, and is associated with endothelial dysfunction.34 Increased exercise has been shown to reduce inflammation,35, 36 though more research is needed to determine whether this reduced inflammation results from or leads to exercise-related improvements in endothelial function in T2DM.
Several limitations of our study deserve comment. First, while we retained 80% of participants overall, a greater proportion of the exercise group was lost to follow-up. Losses to follow-up had a slightly worse cardiovascular profile compared to completers, which could have decreased our ability to see a significant intervention effect. To address this concern, we imputed missing outcomes and adjusted comparisons for imbalances in baseline characteristics between groups, which produced consistent results. Second, participants were allowed to be taking several medications which could affect vascular health, and this could have decreased the effect of exercise training. However, our findings apply to a clinical population of adults with T2DM who are very likely to be taking these medications; thus, this limitation is offset by greater generalizability. Third, we may have missed transient improvements in FMD that could have occurred between baseline and 6-months due to our assessment schedule. Further, it has been reported in this29 and other37 study populations with T2DM that some subjects do not exhibit a vasodilatory response during FMD testing. We did not account for this phenomenon in the current analysis, but acknowledge that this may have affected results and is a research question in need of more in-depth study. Lastly, we measured FMD using upper arm cuff placement, which differs from some studies and may induce alternate vasodilatory effects in addition to nitric oxide-mediated dilation. However, a recent review concluded that FMD as measured with an upper arm cuff placement was at least as predictive of outcomes as that measured with a forearm cuff placement,38 suggesting that this technique remains clinically relevant for understanding endothelial health. Nonetheless, this difference could be the reason we saw no effect of exercise on FMD.
In conclusion, participation in 6-months of exercise training improved fitness, decreased BMI and body fat, and decreased HbA1c in participants with T2DM and milder forms of hypertension, but did not improve vascular function measured by FMD or the endothelial biomarkers E-selectin, P-selectin, ICAM-1, VCAM-1, or tPA. Despite our negative findings overall, beneficial changes in several circulating biomarkers were associated with exercise-induced improvements in fatness, lipids, glycemic control, and insulin sensitivity in the exercise group. The latter finding suggests that CVD risk factor improvement may mediate improvements in endothelial health with exercise.
Table 3.
Adjusted Pearson’s Correlations (r) of Changes in Vascular and Endothelial Markers with Changes in Cardiovascular Risk Factors among Exercisers (n=49) and Controls (n=63)
| FMD% (r) | E-selectin (r) | P-selectin (r) | ICAM-1 (r) | VCAM-1 (r) | tPA (r) | |
|---|---|---|---|---|---|---|
| BMI, kg/m2 | ||||||
| Exercise | −0.11 (0.47) | 0.12 (0.40) | 0.29 (0.04) | 0.20 (0.17) | 0.12 (0.40) | 0.23 (0.11) |
| Usual Care | 0.02 (0.89) | 0.22 (0.08) | 0.01 (0.91) | 0.05 (0.72) | 0.04 (0.73) | 0.05 (0.67) |
|
| ||||||
| Body fat, % | ||||||
| Exercise | −0.06 (0.70) | 0.05 (0.76) | −0.02 (0.88) | 0.26 (0.07) | 0.03 (0.83) | 0.28 (0.05) |
| Usual Care | −0.06 (0.63) | −0.11 (0.38) | −0.21 (0.10) | −0.19 (0.14) | −0.27 (0.03) | 0.03 (0.80) |
|
| ||||||
| Visceral fat, cm2 | ||||||
| Exercise | −0.10 (0.52) | 0.23 (0.12) | 0.00 (0.98) | 0.10 (0.50) | 0.17 (0.27) | 0.38 (<0.01) |
| Usual Care | 0.05 (0.73) | 0.23 (0.08) | −0.04 (0.79) | 0.02 (0.88) | −0.07 (0.60) | 0.04 (0.77) |
|
| ||||||
| LDL cholesterol, mg/dL | ||||||
| Exercise | 0.21 (0.21) | 0.20 (0.22) | −0.10 (0.54) | −0.07 (0.65) | −0.04 (0.79) | 0.01 (0.96) |
| Usual Care | −0.39 (<0.01) | −0.05 (0.71) | −0.18 (0.18) | 0.05 (0.71) | −0.35 (<0.01) | −0.13 (0.33) |
|
| ||||||
| HDL cholesterol, mg/dL | ||||||
| Exercise | −0.14 (0.36) | 0.15 (0.29) | −0.36 (0.01) | 0.09 (0.56) | −0.17 (0.25) | 0.05 (0.70) |
| Usual Care | −0.14 (0.27) | 0.07 (0.56) | −0.02 (0.88) | −0.04 (0.78) | −0.17 (0.19) | 0.07 (0.60) |
|
| ||||||
| Triglycerides, mg/dL | ||||||
| Exercise | −0.10 (0.29) | 0.24 (0.10) | 0.11 (0.44) | 0.30 (0.04) | 0.25 (0.09) | 0.20 (0.17) |
| Usual Care | −0.18 (0.26) | 0.25 (0.05) | −0.11 (0.39) | 0.37 (<0.01) | −0.05 (0.70) | −0.03 (0.81) |
|
| ||||||
| HbA1c, % | ||||||
| Exercise | −0.14 (0.38) | −0.30 (0.04) | 0.04 (0.81) | 0.31 (0.03) | −0.03 (0.86) | −0.04 (0.81) |
| Usual Care | −0.08 (0.55) | 0.02 (0.89) | −0.03 (0.84) | −0.05 (0.68) | 0.13 (29) | −0.02 (0.86) |
|
| ||||||
| QUICKI | ||||||
| Exercise | 0.21 (0.18) | 0.01 (0.94) | −0.15 (0.31) | −0.28 (0.05) | 0.24 (0.12) | −0.38 (<0.01) |
| Usual Care | 0.04 (0.79) | −0.28 (0.03) | 0.25 (0.06) | −0.03 (0.84) | 0.00 (0.98) | 0.02 (0.87) |
|
| ||||||
| VO2peak, mL/kg·min | ||||||
| Exercise | 0.10 (0.52) | 0.09 (0.56) | −0.23 (0.11) | −0.32 (0.03) | −0.18 (0.21) | −0.06 (0.67) |
| Usual Care | 0.20 (0.13) | 0.13 (0.33) | 0.16 (0.21) | 0.06 (0.68) | 0.14 (0.30) | 0.16 (0.23) |
Abbreviations: BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; QUICKI, quantitative insulin sensitivity check index; VO2peak, peak oxygen uptake
Acknowledgments
Acknowledgements and Grants
This work was supported by grants from the National Heart Lung and Blood Institute (R21-HL095157, 12/01/2008 – 12/01/2010), the National Institute for Diabetes, Digestive, and Kidney Disorders (R01 DK062368-04, 02/02/04 – 12/31/10), and Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 3.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 4.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair SN. Physical inactivity and cardiovascular disease risk in women. Med Sci Sports Exerc. 1996;28:9–10. doi: 10.1097/00005768-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Green DJ, Spence A, Halliwill JR, Cable NT, Thijssen DH. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96:57–70. doi: 10.1113/expphysiol.2009.048694. [DOI] [PubMed] [Google Scholar]
- 8.Lenk K, Uhlemann M, Schuler G, Adams V. Role of endothelial progenitor cells in the beneficial effects of physical exercise on atherosclerosis and coronary artery disease. J Appl Physiol. 111:321–328. doi: 10.1152/japplphysiol.01464.2010. [DOI] [PubMed] [Google Scholar]
- 9.Metsios GS, Stavropoulos-Kalinoglou A, Sandoo A, van Zanten JJ, Toms TE, John H, Kitas GD. Vascular function and inflammation in rheumatoid arthritis: the role of physical activity. Open Cardiovasc Med J. 4:89–96. doi: 10.2174/1874192401004020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 13.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 16.Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O’Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- 17.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 18.Jansson JH, Nilsson TK, Johnson O. von Willebrand factor, tissue plasminogen activator, and dehydroepiandrosterone sulphate predict cardiovascular death in a 10 year follow up of survivors of acute myocardial infarction. Heart. 1998;80:334–337. doi: 10.1136/hrt.80.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonjes A, Scholz M, Fasshauer M, Kratzsch J, Rassoul F, Stumvoll M, Bluher M. Beneficial effects of a 4-week exercise program on plasma concentrations of adhesion molecules. Diabetes Care. 2007;30:e1. doi: 10.2337/dc06-1760. [DOI] [PubMed] [Google Scholar]
- 21.Zoppini G, Targher G, Zamboni C, Venturi C, Cacciatori V, Moghetti P, Muggeo M. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2006;16:543–549. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 23.Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Goto Y, Yoshimasa Y, Miyamoto Y. Effect of Exercise Intervention on Endothelial Function and Incidence of Cardiovascular Disease in Patients with Type 2 Diabetes. J Atheroscler Thromb. 2010 doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- 24.Miche E, Herrmann G, Nowak M, Wirtz U, Tietz M, Hurst M, Zoller B, Radzewitz A. Effect of an exercise training program on endothelial dysfunction in diabetic and non-diabetic patients with severe chronic heart failure. Clin Res Cardiol. 2006;95 (Suppl 1):i117–124. doi: 10.1007/s00392-006-1106-z. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HR, Min KW, Ahn HJ, Seok HG, Lee JH, Park GS, Han KA. Effects of Aerobic Exercise vs. Resistance Training on Endothelial Function in Women with Type 2 Diabetes Mellitus. Diabetes Metab J. 2011;35:364–373. doi: 10.4093/dmj.2011.35.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrosielski D, Barone Gibbs B, Ouyang P, Bonekamp S, Clark JM, Wang N, Silber HS, Shapiro EP, Stewart KJ. Effect of Exercise on Blood Pressure in Type 2 Diabetes: A Randomized Controlled Trial. Journal of General Internal Medicine. 2012 doi: 10.1007/s11606-012-2103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 29.Barone Gibbs B, Dobrosielski D, Lima M, Bonekamp S, Stewart KJ, Clark JM. The association of arterial shear and flow-mediated dilation in diabetes. Vasc Med. 2011 doi: 10.1177/1358863X11411361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003–5012. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaun MI, Dipp T, da Rossato SJ, Wilhelm EN, Pinto R, Rech A, Plentz RD, Homem de Bittencourt PI, Reischak-Oliveira A. The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct. 2011;29:534–542. doi: 10.1002/cbf.1781. [DOI] [PubMed] [Google Scholar]
- 32.Dunstan DW, Mori TA, Puddey IB, Beilin LJ, Burke V, Morton AR, Stanton KG. A randomised, controlled study of the effects of aerobic exercise and dietary fish on coagulation and fibrinolytic factors in type 2 diabetics. Thromb Haemost. 1999;81:367–372. [PubMed] [Google Scholar]
- 33.Hatunic M, Finucane F, Burns N, Gasparro D, Nolan JJ. Vascular inflammatory markers in early-onset obese and type 2 diabetes subjects before and after three months’ aerobic exercise training. Diab Vasc Dis Res. 2007;4:231–234. doi: 10.3132/dvdr.2007.045. [DOI] [PubMed] [Google Scholar]
- 34.Esposito K, Ciotola M, Giugliano D. Mediterranean diet, endothelial function and vascular inflammatory markers. Public Health Nutr. 2006;9:1073–1076. doi: 10.1017/S1368980007668529. [DOI] [PubMed] [Google Scholar]
- 35.Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G. Effect of an Intensive Exercise Intervention Strategy on Modifiable Cardiovascular Risk Factors in Subjects With Type 2 Diabetes Mellitus: A Randomized Controlled Trial: The Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 36.Yates T, Davies MJ, Gorely T, Talbot D, Bull F, Sattar N, Khunti K. The effect of increased ambulatory activity on markers of chronic low-grade inflammation: evidence from the PREPARE programme randomized controlled trial. Diabet Med. 27:1256–1263. doi: 10.1111/j.1464-5491.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 37.Irace C, Tschakovsky ME, Carallo C, Cortese C, Gnasso A. Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis. 2008;200:439–445. doi: 10.1016/j.atherosclerosis.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]