Summary
Aggregatibacter actinomycetemcomitans leukotoxin (Ltx) is a repeats-in-toxin (RTX) cytolysin that kills human leukocyte function-associated antigen-1 (LFA-1; αL/β2)-bearing cells. In order to determine whether the αL portion of the heterodimer is involved in Ltx recognition, we transfected human, mouse and bovine αL cDNAs into J-β2.7, an αL-deficient cell line, and looked for restoration of Ltx susceptibility. Cells expressing either bovine or human αL in conjunction with human β2 were efficiently killed by Ltx, an indication that bovine αL could substitute for its human counterpart in critical regions used by Ltx for attachment to LFA-1. On the other hand, cells expressing murine αL and human β2 were not susceptible to the lethal effects of Ltx indicating that the toxin recognition sites are not present in the corresponding mouse sequence. To further identify the region(s) of αL recognized by Ltx, we constructed and evaluated a panel of chimeric human/murine αL genes in J-β2.7 cells. Analysis of the αL mutant panel showed that the presence of human N-terminal 128 amino acids on a mouse CD11a background, a region that includes β-sheets 1 and 2 of the β-propeller of the human αL chain, was sufficient for Ltx cytolysis.
Introduction
Repeats-in-toxin (RTX) toxins are bacterial proteins synthesized by a diverse group of Gram-negative pathogens and, as a model, we have been using Aggregatibacter actinomycetemcomitans (Nørskov-Lauritsen and Kilian, 2006) leukotoxin (Ltx) to study the mechanism(s) by which these toxins kill cells. Studies from several laboratories have reported that expression of leukocyte function-associated antigen-1 (LFA-1) is necessary for RTX cytolysins to kill cells (Lally et al., 1997; Wang et al., 1998; Ambagala et al., 1999; Li et al., 1999; Jeyaseelan et al., 2000). LFA-1 is uniquely expressed on the cell surfaces of innate and adaptive immune cells which explains, in part, the restricted cytotoxicity exhibited by these toxins for cells of haematopoietic origin. Organisms that produce these cytolysins have a restricted pattern of host colonization and these long-standing associations with a single species have led to RTX that will recognize and kill LFA-1 bearing cells of that species. For example, A. actinomycetemcomitans colonizes man, the Great Apes, and Old World monkeys and the toxin produced by a human strain of A. actinomycetemcomitans is selective for cells from these species (Taichman et al., 1987) while Mannheimia haemolytica, an organism which colonizes cattle, produces an RTX toxin that will only kill bovine LFA-1-bearing cells (Shewen and Wilkie, 1982; Wang et al., 1998) and not their human counterparts. The unique specificity exhibited by RTX cytolysins has lent itself to the production of chimeric toxins for structure/function studies that identified the non-apeptide GGXG(N/D)DX(L/I/F)X (where X represents any amino acid) repeats (Felmlee et al., 1985) as the region involved in recognition of human target cells (Lally et al., 1994).
Function-associated antigen-1 is formed by the non-covalent association of α (αL, CD11a) and β (β2, CD18) subunits. CD11a is the larger (Mr = 170 000) polypeptide. It contains seven β-sheet repeats in the N-terminus which have been predicted to fold into a β-propeller domain. The ligand-binding (I or inserted domain) is located between β-sheets 2 and 3 of the putative β-propeller (Huang and Springer, 1997). The CD18 subunit (Mr = 95 000) has a 677 amino acid extracellular domain with a 23 amino acid cytoplasmic tail. The expressed protein has a high cysteine content, especially in a 186 amino acid repeat region that contains four repeats of an 8-cysteine motif, which gives the protein a very rigid structure (Kishimoto et al., 1987; Law et al., 1987; Stephens et al., 1995). The cysteine-rich region is believed to play a regulatory role because the epitopes of many antibodies that activate ligand binding map to this region (Stephens et al., 1995; Huang et al., 2000; Lu et al., 2001).
Subtle similarities and differences exist in the LFA-1 amino acid sequences among species and these have proved useful for the analysis of integrin function. Interspecies LFA-1 chimeras have been used to explore complementation in patients with leukocyte adhesion deficiency (Marlin et al., 1986), map anti-LFA-1 mAb epitopes (Champe et al., 1995; Stephens et al., 1995) and study interactions of LFA-1 with its ICAM-1 ligand (Huang and Springer, 1995). In our current study, we have made use of a series of mouse/human and bovine/human CD11a/CD18 chimeras to identify regions of the α chain of the LFA-1 molecule that are critical for Ltx recognition or binding. The human α chain (CD11a) is phylogenetically conserved when compared with both its mouse (72% identity) and its bovine (77% identity) counterparts (Kaufmann et al., 1991; Fett et al., 2004), allowing for the construction of chimeric CD11a cDNAs and transfection of these chimeric genes into a T lymphoblastoid CD11a-deficient cell line, J-β2.7 (Weber et al., 1997), which expresses neither component of α/β heterodimer on its cell surface and is impervious to Ltx-mediated cytotoxicity. Transfection of human, bovine or mouse CD11a cDNA into J-β2.7 mutant cells all resulted in LFA-1 expression. However, only cells expressing human or bovine CD11a were sensitive to Ltx. This observation indicated that mouse/human but not bovine/human CD11a chimeras were capable of providing information concerning the specific region(s) of the human LFA-1 heterodimer that is necessary for Ltx recognition/binding. Using a panel of mouse/human chimeras we demonstrate that the N-terminal 128 residues of the alpha chain are critical for Ltx-mediated cytotoxicity.
Results and discussion
Transfection of J-β2.7 cells with mouse or bovine CD11a cDNA results in expression of chimeric LFA-1 heterodimers
Expression of human LFA-1 on the target cells is necessary for Ltx cytotoxicity (Lally et al., 1997). In order to understand the nature of the interaction of the toxin with this cell surface heterodimeric protein, we made use of a Jurkat cell line, Jn.9, and its LFA-1-negative mutant, J-β2.7 (Weber et al., 1997) to identify regions of the LFA-1 heterodimer recognized by A. actinomycetemcomitans Ltx. J-β2.7 cells are derived from Jurkat (Jn.9) cells by ethylmethane sulphonate mutagenesis and a negative selection scheme (Weber et al., 1997). As a result of this process, the cells have lost surface expression of both the CD11a and CD18 subunits. Analysis of J-β2.7 cells has shown that they do not synthesize the CD11a subunit. CD18 mRNA, however, is present within the cell suggesting that the CD18 protein may require association with CD11a in the endoplasmic reticulum before being transported to the cell surface (Weber et al., 1997). The human αL subunit is capable of combining with the human β component present in J-β2.7 cells (Weber et al., 1997; 1999; Leitinger and Hogg, 2000; Lu et al., 2001), such that transfection of human CD11a cDNA into J-β2.7 cells results in expression of the LFA-1 heterodimer on the cell surface.
Our experimental objectives required that the CD18 present in these cells be able to combine with heterologous CD11a and produce a chimeric LFA-1 on the cell surface of J-β2.7 cells. Dimerization of the αβ components of LFA-1 is critical for post-translational modification such as glycosylation and folding that lead to transportation of the heterodimer to the cell surface, as shown by previous studies with the J-β2.7 line (Weber et al., 1997; 1999; Leitinger and Hogg, 2000). Those studies, however, were limited to the expression of human α and β components on the cell surface whereas our experimental goal was to express a chimeric LFA-1 molecule composed of one protein derived from either mouse or bovine cDNA transgene while the other component is translated from the constitutive human gene. To determine whether this goal was achievable, J-β2.7 cells were transfected with either mouse (mCD11a), or human (hCD11a) CD11a cDNA. Transfectants and controls were stained with anti-human CD11a or anti-mouse CD11a antibody and anti-human CD18 antibody and examined by confocal fluorescence microscopy (Fig. 1). Incubation of wild-type (wt) Jn.9 cells stained with both CD11a and CD18 antibodies resulted in red and green signals, respectively (Fig. 1A, Jn.9). When the two confocal images were superimposed or merged a yellow signal, consistent with the colocalization of α and β components of the LFA-1 heterodimer, was observed. As expected, J-β2.7 cells (Fig. 1A, J-β2.7) failed to demonstrate cell surface localization with either CD11a or CD18 while J-β2.7 cells transfected with hCD11a (Fig. 1A, J-β2.7/hCD11a) showed expression and colocalization of the heterodimer. Transfection of J-β2.7 with mouse CD11a (mCD11a) resulted in colocalization of the mouse CD11a subunit with the human CD18 subunit on the cell surface (Fig. 1A, J-β2.7/mCD11a). Flow cytometric analysis (Fig. 1B) of the mouse/human LFA-1 (J-β2.7/mCD11a) chimera demonstrated that LFA-1 levels were similar to both wt Jn.9 cells and J-β2.7 cells transfected with human CD11a (J-β2.7/hCD11a) because J-β2.7/mCD11a cells reacted with both anti-human CD18 mAb (TS1/18) and an anti-mouse CD11a mAb (M17/4).
Fig. 1.
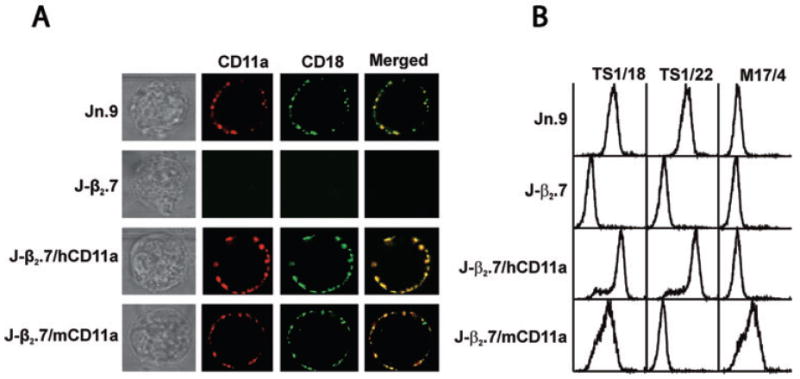
Expression of LFA-1 in CD11a transfected J-β2.7 cells.
A. Jn.9 (wt), J-β2.7 and J-β2.7 cells transfected with human (J-β2.7/hCD11a) or mouse (J-β2.7/mCD11a) CD11a were stained with anti-human CD11a (Jn.9, J-β2.7 and J-β2.7/hCD11a) or anti-mouse CD11a (J-β2.7/mCD11a) and anti-human CD18 (all cells) and visualized by confocal microscopy demonstrating the restoration of human CD18 (green), as well as human or mouse CD11a (red) expression on the mutant J-β2.7 cell surface. Superimposing the two images (merged) gave a yellow signal indicative of colocalization of the CD11a and CD18 molecules, hence revealing the formation of the LFA-1 heterodimer.
B. FACS analysis of 10 000 cells in each group using anti-human CD18 mAb (TS1/18) and anti-human CD11a mAb (TS1/22) confirmed that transfecting human CD11a (J-β2.7/hCD11a) into mutant J-β2.7 restored expression of human CD11a and human CD18 on the cell surface equivalent to that of wild-type Jn.9. Analyses of the mouse CD11a transfected line (J-β2.7/mCD11a) using anti-mouse CD11a (M17/4), anti-human CD11a (TS1/22) and anti-human CD18 (TS1/18) demonstrated that transfection with the mouse gene restored human CD18 on the cell surface in conjunction with mouse, but not human, CD11a.
In a separate experiment, a bovine CD11a (bCD11a) construct was transfected into J-β2.7 cells and flow cytometric analysis with anti-human CD18 mAb TS1/18, anti-human CD11a mAb TS1/22 and anti-bovine CD11a mAb MUC76A used to detect the expression of human CD18, as well as human and bovine CD11a (Fig. 2A, J-β2.7/bCD11a). As expected, our wt human Jn.9 reacted with both human antibodies, but not bovine MUC76A, and the mutant J-β2.7 did not respond to any of the anti-LFA-1 antibodies. BL3, a bovine B-lymphosarcoma cell line (ATCC CRL-8037), served as a bovine LFA-1 control and reacted with anti-bovine CD11a (MUC76A; Fig. 2A). Our bovine CD11a/human CD18 transfectant (J-β2.7/bCD11a) however, while not reacting to TS1/22 as expected, did not react to bovine anti-CD11a MUC76A, and its reaction to human TS1/18, while positive, was less than stellar. (Fig. 2A).
Fig. 2.
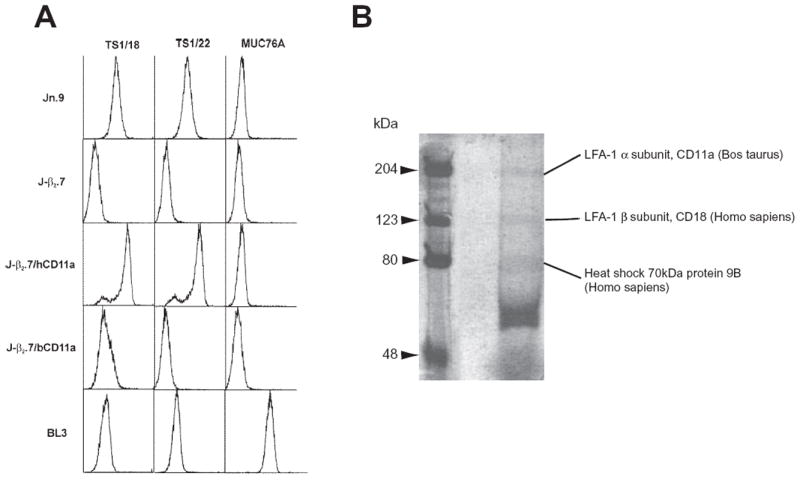
Expression of bovine CD11a/human CD18 heterodimer on the cell surface of mutant J-β2.7 cells.
A. Flow cytometric analyses of 10 000 J-β2.7 cells transfected with bovine CD11a (J-β2.7/bCD11a) showed restoration of human CD18 (TS1/18) to the cell surface. As expected, J-β2.7/bCD11a did not react with human CD11a (TS1/22), however, the cell line was also not reactive with anti-bovine CD11a antibody (MUC76A). Wild-type Jn.9, mutant J-β2.7, human CD11a transfected J-β2.7 (J-β2.7/hCD11a) and bovine BL3 served as controls.
B. 2 × 108 J-β2.7 cells transfected with bovine CD11a were lysed and immunoprecipitated with anti-human CD18 (TS1/18), run on a 7.5% SDS PAGE gel and stained with Colloidal Blue staining kit (Novex, Invitrogen) according to manufacturer’s instructions revealing bands corresponding to bovine CD11a and human CD18 (see Table 1).
Anti-bovine LFA-1 antibodies are not as abundant, nor as well characterized, as human and mouse reagents. As a result, we have demonstrated that J-β2.7/bCD11a cells express bovine CD11a as part of a bovine/human LFA-1 heterodimer by solubilizing and immunoprecipitating the cells with TS1/18 mAb followed by SDS-PAGE electrophoresis and staining with colloidal blue. The resulting gel contained bands at 170, 95 and 70 kDa (Fig. 2B). To verify that the bands present at 170 and 95 kDa were bovine CD11a and human CD18, respectively, the visible bands were cut from the gel, digested in-gel with trypsin (Speicher et al., 2000) and subjected to liquid chromatography – tandem mass spectrometry (LC-MS/MS) analysis (Tang et al., 2005). The 170 kDa band (Table 1) was identified as lymphocyte function-associated antigen 1 alpha subunit CD11a (Bos taurus). Analysis of identified peptides using BLAST against the human NCBI database confirmed that 14 of the 17 peptides identified were bovine sequences that were not identical to the corresponding human sequences. The CD11a peptides identified were distributed between amino acid residues 91 and 1156, which spanned more than 90% of the intact protein sequence. Together with the observed molecular weight, the MS/MS data suggest that a full length protein was isolated in this immuno-complex. As expected, ITGB2 protein (Homo sapiens) was unambiguously identified as the major component of the 95 kDa band consistent with the presence of the human CD18 gene in J-β2.7 and use of the TS1/18 mAb to precipitate the complex. An additional minor protein (SFPQ) was detected in the 95 kDa band, although the function and the interaction specificity of this protein are uncertain at the present moment. Analysis of the 70 kDa band revealed the presence of multiple proteins, including two heat shock proteins. These data strongly suggested that transfecting murine or bovine CD11a into J-β2.7 results in expression of chimeric LFA-1 on the cell surface, but that a change in conformation may occur, rendering certain epitopes no longer accessible to antibody.
Table 1.
Mass spectrometry identification of TS1/18 immuno-complexes from J-β2.7/bCD11a cells.
| Band | Accession no. | Protein name | No. unique peptides | Coverage (%) |
|---|---|---|---|---|
| 1 | 58294978 | Lymphocyte function-associated antigen 1 alpha subunit CD11a (Bos taurus) | 17 | 21 |
| 2 | 13543407 | ITGB2 protein (Homo sapiens) | 20 | 38 |
| 29881667 | SFPQ protein (Homo sapiens) | 6 | 13 | |
| 3 | 16741727 | Heat shock 70 kDa protein 8 (Homo sapiens) | 9 | 15 |
| 24234688 | Heat shock 70 kDa protein 9B (Homo sapiens) | 12 | 24 | |
| 48145611 | FUS (Homo sapiens) | 5 | 10 | |
| 49457432 | G22P1 (Homo sapiens) | 8 | 17 | |
| 226021 | Growth regulated nuclear 68 protein (Homo sapiens) | 3 | 5 | |
| 183613 | Granulin (Homo sapiens) | 3 | 6 | |
| 16905456 | Ribonucleoprotein (Homo sapiens) | 2 | 4 |
Cells expressing chimeric LFA-1 heterodimers composed of a bovine α chain/human β chain are susceptible to Ltx-mediated cytolysis while those expressing a murine α chain/human β chain are not
To determine whether either bovine or murine CD11a expressed with human CD18 in the form of a chimeric LFA-1 could make cells susceptible to Ltx, we incubated J-β2.7/bCD11a and J-β2.7/mCD11a cell lines and the appropriate controls with Ltx and assessed cell viability via trypan blue (Fig. 3). Wild-type Jurkat (Jn.9) cells express LFA-1 on the cell surface and are killed by the toxin (95.35% ± 3.44) while LFA-1-deficient J-β2.7 cells which do not express the integrin heterodimer are unaffected (0.19% ± 0.19). Transfection of human CD11a cDNA (J-β2.7/hCD11a) into J-β2.7 cells restores Ltx susceptibility to near wt levels (90.92% ± 1.64) while mutants expressing mouse CD11a gene (J-β2.7/mCD11a) were not affected (6.13% ± 2.58) by culturing with Ltx. On the other hand, J-β2.7 cells transfected with a bovine CD11a construct (J-β2.7/bCD11a) were killed by Ltx (81.89% ± 5.76) demonstrating that while mouse CD11a does not substitute for human CD11a when paired with human CD18, a chimeric LFA-1 composed of bovine CD11a and human CD18 is capable of binding Ltx and initiating the cell death process.
Fig. 3.
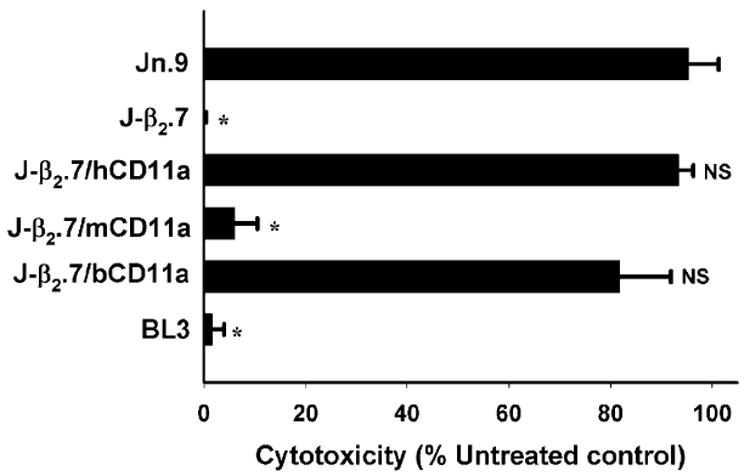
Bovine, but not mouse, CD11a substitutes for human CD11a. Wild-type Jn.9 cells, mutant J-β2.7 cells and cells expressing human (J-β2.7/hCD11a), mouse (J-β2.7/mCD11a) or bovine (J-β2.7/bCD11a) CD11a on the cell surface were subjected to Ltx and viability determined via trypan blue exclusion assay (see Experimental procedures). Bovine leukaemia cells (BL3) served as a control. Transfection of human or bovine, but not mouse, CD11a into mutant J-β2.7 restored susceptibility to Ltx equal to that of the wild-type Jn.9. (* significant; NS: not significant when compared with wild-type Jn.9 P < 0.001).
Leukotoxin is species specific and will not kill lymphocytes of bovine origin such as BL3 cells. BL3 cells express both bovine CD11a (Fig. 2) and bovine CD18 (data not presented) on the cell surface. While impervious to Ltx, BL3 is readily killed by the related bovine RTX toxin secreted by Mannheimia (Pasteurella) haemolytica (Lkt) (Lally et al., 1994). The ability of Ltx to kill J-β2.7/bCD11a, which has a bovine alpha chain and human beta chain, but not BL3, which contains both bovine LFA-1 chains, provides initial evidence that although bovine CD11a can substitute for its human counterpart, the toxin is also recognizing additional sequences on human CD18. Human and mouse CD11a are approximately 72% homologous (Kaufmann et al., 1991) while bovine and human are 77% homologous (Fett et al., 2004), so the fact that bovine but not mouse CD11a can substitute for the human gene may be due to the difference in similarity, or to the presence of a very specific residue(s) in a critical region(s).
Leukotoxin recognizes the N-terminal 128 residues of human CD11a
The inability of Ltx to kill cells expressing mouse CD11a combined with human CD18 provided a useful platform upon which to localize a region of the α subunit necessary for Ltx recognition. Human and mouse CD11a molecules are not only homologous, but also easily divisible into functional domains (Larson et al., 1989; Springer, 1997; Yalamanchili et al., 2000). The α subunit consists of a 1063-amino acid extracellular domain, a 29-amino acid transmembrane (tm) region and a 53-amino acid cytoplasmic tail (Larson et al., 1989). The N-terminus of the α subunit contains seven β-sheet repeats (W1-7) of ~60 amino acids, which have been predicted to fold into a β-propeller domain (Huang and Springer, 1997). Inserted between β-sheets 2 and 3 of this putative β-propeller is an additional domain of ~200 amino acids known as the I (inserted) domain. There is abundant evidence that the I domain contains the major ligand binding site for Intracellular Adhesion Molecule 1 (ICAM-1) (Landis et al., 1994; Huang and Springer, 1995). The crystal structure of the I domain has been solved (Qu and Leahy, 1995) and it consists of a five-stranded parallel β-sheet core, surrounded by α-helices; and while the β-propeller domain requires association with CD18 to achieve the correct tertiary structure, the I domain folds independently (Huang and Springer, 1997).
We divided the α chain into specific domains and constructed a series of chimeric cDNAs by either deleting portions of the human CD11a or exchanging human integrin domains with their mouse counterparts, transfecting them into the J-β2.7 cell mutant, and examining the cells for cell surface expression of LFA-1 and Ltx susceptibility. Transfection of either human (J-β2.7/hCD11a) or mouse (J-β2.7/mCD11a) CD11a genes into J-β2.7 restored expression of both human CD18 and human or mouse CD11a on the cell surface (Table 2) as determined by antibody recognition, and human, but not mouse, CD11a restored Ltx-mediated cytotoxicity (Fig. 4).
Table 2.
Human/mouse chimera antibody recognition.
| TS1/18 human CD 18 | TS1/22 human I-domain | G25.2 human β-propeller W5-W7 | TS2/4 human β-propeller residues 1–57 | M17/4 mouse CD11a | |
|---|---|---|---|---|---|
| Jn.9 | + | + | + | + | − |
| J-β2.7 | − | − | − | − | − |
| J-β2.7/empty vector | − | − | − | − | − |
| J-β2.7/hCD11a | + | + | + | + | − |
| J-β2.7/mCD11a | + | − | − | − | + |
| J-β2.7/hΔDICD11a | + | − | + | + | − |
| J-β2.7/h654CD11a | + | + | − | + | − |
| J-β2.7/m654CD11a | + | − | − | − | − |
| J-β2.7/h319CD11a | + | + | − | + | − |
| J-β2.7/m319CD11a | +/− | +/− | − | − | +/− |
Fig. 4.
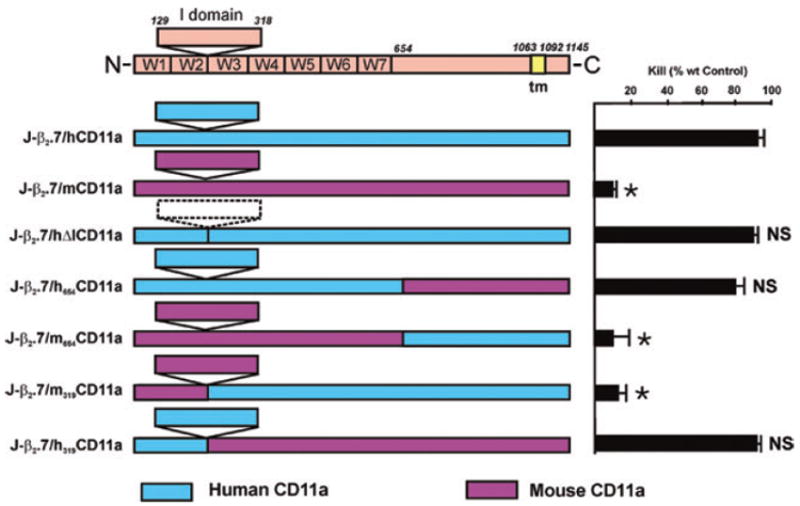
Human CD11a specificity for Ltx resides in the N-terminal 128 amino acids. Human and mouse CD11a sequences were aligned and human domains were systematically replaced with their mouse counterparts. The resultant chimeric human/mouse CD11a molecules were transfected into the Ltx-resistant mutant J-β2.7 cell line, and tested for Ltx susceptibility demonstrating that only cells containing human CD11a residues 1–128 were Ltx sensitive. Cytotoxicity representative of at least four assays and shown as per cent wild-type (Jn.9) control. (*Significant; NS, not significant when compared with J-β2.7/hCD11a P < 0.001).
The initial construct consisted of deleting the inserted or I domain (J-β2.7/hΔICD11a; residues 129–318) from human alpha chain. This construct contained all of the residues of human CD11a, except for the ones purported to be the binding site for ICAM-1 (Landis et al., 1994; Leitinger and Hogg, 2000) and, as expected, this construct was detected by all antibodies that recognize human CD11a/CD18 (Table 2) except mAb TS1/22 which detects an epitope within the inserted (I) domain (Huang and Springer, 1997).
Our previous results indicated that TS1/22, a monoclonal antibody that purportedly binds to the I domain, inhibits Ltx-mediated cytotoxicity (Lally et al., 1997). We therefore had assumed that the I domain would be involved in Ltx recognition. However, Ltx sensitivity was not diminished when J-β2.7/hΔICD11a was compared with the wt gene (J-β2.7/hCD11a: Fig. 4) indicating, to the contrary, that the I domain is not involved in Ltx/LFA-1 interaction. While TS1/22 is reported to recognize an I domain epitope between residues 248–303 (Huang and Springer, 1997), another report places it within the linker region between the β-propeller domain and the I domain (residues I126 and N129) of human CD11a (Champe et al., 1995). The ability of TS1/22 to inhibit Ltx-mediated cytotoxicity could therefore be due to steric hindrance or involvement with Ile126 because residues 129–318 were destroyed in construction of our I deletion mutant. Several additional observations support the current experiment that the I region is not involved Ltx cytolysis. First, preincubation of Ltx or target cells with solubilized ICAM-1 does not inhibit cytotoxicity (data not shown) and KL/4 cells (Ortlepp et al., 1995) do not bind ICAM-1 unless activated, yet are still susceptible to Ltx (Lally et al., 1997).
Having ruled out the I domain as the region responsible for Ltx/LFA-1 interaction, we turned our attention to separating the β-propeller domain from the C-terminus via two constructs (i) J-β2.7/h654CD11a, which contains human sequence from the N-terminus to residue 654 (located approximately 30 residues past the β-propeller domain of CD11a) and mouse CD11a sequence for the remainder of the gene to the C-terminus and (ii) J-β2.7/m654CD11a, the flipped version with mouse residues through the β-propeller domain and human sequences at the C-terminus. While J-β2.7/h654CD11a reacted as expected to mAb TS1/18, TS1/22 and TS2/4, it was not detected by monoclonal antibody G25.2 (Table 2). This antibody recognizes β-propeller domain W5 to W7 (Huang and Springer, 1997) and thus should have reacted with J-β2.7/h654CD11a. Lack of antibody reaction in the otherwise active construct may indicate a conformational change in this region that is irrelevant to Ltx recognition of LFA-1.
Reverse construct J-β2.7/m654CD11a which contains mouse residues at the N-terminus and human residues at the carboxy end reacted, as expected, with anti-human CD18 (mAb TS1/18) but not with any of the antibodies (mAbs TS1/22, G25.2, TS2/4) to the human CD11a domains that were replaced by their mouse counterpart (Table 2). However, it also did not react to anti-mouse CD11a mAb M17/4, and although controversy exists about whether mAb M17/4 sees the I domain (residues 250–303) or the linker between the β-propeller domain and the I domain (residues I126 and N129) of mouse CD11a (Champe et al., 1995; Huang and Springer, 1995), both of these epitopes are present in J-β2.7/m654CD11a, so conformational changes induced by chimera formation may make some of these epitopes inaccessible to antibody, and may explain the controversy that exists in the literature when trying to pinpoint CD11a antibody binding epitopes. Checking for susceptibility of the β-propeller domain chimeras to Ltx (Fig. 4) demonstrated that replacing the region between the β-propeller domain and the C-terminus with mouse residues (J-β2.7/h654CD11a) did not alter Ltx-susceptibility. Replacement of the β-propeller with corresponding mouse residues (J-β2.7/m654CD11a), however, resulted in abrogation of Ltx-mediated cytotoxicity indicating that the β-propeller domain is crucial for the Ltx/LFA-1 interaction, but the C-terminus does not appear to be involved.
Having demonstrated that the β-propeller domain is critical to Ltx function, the repeats within the β-propeller domain on either side of the I domain were then separated and further evaluated. The β-propeller domain contains seven four-stranded (represented as W1-7 from N- to C-terminal) β-sheets arranged in a torus around a pseudosymmetry axis (Huang and Springer, 1997; Zang et al., 2000). To probe the β-propeller region, a silent EcoRV was engineered into a highly conserved region (residues Q315 to S334) which is 100% identical in human, bovine and mouse CD11a and used as a splice site to swap portions of the β-propeller domain (see Table S1 in Supplementary material). This resulted in two constructs: J-β2.7/h319CD11a which contains the human N-terminal sequences including β-sheets 1 and 2 and J-β2.7/m319CD11a which contains the human C-terminus including β-sheets 3 to 7.
J-β2.7/h319CD11a reacted as expected to our panel of antibodies (Table 2) but construct J-β2.7/m319CD11a exhibited low antibody reactivity which dropped upon cell passage and, despite several attempts at transfection and subcloning, proved too unstable for adequate characterization. This is consistent with a previous report that replacement of the first 117 residues, as well as the N-terminal 359 residues, of human CD11a with mouse residues (chimera m359h) resulted in chimeras that did not express on the cell surface (Huang and Springer, 1995).
Adding 319 N-terminal human residues to the mouse CD11a gene (J-β2.7/h319CD11a) restored Ltx sensitivity to the J-β2.7 mutant (Fig. 4). Ltx, on the other hand, was not toxic to the construct that contained mouse residues on the N-terminal end (J-β2.7/m319CD11a) but it was impossible to determine whether the instability of the molecule contributed to this result, although the observed instability would appear to substantiate the proposal that this region is extremely important to LFA-1 function. The ability of first 319 residues of human CD11a to restore Ltx recognition to the mouse molecule, together with the observation that residues 129–318 of the I domain could be deleted without affecting Ltx recognition thereby narrowed Ltx specificity to the N-terminal 128 amino acids.
Models of human CD11a suggest that these residues fold to form W1 and W2 of the CD11a β-propeller. In molecular models of the β-propeller, W1 is structurally contiguous with residues forming W7 (Springer, 1997; Zang et al., 2000), therefore we cannot rule out that a portion of W7 is involved in Ltx recognition. However, the inability of mAb TS2/4, which maps to the first 57 residues of CD11a (Huang and Springer, 1997; Zang et al., 2000), to inhibit Ltx-mediated cytotoxicity (data not presented) suggests that W7 is most likely not critical to toxin recognition. If this supposition is correct, then the area recognized by Ltx is somewhere between residues 58–128, a region that encompasses the 4th β-strand of W1 as well as all four β strands of W2 (Zang et al., 2000).
Attempts to produce cells expressing chimeric CD11a that probe this region have not been successful thus far. The reasons for this problem are not clear but is probably linked to the fact that following translation, the components of the LFA-1 heterodimer undergo preliminary folding in the endoplasmic reticulum before transport to the cell surface (Weber et al., 1997). The tertiary structure of seven-bladed β-propellers is complex (Murzin, 1992) and based upon molecular modelling studies W1 is projected to be closely packed with its residues exhibiting a preference for small amino acid side chains. On the other hand, W2 and W3 are completely buried and probably hydrophobic while W4 is an amphipathic edge strand (Springer, 2002). Furthermore, the lower half of W2 contacts the I-like domain of β-strand 6 of CD18, the β chain of the LFA-1 heterodimer (Zang et al., 2000). It is possible that in designing our splice sites in this region we and others (Champe et al., 1995; Huang and Springer, 1995) may be interfering with the proper LFA-1 folding.
Although the mouse α/human β chimeras demonstrated that the α chain is necessary for Ltx activity, the fact that J-β2.7/bCD11a, the heterodimer containing bovine αL and human β2 genes, was efficiently killed by Ltx while bovine BL3 cells were not (Fig. 3), combined with previous studies revealing that anti-CD18 antibodies inhibit Ltx-mediated cytotoxicity (Lally et al., 1997), indicate that the human β chain is most likely also involved in Ltx recognition. The β-propeller domain of the α chain requires association with CD18 to achieve the correct tertiary structure (Huang and Springer, 1997), so perhaps while bovine CD11a can substitute for its human counterpart, it can only achieve correct folding for Ltx recognition on a human β2 scaffold or that β2 folding (Huang et al., 1997) is dependent upon a conserved region shared by bovine and human CD11a but not by the corresponding mouse segment. Conformational movements are thought to occur at the interface between β-sheets 2 and 3 of αL and the I-like domain of β2 (Zang et al., 2000) and calcium involvement at the αL/β2 interface of the subunits may be important for integrin activation (Dransfield et al., 1992). Ltx is a calcium binding protein (Lally et al., 1991) and we have previously shown that its repeat region (residues 688–941) is responsible for target cell recognition (Lally et al., 1994). Analyses of the repeat domain shows a novel ‘parallel β-roll’ structure in which successive β strands are wound in a right-hand spiral and Ca2+ ions are bound within the turns between the strands (Lally et al., 1991; Lally and Kieba, 1994). Here we localize the Ltx interaction with its LFA-1 receptor to the first 128 residues, namely β-sheets 1 and 2 of the β-propeller domain of the α chain of LFA-1. The next challenge will be to determine which part of the β-chain is involved in the Ltx recognition process and how the interplay between the α and β subunits of LFA-1 with the calcium binding repeat region of the toxin contribute to downstream events that lead to cell death.
Experimental procedures
Antibodies and reagents
TS1/18 against human CD18, TS1/22 against residues 248–303 of human CD11a I domain, TS2/4 against residues 1–57 of the human CD11a β-propeller (W1) domain (Huang and Springer, 1997), and M17/4 against mouse CD11a were from ATCC (Manassas, VA). G25.2 to β-propeller W5-W7 (Huang and Springer, 1997) was purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA), MUC76A against bovine CD11a was purchased from VMRD (Pullman, WA), mouse anti-human CD18 clone MEM 48 and FITC labelled anti-mouse secondary antibodies were from Southern Biotech (Birmingham, AL), purified TS1/18 was from Pierce/Endogen (Rockford, IL), biotin conjugated goat anti-human CD11a polyclonal antibody was purchased from Genetex (San Antonio, TX), biotin conjugated rat anti-mouse CD11a was purchased from BD Biosciences Pharmingen (Chicago, IL), and AlexaFluor® 488-conjugated goat anti-mouse and AlexaFluor® 647 conjugated streptavidin from Molecular Probes (Eugene OR), Phorbol 12-myristate 13-acetate (PMA) and polymyxin B sulphate were purchased from Sigma-Aldrich (St. Lois, MO), and the Colloidal Blue staining kit was purchased from Invitrogen (Carlsbad, CA).
Cell lines
J-β2.7, a CD11a deficient cell line, was derived from Jurkat (Jn.9) by mutagenesis with ethylmethane sulphonate (Weber et al., 1997) and maintained in RPMI 1640 medium (Mediatech Cellgro, Herndon, VA) containing 10% fetal calf serum, 0.1 mM MEM non-essential amino acids, 1× MEM vitamin solution, 2 mM l-glutamine and 50 μg ml−1 gentamicin (Gibco, Invitrogen, Grand Island, NY) at 37°C under 5% CO2. BL3 cells were obtained from American Type Culture Collection (Bethesda, Maryland) and maintained in 50% Leibovitz L-15, 50% DMEM (Mediatech Cellgro, Herndon, VA), 20% fetal calf serum, 2 mM l-glutamine and 1% sodium pyruvate under 5% CO2.
Plasmid constructs
Human and mouse CD11a genes were obtained from Martyn Robinson (Celltech, Slough, UK). Chimeric mouse/human CD11a genes h654m (Human N-terminal CD11a to residue 654, mouse CD11a residue 654 to termination) and m654h (N-terminal mouse CD11a to residue 654 and human CD11a residue 654 to termination), were a kind gift of Timothy A Springer (Harvard).
To construct hΔICD11a, the I region of human CD11a was deleted via polymerase chain reaction (PCR) by splicing residue G128 to residue S319 by way of an in-frame Hindlll restriction sites as described previously (Leitinger and Hogg, 2000).
h319CD11a and m319CD11a were constructed by engineering a silent EcoRV at the human/mouse or mouse/human splice site and stitching into the vector with the help of restriction enzymes NheI and NotI (see Table S1 in Supplementary material). PCR was performed under the following conditions: 30 cycles at 94°C for 15 s., 55°C for 1 min 15 s, 72°C for 2 min 15 s, followed by an extension at 72°C for 8 min 15 s.
Bovine CD11a was engineered via PCR from PMA-stimulated BL3 cells and oligomers described previously (Fett et al., 2004) except the cycling parameters were modified to: 94°C for 1 min; followed by four cycles at 94°C for 30 s, 70°C for 30 s and 68°C for 3 min 30 s; followed by four cycles at 94°C for 30 s, 65°C for 30 s and 68°C for 3 min 30 s, followed by 30 cycles at 94°C for 30 s, 60°C for 30 s and 68°C for 3 min 30 and a final extension at 68°C for 10 min.
All the genes were excised by restriction digest or PCR and cloned into the pcDNA® expression vector system (Invitrogen, Carlsbad, CA) under the control of the Pcmv promoter and were sequenced before transfection.
Transfections
J-β2.7 cells in log phase were washed once and 8 × 106 cells were resuspended in 0.7 ml of RPMI media in a 4 mm cuvette. The cell suspensions were mixed with 25 μg of a recombinant plasmid containing the appropriate integrin cDNA insert and electroporated at 240 V and 960 μF. (Weber et al., 1997) on a Gene Pulser XCell® (Bio-Rad Laboratories, Hercules, CA). The transfected cells were then cultured in complete RPMI 1640 medium for 2 days and transferred to RPMI 1640 supplemented with 20% FCS and 1 mg ml−1 G418 or 5 μg ml−1 Blasticidin® to select for stable transfectants.
Selection of positive clones by flow cytometry
Flow cytometry with TS1/22 or TS1/18 mAbs (ATCC, Manassas, VA) and FITC-labelled anti-mouse secondary antibodies (Southern Biotech, Birmingham, AL) was used to isolate J-β2.7 cells expressing transfected integrins. The normal Poisson distribution of integrin expression present in any clonal cell line allowed us to isolate and enrich mouse × human, bovine × human and human × human transfectants that had equal expression of the LFA-1 heterodimer on their cell surface as judged by their reactivity to our antibody panel.
Confocal microscopy
Fluorescent microscopy has been described previously (Fong et al., 2006). Briefly, CD11a was detected by incubating 3 × 106 cells with biotin-conjugated goat anti-human CD11a or rat anti-mouse CD11a antibody for 30 min at 37 C, and then adding Alexa-Fluor-647®-streptavidin. Human CD18 was detected with monoclonal anti-human CD18 followed by Alexa-Fluor-488® goat anti-mouse antibody. Images were acquired with a Nikon Eclipse TE300 microscope (100 × objective), connected to a Bio-Rad Radiance® Confocal Scanning System using an argon laser (γ = 488) and/or a red diode laser (γ = 637) and analysed using the Laser Sharp 2000® Software (Bio-Rad Laboratories, Hercules, CA).
Leukotoxin purification
Aggregatibacter actinomycetemcomitans (strain JP2) bacteria were inoculated into 24 l of brain heart infusion media (DIFCO) and incubated overnight at 37 degrees. The cells were harvested using a 0.45 μM Pellicon cassette (Millipore) and then incubated for 1 h on an orbital shaker in 0.01 M Phosphate pH 6.5 containing 4% polymyxin B sulphate and protease inhibitors, then centrifuged at 9700 g, 4°C for 30 min. The cell pellet was discarded and the supernatant filtered, passed over a HiTrap SP® column (Pharmacia) and eluted in 0.01 M Phosphate/60% NaCl. Purity was verified by Western and cytotoxicity inhibition using anti-Ltx mAb107 (Lally et al., 1994).
Cytotoxicity
Cell viability was determined using the trypan blue exclusion assay. Cells in PBS/0.1% BSA were incubated in triplicate with 10−8 M Ltx for 3 h. An equal volume of 0.4% trypan blue in PBS was added and the viable cells counted. Per cent kill was determined by calculating the per cent difference between live Ltx treated and untreated cells and subtracting from 100. Ltx heated to 70°C for an hour served as a negative control.
Protein identification by mass spectrometry
2 × 108 J-β2.7 cells transfected with bovine CD11a were lysed, immunoprecipitated with anti-human CD18 (TS1/18) run on a 7.5% SDS-PAGE gel and stained with colloidal blue. The stained gel slices were excised and digested in-gel with trypsin as previously described (Speicher et al., 2000). Tryptic peptides were analysed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on an LTQ-FT mass spectrometer (Thermo Electron, San Jose, CA) coupled with a NanoLC pump (Eksigent Technologies, Livermore, CA) and autosampler essentially as previously described (Tang et al., 2005). Peptide sequences were interpreted from MS/MS spectra by searching against the National Center for Biotechnology Information (NCBI) non-redundant database (June 2006) using SEQUEST in the Proteomics Browser suite of programs (Thermo Electron, San Jose, CA). Outputs from SEQUEST searches were filtered using Xcorr ≥ 1.9 (z = 1), 2.2 (z = 2), 3.75 (z = 3) AND ΔCn ≥ 0.1, and protein identifications manually verified. Keratins, trypsin and other common contaminants were removed from the data sets.
Supplementary Material
PCR primer sequences used for constructing chimeras.
Acknowledgments
We thank Dr Martyn Robinson (Celltech) for human and mouse CD11a cDNAs and Dr Timothy A. Springer (Harvard) for plasmids h654m and m654h. We thank Sylvia Decker and Bridget J. Gallagher for expert help with confocal microscopy and image analyses. Dr Joel Rosenbloom provided us with many helpful suggestions and criticisms during the preparation of the manuscript. This work was supported by DE09517 and DE12305.
Footnotes
Supplementary material
The following supplementary material is available for this article online:
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1462-5822.2007.00989.x
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ambagala TC, Ambagala AP, Srikumaran S. The leukotoxin of Pasteurella haemolytica binds to β2 integrins on bovine leukocytes. FEMS Microbiol Lett. 1999;179:161–167. doi: 10.1111/j.1574-6968.1999.tb08722.x. [DOI] [PubMed] [Google Scholar]
- Champe M, McIntyre BW, Berman PW. Monoclonal antibodies that block the activity of leukocyte function-associated antigen 1 recognize three discrete epitopes in the inserted domain of CD11a. J Biol Chem. 1995;270:1388–1394. doi: 10.1074/jbc.270.3.1388. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T, Pellett S, Welch RA. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett T, Zecchinon L, Baise E, Desmecht D. The bovine (Bos taurus) CD11a-encoding cDNA: molecular cloning, characterisation and comparison with the human and murine glycoproteins. Gene. 2004;325:97–101. doi: 10.1016/j.gene.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Fong KP, Pacheco CMF, Otis LL, Baranwal S, Kieba IR, Harrison G, et al. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Springer TA. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1) J Biol Chem. 1995;270:19008–19016. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- Huang C, Springer TA. Folding of the β-propeller domain of the integrin αL subunit is independent of the I domain and dependent on the β2 subunit. Proc Natl Acad Sci USA. 1997;94:3162–3167. doi: 10.1073/pnas.94.7.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Lu C, Springer TA. Folding of the conserved domain but not of flanking regions in the β2 integrin subunit requires association with the α subunit. PNAS. 1997;94:3156–3161. doi: 10.1073/pnas.94.7.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zang Q, Takagi J, Springer TA. Structural and functional studies with antibodies to the integrin β2 subunit. A model for the I-like domain. J Biol Chem. 2000;275:21514–21524. doi: 10.1074/jbc.M002286200. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan S, Hsuan SL, Kannan MS, Walcheck B, Wang JF, Kehrli ME, et al. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect Immun. 2000;68:72–79. doi: 10.1128/iai.68.1.72-79.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y, Tseng E, Springer TA. Cloning of the murine lymphocyte function-associated molecule-1 alpha-subunit and its expression in COS cells. J Immunol. 1991;147:369–374. [PubMed] [Google Scholar]
- Kishimoto TK, O’Connor K, Lee A, Roberts TM, Springer TA. Cloning of the β subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987;48:681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Lally ET, Kieba IR. Molecular biology of Actinobacillus actinomycetemcomitans leukotoxin. In: Hamada S, Genco RJ, Lehner T, editors. Molecular Pathogenesis of Periodontal Disease. Washington, DC: American Society For Microbiology; 1994. pp. 69–82. [Google Scholar]
- Lally ET, Kieba IR, Taichman NS, Rosenbloom J, Gibson CW, Demuth DR, et al. Actinobacillus actinomycetemcomitans leukotoxin is a calcium-binding protein. J Periodontal Res. 1991;26:268–271. doi: 10.1111/j.1600-0765.1991.tb01655.x. [DOI] [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J Biol Chem. 1994;269:31289–31295. [PubMed] [Google Scholar]
- Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- Landis RC, McDowall A, Holness CL, Littler AJ, Simmons DL, Hogg N. Involvement of the ‘I’ domain of LFA-1 in selective binding to ligands ICAM-1 and ICAM-3. J Cell Biol. 1994;126:529–537. doi: 10.1083/jcb.126.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RS, Corbi AL, Berman L, Springer T. Primary structure of the leukocyte function-associated molecule- 1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989;108:703–712. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SK, Gagnon J, Hildreth JE, Wells CE, Willis AC, Wong AJ. The primary structure of the β-subunit of the cell surface adhesion glycoproteins LFA-1, CR3 and p150,95 and its relationship to the fibronectin receptor. EMBO J. 1987;6:915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hogg N. Effects of I domain deletion on the function of the β2 integrin lymphocyte function-associated antigen-1. Mol Biol Cell. 2000;11:677–690. doi: 10.1091/mbc.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Clinkenbeard KD, Ritchey JW. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol. 1999;67:91–97. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- Lu C, Ferzly M, Takagi J, Springer TA. Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J Immunol. 2001;166:5629–5637. doi: 10.4049/jimmunol.166.9.5629. [DOI] [PubMed] [Google Scholar]
- Marlin SD, Morton CC, Anderson DC, Springer TA. LFA-1 immunodeficiency disease. Definition of the genetic defect and chromosomal mapping of α and β subunits of the lymphocyte function-associated antigen 1 (LFA-1) by complementation in hybrid cells. J Exp Med. 1986;164:855–867. doi: 10.1084/jem.164.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin AG. Structural principles for the propeller assembly of β-sheets: the preference for seven-fold symmetry. Proteins. 1992;14:191–201. doi: 10.1002/prot.340140206. [DOI] [PubMed] [Google Scholar]
- Nørskov-Lauritsen N, Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb nov., Aggregatibacter aphrophilus comb nov & Aggregatibacter segnis comb nov & emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int J Syst Evol Microbiol. 2006;56:2135–2146. doi: 10.1099/ijs.0.64207-0. [DOI] [PubMed] [Google Scholar]
- Ortlepp S, Stephens PE, Hogg N, Figdor CG, Robinson MK. Antibodies that activate β-2 integrins can generate different ligand binding states. Eur J Immunol. 1995;25:637–643. doi: 10.1002/eji.1830250302. [DOI] [PubMed] [Google Scholar]
- Qu A, Leahy DJ. Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, αLβ2) integrin. Proc Natl Acad Sci USA. 1995;92:10277–10281. doi: 10.1073/pnas.92.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewen PE, Wilkie BN. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher KD, Kolbas O, Harper S, Speicher DW. Systematic analysis of peptide recoveries from in-gel digestions for protein identifications in proteome studies. J Biomol Tech. 2000;11:74–86. [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Predicted and experimental structures of integrins and β-propellers. Curr Opin Struct Biol. 2002;12:802–813. doi: 10.1016/s0959-440x(02)00384-6. [DOI] [PubMed] [Google Scholar]
- Stephens P, Romer JT, Spitali M, Shock A, Ortlepp S, Figdor CG, Robison MK. KIM127, an antibody that promotes adhesion, maps to a region of CD18 that includes cysteine-rich repeats. Cell Adhes Commun. 1995;3:375–384. doi: 10.3109/15419069509081292. [DOI] [PubMed] [Google Scholar]
- Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J, et al. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Tang HY, Ali-Khan N, Echan LA, Levenkova N, Rux JJ, Speicher DW. A novel four-dimensional strategy combining protein and peptide separation methods enables detection of low-abundance proteins in human plasma and serum proteomes. Proteomics. 2005;5:3329–3342. doi: 10.1002/pmic.200401275. [DOI] [PubMed] [Google Scholar]
- Wang JF, Kieba IR, Korostoff J, Guo TL, Yamaguchi N, Rozmiarek H, et al. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb Pathog. 1998;25:317–331. doi: 10.1006/mpat.1998.0236. [DOI] [PubMed] [Google Scholar]
- Weber KS, York MR, Springer TA, Klickstein LB. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the αL and β2 subunits are interdependent for cell surface expression. J Immunol. 1997;158:273–279. [PubMed] [Google Scholar]
- Weber KS, Klickstein LB, Weber C. Specific activation of leukocyte β2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the α subunit cytoplasmic domains. Mol Biol Cell. 1999;10:861–873. doi: 10.1091/mbc.10.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili P, Lu C, Oxvig C, Springer TA. Folding and function of I domain-deleted Mac-1 and lymphocyte function-associated antigen-1. J Biol Chem. 2000;275:21877–21882. doi: 10.1074/jbc.M908868199. [DOI] [PubMed] [Google Scholar]
- Zang Q, Lu C, Huang C, Takagi J, Springer TA. The top of the inserted-like domain of the integrin lymphocyte function-associated antigen-1 β subunit contacts the α subunit β-propeller domain near β-sheet 3. J Biol Chem. 2000;275:22202–22212. doi: 10.1074/jbc.M002883200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primer sequences used for constructing chimeras.


