Abstract
Introduction
Pervious biochemical and hemodymanic studies have highlighted the important role of εPKC in cardioprotection during ischemic preconditioning. However, little is known about the electrophysiological consequences of εPKC modulation in ischemic hearts. Membrane permeable peptide εPKC selective activator and inhibitor were used to investigate the role of εPKC modulation in reperfusion arrhythmias.
Methods
Protein transduction domain from HIV- TAT was used as a carrier for peptide delivery into intact Langendorff perfused guinea pig hearts. Action potentials were imaged and mapped (124 sites) using optical techniques and surface ECG was continuously recorded. Hearts were exposed to 30 min stabilization period, 15 min of no-flow ischemia, followed by 20 min reperfusion. Peptides (0.5 μM) were infused as follows: a) control (vehicle-TAT peptide; TAT-scrambled ψεRACK peptide); b) εPKC agonist (TAT-ψεRACK); c) εPKC antagonist (TAT-εV1).
Results
Hearts treated with εPKC agonist ψεRACK had reduced incidence of ventricular tachycardia (VT, 64%) and fibrillation (VF, 50%) compared to control (VT, 80%, p<0.05) and (VF, 70%, P<0.05). However, the highest incidence of VT (100%, P<0.05) and VF (80%) occurred in hearts treated with εPKC antagonist peptide εV1 compared to control and to εPKC agonist ψεRACK. Interestingly, at 20 min reperfusion, 100% of hearts treated with εPKC agonist ψεRACK exhibited complete recovery of action potentials compared to 40% (p<0.05) of hearts treated with εPKC antagonist peptide, εV1 and 65% (P<0.5) of hearts in control. At 20 min reperfusion, maps of action potential duration from εPKC agonist ψεRACK showed minimal dispersion (48.2±9 ms) compared to exacerbated dispersion (115.4±42 ms, P<0.05) in εPKC antagonist and control (67±20 ms, P<0.05). VT/VF and dispersion from hearts treated with scrambled agonist or antagonist peptides were similar to control.
In conclusion
the results demonstrate that εPKC activation by ψεRACK peptide protects intact hearts from reperfusion arrhythmias and affords better recovery. On the other hand, inhibition of εPKC increased the incidence of arrhythmias and worsened recovery compared to controls. The results carry significant therapeutic implications for the treatment of acute ischemic heart disease by preconditioning-mimicking agents.
Keys Words: cardiac electrophysiology, Protein Kinase C, reperfusion arrhythmia, optical mapping
Introduction
Recently, the identification of novel therapeutic targets for the prevention of ischemia/reperfusion-induced cardiac injury has gained considerable attention due to the importance of cardiovascular disease as a public healthcare problem worldwide [1]. Ischemic preconditioning is a powerful phenomena which confers protection to hearts subjected to brief episodes of ischemia prior to the subsequent ischemic insult [2; 3]. There is a strong evidence supporting the involvement of εPKC activation in preconditioning [4; 5; 6]. If indeed activation of εPKC is required for cardioprotection, an εPKC selective peptide activator would be expected to act as a preconditioning mimetic. Although hemodynamic and biochemical data using an εPKC-selective agonist in mouse [5; 7] and porcine [8] hearts demonstrated that εPKC translocation is required for protection from ischemic injury, the detailed electrophysiological effects of εPKC isozyme modulation in intact hearts is lacking. In this study, we delivered selective membrane permeable peptide εPKC modulators (activator and inhibitor) into intact whole hearts to investigate the electrophysiological role of PKC isozymes in reperfusion arrhythmias. Protein transduction domain from HIV-TAT peptide [8; 9] was used as a carrier for peptide delivery and optical mapping technique was used to record action potentials in Langendorff perfused guinea pig hearts.
Material and Methods
Surgical Procedure and Experimental Setup
All experimental protocols were approved by the Animal Studies Subcommittee of the Department of Veterans Affairs, New York Harbor Healthcare System and carried out in accordance with EU Directive 2010/63/EU for animal experiments.
The details of the surgical procedure have been described elsewhere [10; 11]. Briefly, Dunkin-Hartley guinea pigs of either sex, weighing between 300 and 400 g, were anesthetized by intraperitoneal injection of sodium pentobarbital (30 mg/kg) and heparinized (1000 U/kg). A mid-thoracotomy was performed and the hearts were rapidly excised and placed in cold oxygenated Tyrode’s solution containing 10,000 U/l heparin. The excised heart was rapidly cannulated at the aorta and retrogradely perfused in a modified Langendorff apparatus. The Tyrode’s solution contained (in mM): NaCl 130, KCI 4.75, CaCI2 1.0, MgSO4 1.2, NaHCO3 12.5, and glucose 15.0. The solution was continuously bubbled with 95%-O2/5%-CO2 through a fritted glass tube. Temperature was maintained at 36±0.3°C by monitoring the temperature of the perfusate within the closed chamber.
Langendorff Preparation and Perfusion Chamber
Experiments were started after the heart was stabilized and a steady perfusion pressure of 60–70 mm Hg during sinus rhythm was maintained. A custom designed perfusion chamber was used for studying intact hearts using optical recording methods [10; 12; 13; 14]. Bipolar surface electrograms were recorded using Teflon-coated silver wires (250-μm diameter) exposed at the tip and chlorided with an interpolar distance of 500 μm and positioned separately on the epicardial surface.
Fluorescent Dye Staining
A dye, di-4-ANEPPS (Molecular Probes; Eugene, OR), was used as the potentiometric fluorescent probe. Dye fluorescence was measured at wavelengths above the 645-nm cutoff filter when excited with a 520±20 nm interference filter [10; 11; 12; 13; 14]. Hearts were stained by gradual injection of 40 to 60 μl from a 2.5 mM stock solution of dye into a 5 ml bubble trap situated directly above the aortic cannula. The final dye concentration was approximately 1.8 μM; 10 to 15 minutes was allowed for the staining to complete. For longer protocols for which photobleaching and/or dye washout may reduce the optical signal amplitudes, hearts were restrained with smaller amounts of dye (5–10 μl) to restore the original signal-to-noise ratio.
Instrument Setup
Details of the optical and recording apparatus have been described elsewhere [10; 13; 14]. The epicardial surface was illuminated with light from two 45 W tungsten halogen lamps, which was collimated and passed through 520±20 nm interference filters. A 45° mirror in the optical apparatus was used to focus the grid pattern on the region of interest using a 35 mm camera lens (50 mm, F1:1.4, Nikon). Epi-fluorescent light from the stained heart was collected, projected through a 645 nm cutoff filter, and focused to form an image of the heart on a 12×12 element photodiode array. 124 diodes were current to voltage converted and sampled.
Experimental Design
Hearts were subjected to a 30-min control stabilization period, followed by 15-min no-flow ischemia and then 20-min reperfusion. Optical recordings were performed every 2–5 min during the stabilization/perfusion period, every 2–3 min during ischemia, immediately following reperfusion and every 2–3 min thereafter. Permeable peptide (0.5 μM) modulators of εPKC isozyme, their scrambled peptides or vehicles were administered for 30-min prior to ischemia. The membrane permeable peptides, ψεRACK; (εV1–7 [HDAPIGYD]), which activates εPKC translocation and function [5; 15] and εV1–2 [EAVSLKPT], which inhibits the translocation and function [5; 15]of εPKC were conjugated to TAT peptide [YGRKKRRQRRR] via cysteine bond at the N-terminus for permeability [6; 8]. For controls, we used scrambled peptides for ψεRACK [CPDYHDAGI], εV1–2 [LSETKPAV]) and vehicle alone (TAT-peptide). Effective delivery of εPKC modulator peptides to intact hearts has been tested and demonstrated [8]. The peptides were synthesized from Genemed Synthesis, South San Francisco, CA and at the Protein and Nucleic Acid Facility, Stanford University, Stanford, CA. All peptides used were over 90% pure.
Signal Acquisition and Data Analysis
Activation time was defined as the peak temporal derivative of the action potential upstroke and recovery was defined as the point of maximum second derivative during repolarization. VT was defined as a successive run of at least 10 premature ventricular contractions. Data are presented as means ± SE. The number of experiments (n) indicates the number of hearts used. Arrhythmia and optical mapping data from peptides treated and controls are compared by paired or unpaired Student’s t-test as appropriate. A value of P<0.05 was considered significant.
Results
A total of 41 hearts were used as follows: 11 control hearts which did not receive any treatment; 8 hearts were treated with εPKC agonist peptide, ψεRACK; 8 hearts were treated with εPKC antagonist peptide, εV1; 6 hearts were treated with scrambled εPKC agonist ψεRACK (negative control); 4 hearts were treated with scrambled εPKC antagonist peptide (negative control) and 4 hearts were treated with TAT alone (vehicle).
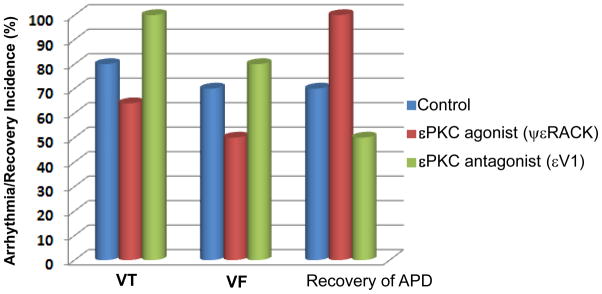
Hearts treated with εPKC agonist ψεRACK had significantly reduced incidence of ventricular tachycardia, VT (64%) and fibrillation, VF (50%) compared to control VT (80%, p<0.05) and VF (70%, P<0.05). However, the highest incidence of VT (100%, P<0.05) and VF (80% (P<0.05) occurred in hearts treated with εPKC antagonist peptide εV1 compared to control and to εPKC agonist ψεRACK (Figure 1). At the end of reperfusion (20 min), hearts treated with εPKC agonist ψεRACK had 100 % recovery of action potential duration measured at 90% repolarization (APD90) compared to 40% (P<0.05) of hearts treated with εPKC antagonist peptide εV1 and 65% (P<0.05) in control hearts (Figure 1). The incidence of arrhythmias and APD90 recovery in hearts treated with scrambled peptides or vehicle was similar to control hearts (Table 1).
Figure 1. Incidence of ventricular arrhythmia and recovery of action potentials during reperfusion in guinea-pig hearts.
The incidence of ventricular tachycardia (VT), ventricular fibrillation (VF) and recovery of action potential duration at 90% (APD90) at 20 min reperfusion are shown for untreated control, εPKC agonist ψεRACK (0.5 μM) and εPKC antagonist εV1 (0.5 μM) hearts. Hearts treated with εPKC agonist ψεRACK had the lowest incidence of VT/VF and complete recovery of APD90 compared with hearts treated with εPKC antagonist εV1 and control.
Table 1.
Summary of arrhythmic events
| N | VT(%) | VF(%) | Recovery of APD90 (%) | |
|---|---|---|---|---|
| Control | 11 | 80 | 70 | 65 |
| ε-PKC agonist ψ εRACK | 8 | 64 | 50 | 100 |
| ε-PKC antagonist εV1 | 8 | 100 | 80 | 40 |
| Scrambled ε-PKC agonist ψ εRACK | 6 | 85 | 65 | 60 |
| Scrambled ε-PKC antagonist εV1 | 4 | 90 | 70 | 80 |
| TAT-vehicle | 4 | 75 | 70 | 70 |
VT: Ventricular Tachycardia; VF: Ventricular Fibrillation; APD90: Action Potential Duration at 90% repolarization
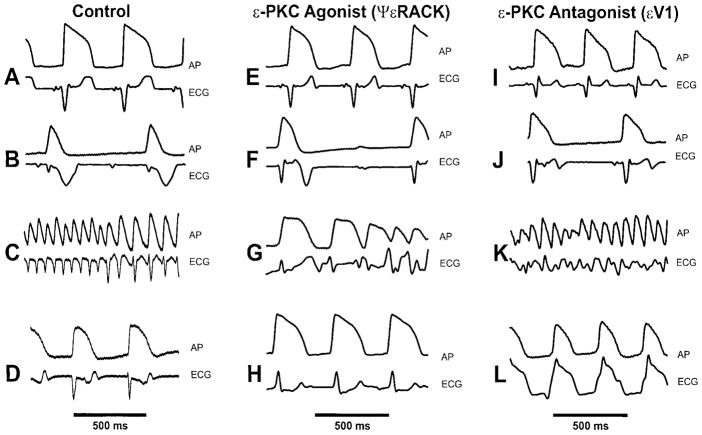
Figure 2 illustrates selected action potentials and surface ECG recorded from control, εPKC agonist ψεRACK and εPKC antagonist εV1 during stabilization period (panels A,E, I), 15 min of ischemia (panels B, F, J), during early reperfusion (panels C,G, K) and at the end of 20 min reperfusion (panels D,H, L). Ischemia resulted in action potential shortening in control, εPKC agonist ψεRACK and εPKC antagonist εV1 (panels B, F and J respectively). Early reperfusion caused VF in both control (panel C) and εPKC antagonist εV1 (panel K), but only a self terminating VT run (panel G) in the εPKC agonist ψεRACK. At the end of reperfusion (20 min), complete recovery of action potential (panel H) was observed in the εPKC agonist ψεRACK, however a slow VT was still present in the εPKC antagonist εV1 (panel L) and incomplete recovery of action potentials in control (panel D) was noted.
Figure 2. Action potentials and electrocardiograms from guinea pig hearts.
Action potentials and ECGs were recorded from untreated control, εPKC agonist ψεRACK and εPKC antagonist hearts. In the control, (A) represents recordings at 30 min stabilization period, (B) at 15 min global ischemia, (C) at 10 sec of reperfusion with an onset of ventricular fibrillation (VF) and (D) at 20 min reperfusion demonstrating partial recovery of action potentials. In the εPKC agonist ψεRACK (0.5 μM), (E) represents recordings at 30 min stabilization period, (F) at 15 min global ischemia, (G) at 30 seconds reperfusion with self terminated ventricular tachycardia (VT) and (H) at 20 min reperfusion demonstrating full recovery of action potentials. In the εPKC antagonist εV1 (0.5 μM), (I) represents recordings at 30 min stabilization period, (J) at 15 min global ischemia, (K) at 20 seconds reperfusion showing VT and (L) at 20 min reperfusion demonstrating poor recovery of action potential and ECG with VT still present.
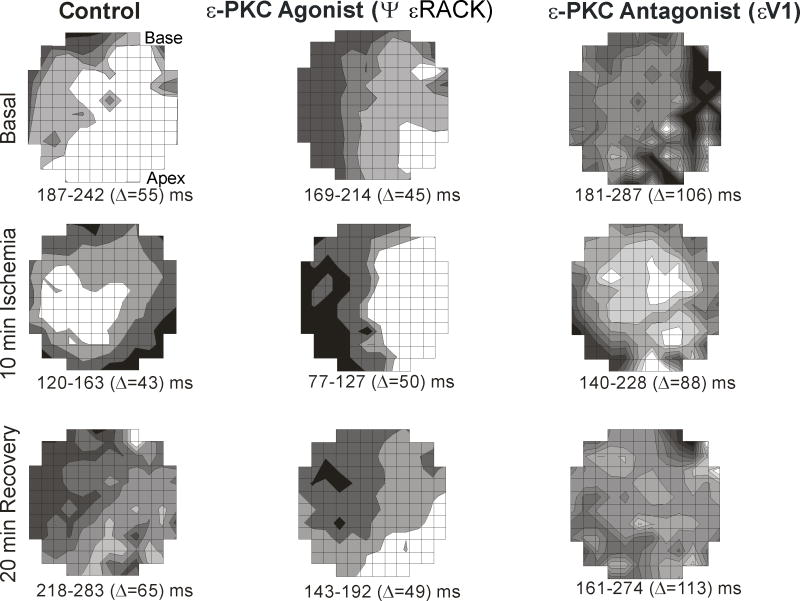
To further characterize the electrophysiologic properties of εPKC modulation, action potential duration activation maps were obtained. In these maps (Figure 3), each shaded zone represents an isochronal region activated at successive 10 ms intervals. The high incidence of VT/VF in the control and εPKC antagonist εV1 correlated with the high dispersion of APD90 during reperfusion compared to εPKC agonist ψεRACK which showed minimal dispersion. At 20 min reperfusion, the average dispersion of APD90 in the εPKC agonist ψεRACK, εPKC antagonist εV1 and control was 48.2±9 ms, 115.4±42 ms and 67±20 ms respectively (p<0.05 when compared to control). Figure 3 shows that APD90 dispersion at 20 min reperfusion was 65 ms, 49 ms and 113 ms during control, εPKC agonist ψεRACK and εPKC antagonist εV1 respectively. Dispersion APD90 was the highest in the εPKC antagonist εV1 compared to control and εPKC agonist ψεRACK during not only basal condition, but also during ischemia and reperfusion. Figure 3 shows ADP90 dispersion of 106 ms, 88 ms and 113 ms during basal condition, 10 min ischemia and at 20 min reperfusion respectively. On the other hand, APD90 dispersion in control and εPKC agonist ψεRACK were 55 ms, 43 ms and 65 ms vs. 45 ms, 50 ms and 49 ms during basal condition, 10 min ischemia and 20 min reperfusion respectively.
Figure 3. Maps illustrating spatial distribution of action potentials recorded from guinea pig hearts.
The maps were obtained at 10 ms isochrones of action potential duration at 90% (APD90) during basal conditions, 10 min ischemia and 20 min reperfusion. During basal conditions (prior to ischemia), the control untreated hearts and εPKC agonist ψεRACK treated hearts, APD90 had less dispersion of 55 ms and 45 ms, respectively compared with εPKC antagonist εV1 treatment (106 ms). During ischemia, the εPKC antagonist εV1 treatment resulted in the worst spatial heterogeneity of the shortening of APD90 with the highest dispersion (88 ms) compared to control (43 ms) and εPKC agonist ψεRACK (50 ms). At the end of the 20 min reperfusion, εPKC agonist ψεRACK) treatment resulted in full recovery of APD90 dispersion (49 ms) similar to the dispersion prior to ischemia under basal conditions (45 ms). However, in the εPKC antagonist εV1 treated hearts, APD90 dispersion was the worst (113 ms) compared to untreated control (65 ms) and to εPKC agonist εV1 (49 ms).
Discussion
The primary finding of this study is that in the intact isolated heart, εPKC activation by ψεRACK peptide (εPKC agonist) resulted in a significant reduction of the incidence of reperfusion induced VT/VF likely by reducing APD dispersion and by affording better action potential recovery. This novel anti-arrhythmic effect of the ψεRACK peptide has significant therapeutic implications for patients with ischemic heart diseases and in the development of new preconditioning mimicking agents.
Comparison with Previous Studies
The findings from this study are consistent with biochemical data demonstrating that ψεRACK peptide is cardioprotective against ischemic injuries when delivered in vivo before the ischemic period [8]. Specifically, we showed that ψεRACK peptide treatment resulted in significant reduction in infarct size, CPK release and improved ejection fraction. More importantly, ψεRACK peptide continuous delivery conferred sustained εPKC activation without desensitization as seen in the settings of adenosine agonists or brief period of ischemic preconditioning [8]. The present data are also consistent with previous work in transgenic mice where moderate in vivo activation of εPKC protects the ischemic heart from reperfusion arrhythmias [7] and moderate in vivo inhibition of εPKC exacerbates the incidence of reperfusion arrhythmia [7]. Collectively, the present electrophysiological data demonstrating that post-reperfusion arrhythmias are significantly reduced by the ψεRACK peptide εPKC agonist together with the above biochemical data showing cardioprotection from myocardial injuries point to a unique and potential novel pharmacological approach in treating both arrhythmias and myocardial injuries related to acute ischemic heart disease.
Potential Mechanism (s) of Anti-arrhythmic Effect of εPKC Activation
Although, previous in vitro and in vivo studies using εPKC modulating peptides and εPKC transgenic mice have shown that εPKC activation significantly improved the hemodynamic of the heart, attenuated cellular damage, and reduced infarction size [5; 8; 16], the role of εPKC in ischemia-reperfusion related ventricular arrhythmias is poorly understood. Using optical maps, we have previously demonstrated that spontaneous Ca oscillations during ischemia/reperfusion resulted in premature ventricular beats that initiated runs of polymorphic VT/VF in a guinea-pig heart [12]. This tachyarrhythmia results in further increase in the level of Ca transient (CaiT). Tachyarrhythmia-induced increase in CaiT and the degeneration of the arrhythmia to VF may be related to the development of fast spontaneous Ca oscillations and/or Ca induced cell-to-cell uncoupling [12]. Using transgenic mice with specific cardiac constitutive activation of εPKC, we found that sustained εPKC resulted in reduced basal Ca current density as well as blunted β-adrenergic activation of Ca current [17] both of which are beneficial in the settings of undesirable catecholamine release during ischemia [18; 19].
PKC regulates several ion channels including Na, K and Ca channels by phosphorylation [20; 21; 22]. Because these channels play critical role in action potential genesis and conduction, alteration in these channels function lead to abnormal electrical activity and arrhythmias. Previous data showed that εPKC activation consistently inhibited Ca and Na channels [20; 22] but increased the K channel (IKs). The combined effect of εPKC activation on these channels may limit the amount of Ca entering the cell during ischemia especially the inhibition of the Ca current (less Ca entering the cell) combined with the activation of the K current, IKs (shortening of action potential thus limiting amount of Ca entry to the cell). Thus intracellular Ca accumulation during ischemia may play an important role in reperfusion arrhythmias through afterdepolarizations [11; 12; 23]. Consistent with the concept that interventions which limit Ca entry to the cell during ischemia, may reduce or eliminate reperfusion arrhythmias, earlier clinical and experimental studies have shown that verapamil, a Ca channel blocker, terminated reperfusion related arrhythmia [24; 25]. However the negative hemodynamic side undermined its usefulness in the clinical settings. Unlike verapamil, ψεRACK peptide does not affect the hemodynamic parameters, heart rate and does not cause desensitization or downregulation of εPKC in a porcine myocardial infarction model [8] making ψεRACK peptide a useful therapeutic agent for patients with ischemic heart disease. Altogether, this study demonstrates that in addition to the cardioprotective effects, εPKC activation by ψεRACK peptide also suppressed the ischemia-reperfusion related ventricular tachyarrhythmia, thus providing a new therapeutic approach using preconditioning mimetic agents to manage patients with ischemic heart diseases.
Highlights.
Optical mapping technique was used in hearts to assess reperfusion arrhythmia.
Peptide specific activator of epsilon PKC had anti-arrhythmic effects.
Peptide specific inhibitor of epsilon PKC had pro-arrhythmic effects.
Results are clinically relevant to the treatment of acute ischemic heart disease.
Acknowledgments
This work has been supported by VA Merit grant and National Heart, Lung and Blood Institute Grant #HL077494 (to M.B).
Footnotes
This paper is dedicated in memory of Dr Mark Restivo
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990;82:609–19. doi: 10.1161/01.cir.82.2.609. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–14. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 5.Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96:12798–803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue Y, Qu Y, Boutjdir M. Protective role of protein kinase C epsilon activation in ischemia-reperfusion arrhythmia. Biochem Biophys Res Commun. 2006;349:432–8. doi: 10.1016/j.bbrc.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 9.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 10.Restivo M, Caref EB, Kozhevnikov DO, El-Sherif N. Spatial dispersion of repolarization is a key factor in the arrhythmogenicity of long QT syndrome. J Cardiovasc Electrophysiol. 2004;15:323–31. doi: 10.1046/j.1540-8167.2004.03493.x. [DOI] [PubMed] [Google Scholar]
- 11.Lakireddy V, Baweja P, Syed A, Bub G, Boutjdir M, El-Sherif N. Contrasting effects of ischemia on the kinetics of membrane voltage and intracellular calcium transient underlie electrical alternans. Am J Physiol Heart Circ Physiol. 2005;288:H400–7. doi: 10.1152/ajpheart.00502.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lakireddy V, Bub G, Baweja P, Syed A, Boutjdir M, El-Sherif N. The kinetics of spontaneous calcium oscillations and arrhythmogenesis in the in vivo heart during ischemia/reperfusion. Heart Rhythm. 2006;3:58–66. doi: 10.1016/j.hrthm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Restivo M, Kozhevnikov DO, Boutjdir M. Optical mapping of activation patterns in an animal model of congenital heart block. Am J Physiol Heart Circ Physiol. 2001;280:H1889–95. doi: 10.1152/ajpheart.2001.280.4.H1889. [DOI] [PubMed] [Google Scholar]
- 14.Himel HDt, Bub G, Yue Y, El-Sherif N. Early voltage/calcium uncoupling predestinates the duration of ventricular tachyarrhythmias during ischemia/reperfusion. Heart Rhythm. 2009;6:1359–65. doi: 10.1016/j.hrthm.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–51. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–75. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 17.Yue Y, Qu Y, Boutjdir M. Beta- and alpha-adrenergic cross-signaling for L-type Ca current is impaired in transgenic mice with constitutive activation of epsilonPKC. Biochem Biophys Res Commun. 2004;314:749–54. doi: 10.1016/j.bbrc.2003.12.155. [DOI] [PubMed] [Google Scholar]
- 18.Schomig A, Dart AM, Dietz R, Mayer E, Kubler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: Locally mediated release. Circ Res. 1984;55:689–701. doi: 10.1161/01.res.55.5.689. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson L. Mechanisms of local noradrenaline release in acute myocardial ischemia. Acta Physiol Scand Suppl. 1987;559:1–85. [PubMed] [Google Scholar]
- 20.Xiao GQ, Qu Y, Sun ZQ, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Na(+) channels. Am J Physiol Cell Physiol. 2001;281:C1477–86. doi: 10.1152/ajpcell.2001.281.5.C1477. [DOI] [PubMed] [Google Scholar]
- 21.Xiao GQ, Mochly-Rosen D, Boutjdir M. PKC isozyme selective regulation of cloned human cardiac delayed slow rectifier K current. Biochem Biophys Res Commun. 2003;306:1019–25. doi: 10.1016/s0006-291x(03)01095-7. [DOI] [PubMed] [Google Scholar]
- 22.Hu K, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Ca(2+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H2658–64. doi: 10.1152/ajpheart.2000.279.6.H2658. [DOI] [PubMed] [Google Scholar]
- 23.Opie LH. Reperfusion injury and its pharmacologic modification. Circulation. 1989;80:1049–62. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Dote K, Sasaki S, Takemoto H, Habara S, Hasegawa D. Intracoronary verapamil rapidly terminates reperfusion tachyarrhythmias in acute myocardial infarction. Chest. 2004;126:702–8. doi: 10.1378/chest.126.3.702. [DOI] [PubMed] [Google Scholar]
- 25.Yu W, Wang JJ, Gan WY, Lin GS, Huang CX. Effects of verapamil preconditioning on cardiac function in vitro and intracellular free Ca2+ and L-type calcium current in rat cardiomyocytes post ischemia-reperfusion injury. Zhonghua Xin Xue Guan Bing Za Zhi. 38:225–9. [PubMed] [Google Scholar]