Abstract
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) inhibits endothelin-1 (ET-1)-induced activation of p44/42 mitogen-activated protein kinase (p44/42 MAPK) and collagen production in cultured rat cardiac fibroblasts (RCFs). However, we do not know whether its inhibitory effect on p44/42 MAPK is due to altered activity of protein tyrosine phosphatases (PTPs), which in turn down-regulate the p44/42 MAPK signaling pathway. Activity of Src homology 2-containing protein tyrosine phosphatase-2 (SHP-2) is downregulated by ET-1 in RCFs; thus, we hypothesized that Ac-SDKP inhibits ET-1-stimulated collagen production in part by preserving SHP-2 activity and thereby inhibiting p44/42 MAPK phosphorylation. When we stimulated RCFs with ET-1 in the presence or absence of Ac-SDKP, we found that a) PTP activity was reduced by ET-1 and b) this effect was counteracted by Ac-SDKP in a dose-dependent fashion. Next, we extracted SHP-2 from RCF lysates by immunoprecipitation and determined that a) ET-1 inhibited SHP-2 by 40% and b) this effect was prevented by Ac-SDKP. However, Ac-SDKP failed to inhibit ET-1-induced p44/42 MAPK phosphorylation in RCFs treated with SHP-2 short hairpin RNA (shRNA); in contrast, in cells transfected with control shRNA, Ac-SDKP’s inhibitory effect on ET-1-induced p44/42 MAPK activation, remained intact. Moreover, the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production was blunted in cells treated with the SHP-1/2 inhibitor NSC-87877. Thus, we concluded that the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production by RCFs is mediated in part by preserving SHP-2 activity and thereby preventing p44/42 MAPK activation. Ac-SDKP or its analogues could represent a new therapeutic tool to treat fibrotic diseases in cardiovascular system.
Keywords: Endothelin-1, cardiac fibroblasts, Src homology 2-containing protein tyrosine phosphatase-2, Ac-SDKP, collagen
Introduction
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), an endogenous tetrapeptide secreted by bone marrow cells [14,31], is widely found in various tissues [21] as well as plasma and circulating mononuclear cells [22] and is a natural substrate for angiotensin-converting enzymes (ACE) [2]. We found that Ac-SDKP inhibited p44/42 mitogen-activated protein kinase (p44/42 MAPK) phosphorylation and collagen production in cultured rat cardiac fibroblasts (RCFs) stimulated with endothelin-1 (ET-1) [23,34] and in the hearts from aldosterone-salt hypertensive rats [18]. Moreover, ET-1-induced collagen production was also inhibited by a specific MAPK kinase (MEK) inhibitor, PD 98059 [23]. However, the molecular mechanisms by which Ac-SDKP blunts p44/p42 activation are unknown.
ET-1 is not only a potent vasoconstrictor but also has very powerful fibrogenic properties. Indeed, it reportedly stimulates collagen production in cardiac fibroblasts [11,12] and also plays an important role in cardiac fibrosis in vivo [1,17]. Activation of ET-1 receptors in cardiac fibroblasts initiates a cascade of well-defined signaling pathways [16]. ET-1 rapidly stimulates MAPK activity in rat mesangial cells via at least two pathways: one is protein kinase C-dependent, while the second involves protein tyrosine kinases [8,28] and has also been shown to enhance fibroblast proliferation and production of collagen I and III [11,12]. While these tyrosine kinase phosphorylations are known to be regulated by protein tyrosine phosphatases (PTPs), it is not clear whether the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production could be mediated by altered PTP activity, thereby blocking p44/42 MAPK phosphorylation; since inhibition of PTP activity is sufficient per se to initiate a complete MAPK activation [33]. PTPs are conventionally thought to represent negative regulation, since they reverse the effects of protein tyrosine kinases; however, one PTP in particular, Src homology 2-1 containing protein tyrosine phosphatase-2 (SHP-2), has been shown to promote Ras activation by growth factors and cytokines [15] although it does negatively regulate the ET-1 signaling pathway in cardiac fibroblasts [4]. In addition, MAPK phosphatases (MKPs) have been shown to be important negative regulators of the MAPK cascade [13]. MKP-1, −2 and −3 are expressed in fibroblasts and negatively regulate p44/42 MAPK phosphorylation [3,10]; however, it is still not clear whether they play any regulatory role in ET-1-induced p44/42 MAPK activation. We hypothesized that Ac-SDKP inhibits collagen production in part by regulating SHP-2 and/or MKP activity and thereby blunting p44/42 MAPK activation in ET-1-stimulated cardiac fibroblasts. To test our hypothesis, we used ET-1-stimulated RCFs to determine whether 1) Ac-SDKP counteracts the regulatory effect of ET-1 on PTP activity, including SHP-2; 2) Ac-SDKP stimulates MKP-2 and/or MKP-3 to downregulate p44/42 MAPK activity; and/or 3) knockdown of SHP-2 by SHP-2 short hairpin RNA (shRNA) or a SHP-2 inhibitor alters the inhibitory effect of Ac-SDKP on p44/42 MAPK phosphorylation and collagen production.
Materials and Methods
Materials
Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT). Low glucose Dulbecco’s modified Eagle’s medium (DMEM) and cell culture materials were obtained from Invitrogen (Carlsbad, CA), ET-1 and Ac-SDKP from Bachem (Torrance, CA), sodium orthovanadate (Na3VO4) from Sigma (Saint Louis, MO), and malachite green-phosphatase assay kits from Promega (Madison, WI). Okadaic acid (OA), protein G plus/protein A-agarose and NSC-87877 (8-hydroxy-7-(6-sulfonaphthalen-2-yl)diazenyl-quinoline-5-sulfonic acid, disodium salt), a Src homology 2-containing protein tyrosine phosphatase (SHP)-1/2 inhibitor, were purchased from Calbiochem (Gibbstown, NJ). SHP-2 shRNA lentiviral particles and their controls along with related transfecting materials were obtained from Santa Cruz (Santa Cruz, CA). The mouse monoclonal antibody against SHP-1 came from BD Transduction Laboratories (Franklin Lakes, NJ), the rabbit polyclonal antibody against SHP-2 or MKP-3 from Santa Cruz who also provided us with normal rabbit IgG, and the rabbit antibodies against p44/42 MAPK, phosphorylated p44/42 MAPK and GAPDH from Cell Signaling Technology (Danvers, MA).
Cell Culture
Primary cultures of cardiac fibroblasts were derived from adult male Sprague-Dawley rats weighing 200 to 250 g (Charles River, Wilmington, MA) using a modified version of Eghbali’s method [9]. Fibroblasts were grown in low glucose DMEM with 10% FBS. Cells at passage 2 or 3 were made quiescent by culturing them in DMEM without FBS for 24 hours before each treatment. Cell viability was determined by counting cells on a hemocytometer in the presence of 0.04 % trypan blue. This protocol was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee (IACUC).
Western blot
Equal amounts of protein were subjected to 10% SDS-PAGE under reducing conditions and electrotransferred to a nitrocellulose membrane. Membranes were treated with blocking buffer (TBS/0.1% Tween 20 containing 5% nonfat dry milk) and incubated with the primary antibody overnight at 4°C. Bound antibodies were visualized using secondary antibodies combined with conjugated horseradish peroxidase (Cell Signaling Technology) and ECL reagent (Amersham Biosciences, Piscataway, NJ). After we located the primary targeted protein, we treated the blots with a stripping buffer (Pierce, Rockford, IL) and reblotted with an antibody against GAPDH or p44/42 MAPK. Band intensity was quantified by densitometry. SHP-2 and MKP-3 were normalized to GAPDH, and phosphorylated p44/42 MAPK to p44/42 MAPK. The final results are expressed as fold increase compared with control.
Protocols
Effect of phosphatase inhibitors on ET-1-stimulated phosphorylation of p44/42 MAPK
In order to test whether PTPs or serine/threonine phosphatases are involved in ET-1-stimulated p44/42 MAPK phosphorylation, we first examined the effect of Na3VO4 or OA on phosphorylation of p44/42 MAPK in cells stimulated with ET-1. Cells with 80% confluence in 6-well plates were pretreated with OA (10 nM) [25,29] or Na3VO4 (100 μM) [29] for 1 hour and then stimulated with ET-1 for 10 - 15 minutes. Whole cell lysates were prepared in 100 μl of a 1:1 mixture of 2 × reducing sample buffer (Bio-Rad) and RIPA lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) and maintained at −72°C until assayed for phosphorylated p44/42 MAPK.
Effect of Ac-SDKP on PTPs
Cells with 80% confluence in 100-mm tissue culture dishes were pre-incubated with 10 nM Ac-SDKP for 30 minutes, followed by stimulation with ET-1 for 10 minutes. They were then washed twice with cold phosphate-free storage buffer (25 mM Tris-HCl, pH 7.0, and 2 mM EDTA) and lysed at 4°C in 170 μl storage buffer containing 0.4% Triton X-100, 0.1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche, Indianapolis, IN). The lysates were centrifuged at 16,000 g for 10 minutes at 4°C, either using the supernatants for PTP assay or storing them at −72°C until assay. Tyrosine phosphatase activity was measured following Promega’s protocol, which involves incubating 4 μg proteins with the reaction solution for 40 minutes at room temperature. Free phosphate turned the sample green, which was quantified by measuring absorbance at 620 nm with a plate reader and expressing PTP activity as a percentage of control.
Effect of Ac-SDKP on SHP-2 and MKP-3 activity
Since MKP-1 is reportedly an immediate early gene product expressed mainly in the nucleus as early as 30 minutes after stimulation with serum [3,27], whereas Ac-SDKP inhibited p44/42 MAPK phosphorylation in the first 5 minutes after fetal calf serum or ET-1 stimulation [23,34], we rejected the possibility that Ac-SDKP could downregulate the p44/42 MAPK signaling pathway via MKP-1. In our pilot study, we were unable to detect SHP-1 and MKP-2 expression in RCFs by Western blot; therefore, we measured SHP-2 and MKP-3 activity instead. Quiescent RCFs in 100-mm tissue culture dishes were pretreated with vehicle or Ac-SDKP for 30 minutes before adding ET-1 to the medium and incubating the samples for 10 minutes. The cells were lysed in 150 μl lysis buffer (25 mM Tris-HCl, 2 mM EDTA, 0.4% Triton-X 100, pH 7.5, and a fresh protease inhibitor cocktail from Roche) at 4°C for 30 minutes. Lysates were centrifuged at 16,000 g for 10 minutes at 4°C and 200 μg protein taken from the supernatants was immunoprecipitated with an anti-SHP-2 or MKP-3 antibody plus protein G plus/protein A-agarose following Santa Cruz’s protocol. The immune complex pellets were assessed for PTP activity using a commercial kit (V2471; Promega, Madison, WI). The pellets were washed with phosphate-free storage buffer and resuspended in 50 μl tyrosine phosphatase reaction buffer, then maintained at room temperature for 40 minutes, shaking the tube to resuspend the beads every 5 to 10 minutes. The pellets were spun at 14,000 g at 4 °C for 5 seconds. The spun supernatants were transferred to 96-well plates and 50 μl molybdate dye/additive mixture added to each well. Free phosphate turned the sample green, which was quantified by measuring absorbance at 620 nm with a plate reader. Absorbance of the sample was normalized to control (normal IgG) by subtracting the background signal of control IgG from lysate immunoprecipitates. The immune complexes on the agarose beads were resuspended in 25 μl 2 × reducing buffer (Bio-Rad, Hercules, CA), boiled and SHP-2 or MKP-3 detected by Western blot.
Effect of Ac-SDKP on SHP-2-regulated phosphorylation of p44/42 MAPK
0.5 × 105 cells per well were seeded in 12-well tissue culture plates in 1 ml low-glucose DMEM with 10% FBS and grown for 18-24 hours until 70% confluence. shRNA lentiviral particles at a multiplicity of infection of 5 (2 × 104 infectious forming units) from Santa Cruz were transfected into RCFs according to the manufacturer’s instructions and positive clones selected with 5 μg/ml puromycin. Transfected cells exhibiting >98% inhibition of SHP-2 expression were pretreated with Ac-SDKP for 30 minutes and then stimulated with ET-1 for 10 minutes. Whole-cell lysates were prepared in 30 μl of a 1:1 mixture of 2 × reducing sample buffer (Bio-Rad) and RIPA lysis buffer and maintained at −72°C until examined for phosphorylated p44/42 MAPK or SHP-2 by Western blot.
Effect of Ac-SDKP on SHP-2-regulated collagen production
To test whether the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production was partly due to increased SHP-2 activity and resultant downregulation of p44/42 MAPK activation, we first attempted to use cells transfected with SHP-2 shRNA but found out they could not survive in low glucose DMEM with only 0.4% FBS for an additional 48 hours. Unfortunately, we were unable to find a specific SHP-2 inhibitor; nevertheless, our inability to detect SHP-1 on Western blot (data not shown) as well as others [30] suggests SHP-1 is not expressed in RCFs. Therefore, we used an SHP-1/2 inhibitor, NSC-87877, to block SHP-2 and found that the cells grew well. Quiescent cells in 6-well plates were cultured in low glucose DMEM with 0.4% FBS and 0.15 mM L-ascorbic acid; they were incubated first in NSC-87877 (50 μM) for 3 hours and then in vehicle or Ac-SDKP (10 nM) for 30 minutes, followed by ET-1 (10−8M) for 48 hours. Collagen content in the conditioned medium was measured by hydroxyproline assay [7,20]. Protein content of cell lysates was measured using Coomassie reagent (Thermo Scientific, Rockford IL). Collagen content was expressed as μg/mg protein, assuming that collagen contains an average of 13.5% hydroxyproline [6].
Statistical Analysis
All variables were tested by analysis of variance (ANOVA) with contrast. Each dose of ET-1 was compared to Ac-SDKP and Hochberg’s method used to determine significance. Control versus ET-1 was considered a single comparison where significance was defined as p < 0.05. Values are expressed as mean ± SEM.
Results
PTPs are involved in ET-1-stimulated phosphorylation of p44/42 MAPK
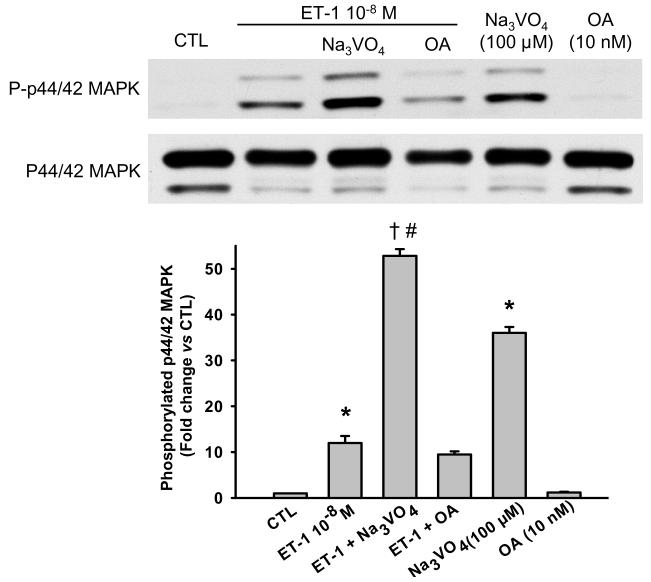
After blocking PTP activity with a nonselective PTP inhibitor, sodium orthovanadate (Na3VO4; 100 μM), both basal and ET-1-stimulated phosphorylated p44/42 MAPK levels increased significantly (Fig. 1); at this dose Na3VO4 did not affect cell viability (4.95 ± 0.1 × 105 in controls vs 4.99 ± 0.16 × 105 cells in Na3VO4-treated cells; n = 3). However, a nonselective serine/threonine inhibitor, OA (10 nM), had no effect, suggesting that PTPs counteract ET-1-stimulated activation of p44/42 MAPK.
Fig. 1.
Effect of phosphatase inhibitors on p44/42 MAPK activation in cardiac fibroblasts treated with ET-1. A nonselective protein tyrosine phosphatases (PTPs), sodium orthovanadate (Na3VO4, 100 μM), significantly increased phosphorylated p44/42 MAPK under both basal condition and ET-1 stimulation, suggesting that PTPs are involved in activation of p44/42 MAPK in cardiac fibroblasts.* p < 0.001, ET-1 or Na3VO4 alone vs control; † p < 0.001, Na3VO4 plus ET-1 vs ET-1 alone; # p < 0.001, Na3VO4 plus ET-1 vs Na3VO4 alone (n = 3 - 4 per group)
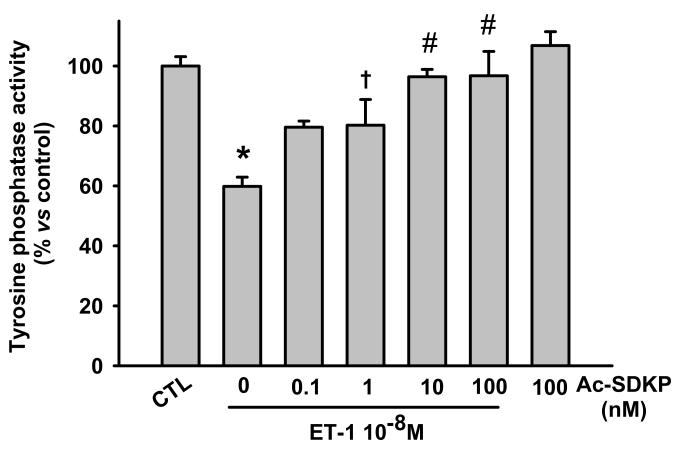
Ac-SDKP blocks ET-1-induced inhibition of PTP activity
We found that ET-1 reduced PTP activity after 10 minutes incubation and Ac-SDKP from 1 to 100 nM prevented this effect in a dose-dependent fashion, whereas Ac-SDKP alone at high dose had no effect (Fig. 2). Therefore, we used 10 nM Ac-SDKP for all further experiments.
Fig. 2.
Effect of Ac-SDKP on protein tyrosine phosphatases (PTP) activity in cardiac fibroblasts treated with ET-1 for 10 minutes. ET-1 significantly reduced PTP activity and Ac-SDKP prevented this reduction in a dose-dependent manner. * p < 0.001, ET-1 alone vs control; † p < 0.05, Ac-SDKP plus ET-1 vs ET-1 alone; # p < 0.001, Ac-SDKP plus ET-1 vs ET-1 alone (n = 3 - 7 per group)
SHP-2 is involved in the response to ET-1 in RCFs, but MKP-3 is not
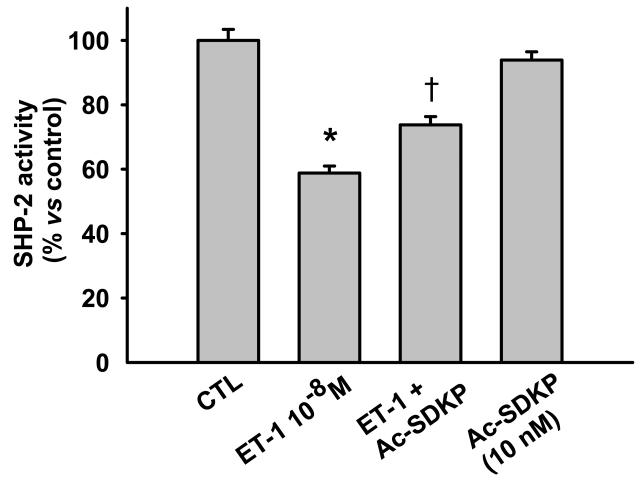
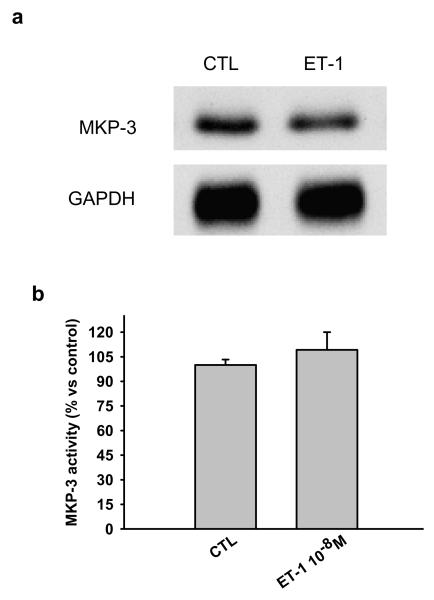
Ac-SDKP counteracts the inhibitory effects of ET-1 on SHP-2 activity. Since SHP-2 reportedly contributes to negative regulation of the ET-1 signaling pathway [4] in cardiac fibroblasts, we questioned whether SHP-2 is involved in regulation of ET-1-stimulated signaling, extracting SHP-2 from RCF lysates by immunoprecipitation and looking for phosphatase activity. We found that SHP-2 activity was significantly decreased 10 minutes after ET-1 stimulation and this decrease was prevented by Ac-SDKP (Fig. 3). To test whether MKP-3 regulates ET-1-induced p44/42 MAPK activation, we examined both expression and activity of MKP-3 and found that RCFs constitutively expressed MKP-3 protein (Fig. 4a); however, we observed no significant differences in MKP-3 activity between control and ET-1-treated cells after 10 minutes incubation (Fig. 4b), ruling out the possibility that MKP-3 could be part of the ET-1 signaling pathway in RCFs.
Fig. 3.
Effect of Ac-SDKP on SHP-2 activity in cardiac fibroblasts treated with ET-1 for 10 minutes. ET-1 significantly reduced SHP-2 activity and this was prevented by Ac-SDKP. * p < 0.001, ET-1 alone vs control; † p < 0.001, Ac-SDKP plus ET-1 vs ET-1 alone (n = 5 per group)
Fig. 4.
Representative Western blot showing a) MAPK phosphatase (MKP)-3 expression and b) activity in cardiac fibroblasts treated with 10−8M ET-1 for 10 minutes. Cells constitutively expressed MKP-3 and there were no significant differences in protein or activity between controls and ET-1-treated cells. (n = 3 - 5 per group)
The inhibitory effect of Ac-SDKP on ET-1-stimulated p44/42 MAPK phosphorylation is blocked in SHP-2 shRNA-treated cells
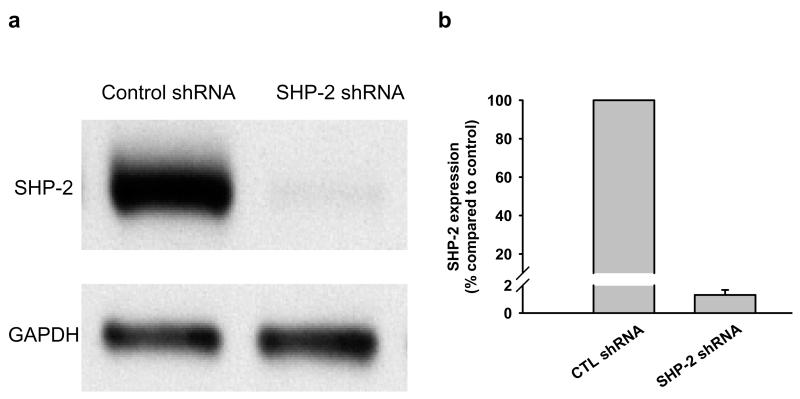
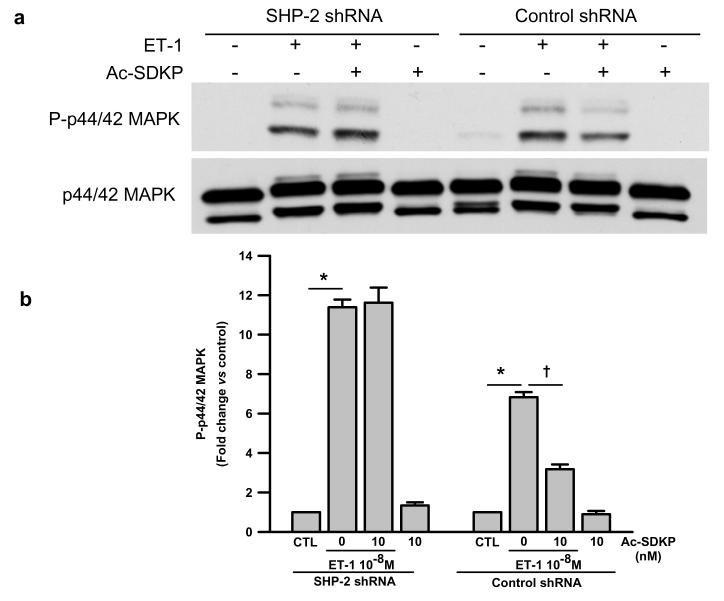
To determine a) whether ET-1-induced p44/42 MAPK activation is related to decreased SHP-2 activity and b) whether this process is influenced by Ac-SDKP, we transfected RCFs with SHP-2 shRNA lentiviral particles. 98.7% of SHP-2 protein expression was blocked in cells transfected with SHP-2 shRNA (Fig. 5); this inhibition resulted in reduced ratio of phosphorylated to total p44/42 MAPK with 0.15 ± 0.05 A.U. in control shRNA and 0.01 ± 0.006 in SHP-2 shRNA-treated quiescent cells (p < 0.05) Fig. 6), indicating that under basal conditions SHP-2 positively regulates p44/42 MAPK phosphorylation in RCFs.
Fig. 5.
a) Representative Western blot for SHP-2 in control- or SHP-2 shRNA-transfected fibroblasts. In cells transfected with SHP-2 shRNA, SHP-2 protein was almost depleted; b) Quantitative analysis of SHP-2 expression in cardiac fibroblasts transfected by control or SHP-2 shRNA. Data is from two individual transfections.
Fig. 6.
a) Representative Western blot and b) quantitative data for phosphorylated p44/42 MAPK levels in cells treated with either control or SHP-2 shRNA and challenged with ET-1 for 10 minutes in the presence or absence of Ac-SDKP. Phosphorylated p44/42 MAPK was normalized to p44/42 MAPK. ET-1 induced an 11- and 6-fold increase in phosphorylated p44/42 MAPK in the SHP-2 knockdown cells and controls, respectively. Ac-SDKP only inhibited ET-1-induced phsophorylated p44/42 MAPK in the controls. These results indicate that the inhibitory effect of Ac-SDKP on p44/42 MAPK activation depends on SHP-2 activity. * p < 0.005, ET-1 vs control; † p < 0.005, Ac-SDKP plus ET-1 vs ET-1 alone (n = 3 per group)
SHP-2 shRNA-transfected cells were pre-incubated with Ac-SDKP for 30 minutes followed by incubation with ET-1 for 10 minutes and phosphorylated p44/42 MAPK was detected by Western blot. We found that ET-1 induced significant phosphorylation of p44/42 MAPK in all cells but this effect was even greater in the cells with SHP-2 knockdown (Fig. 6). Interestingly, Ac-SDKP inhibited ET-1-induced phosphorylated p44/42 MAPK only in cells transfected with control shRNA but not in the cells with SHP-2 knockdown (Fig. 6), suggesting that the inhibitory effect of Ac-SDKP on p44/42 MAPK activation could be mediated by SHP-2.
The inhibitory effect of Ac-SDKP on ET-1-induced collagen production is diminished in cells treated with an SHP1/2 inhibitor
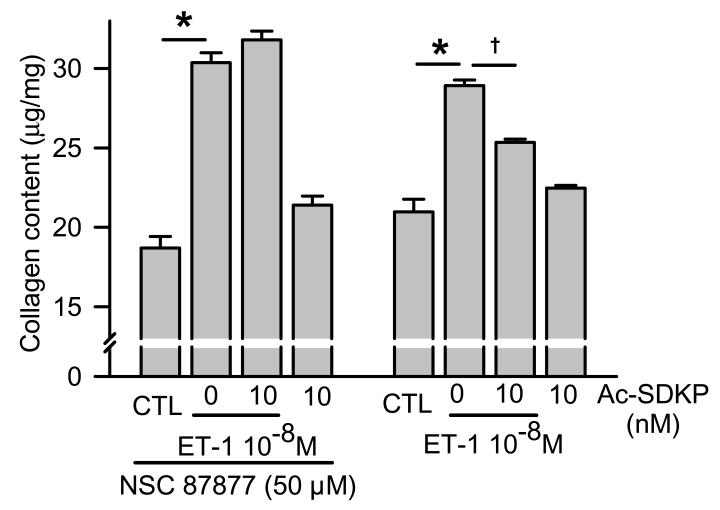
To find out whether the inhibitory effect of Ac-SDKP on ET-1-induced collagen is associated with SHP-2 activity, we first attempted to use cells transfected with SHP-2 shRNA but found out that addition of ET-1 in low glucose media with only 0.4% FBS for an additional 48 hours was associated with massive cell death. Alternatively, we used a SHP-1/2 inhibitor [26], NSC-87877, to block SHP-2 since SHP-1 is not expressed in RCFs [30]. Adding ET-1 for 48 hours significantly increased collagen production with or without preincubation with NSC-87877. We found that 1) basal collagen production was not significantly different in both SHP-2-inhibited and non-inhibited cells and ET-1 induced a similar significant increase in collagen content in both groups of cells, and 2) Ac-SDKP significantly inhibited ET-1-stimulated collagen production in untreated cells but failed to inhibit collagen production in cells treated with NSC-87877 (Fig. 7). These results suggest that 1) SHP-2 does not play an important role in basal collagen production by RCFs but does influence collagen production in ET-1-stimulated cells, and 2) Ac-SDKP inhibits ET-1-stimulated collagen production through mechanisms related to SHP-2.
Fig. 7.
Effects of Ac-SDKP on ET-1-stimulated collagen production in controls and NSC-87877-pretreated fibroblasts. Basal collagen production was similar in both the controls and NSC-87877-treated cells. Ac-SDKP significantly inhibited ET-1-stimulated collagen production in the controls but not in the SHP-2-inhibited cells, suggesting that Ac-SDKP inhibits ET-1-stimulated collagen production through mechanisms related to SHP-2. * p < 0.005, ET-1 vs control; † p < 0.005, Ac-SDKP plus ET-1 vs ET-1 alone (n = 4 per group)
Discussion
We hypothesized that Ac-SDKP inhibits collagen production in part by counteracting the decrease in SHP-2 and/or MKP activity and thereby downregulating p44/42 MAPK in cardiac fibroblasts treated with ET-1. We found that 1) ET-1 stimulation for 10 minutes significantly reduced SHP-2 activity but had no effect on MKP-3, and this reduction was prevented by Ac-SDKP; 2) Ac-SDKP inhibited ET-1-induced p44/42 MAPK phosphorylation in cells transfected with control shRNA but had no effect in cells transfected with SHP-2 shRNA; and 3) Ac-SDKP blunted ET-1-stimulated collagen production in normal cells but not in those treated with an SHP-1/2 inhibitor, NSC-87877. We concluded that in cardiac fibroblasts the inhibitory effect of Ac-SDKP on ET-1-stimulated collagen production may be mediated by preserving SHP-2 activity and thus possibly counteracting p44/42 MAPK activation.
A PTP inhibitor, sodium orthovanadate, markedly increased p44/42 MAPK phosphorylation by itself and ET-1 treatment enhanced this effect; however, a serine/threonine inhibitor, OA, had no effect (Fig. 1). This suggests that PTPs are involved in p44/42 MAPK activation in cardiac fibroblasts stimulated with ET-1, whereas serine/threonine phosphatases are not. Since Chen et al reported that ET-1 transiently inhibited SHP-2 activity of RCFs, increasing phosphorylation of epidermal growth factor receptors and p44/42 MAPK [4], we wanted to confirm whether ET-1 exerts any effect on SHP-2, and also found that ET-1 reduced SHP-2 activity. To demonstrate the role of SHP-2 in activation of p44/42/MAPK, we knocked down SHP-2 with a shRNA and detected phosphorylated p44/42 MAPK, similar to Chen et al [4]. In our study, after SHP-2 depletion ET-1 stimulation induced stronger p44/42 MAPK activation than in SHP-2 intact cells when compared to their own control cells. However, unlike Chen, we found that basal phosphorylated p44/42 MAPK was decreased. In most cells, including embryonic fibroblasts, HEK293 and glioblastoma cells, phosphorylation of p44/42 MAPK (induced by epidermal growth factor) was inhibited when SHP-2 was depleted with either a SHP-2 inhibitor or SHP-2 small interfering RNA [24] [5,32]. Therefore, SHP-2 appears to either positively or negatively regulate growth factor-induced cellular signaling depending on the type of cell and stimulus involved. To determine whether the counteracting effect of Ac-SDKP on ET-1-induced p44/42 MAPK phosphorylation is due to its effect on SHP-2 activity, we blocked the SHP-2 gene by transfecting cells with an SHP-2 shRNA and treated them with ET-1 in the presence or absence of Ac-SDKP. We found that in cells with SHP-2 ShRNA, ET-1 induced significant phosphorylation of p44/42 MAPK; the inhibitory effect of Ac-SDKP on p44/42 MAPK activation was abolished in SHP-2 shRNA-but not in control shRNA-transfected cells, suggesting that Ac-SDKP acts in part by targeting SHP-2.
We previously found that Ac-SDKP inhibited ET-1-induced collagen production by cultured RCFs and this effect was partially blocked by PD 98059, a MEK inhibitor [23], suggesting that Ac-SDKP’s inhibitory effect is mediated in part by inactivating MAPK signaling. In the present study, even though SHP-2 depletion reduced p44/42 MAPK phosphorylation in quiescent cardiac fibroblasts, collagen content was similar between control shRNA- and SHP-2 shRNA-transfected cells, suggesting that p44/42 MAPK does not play a crucial role in collagen production in quiescent cardiac fibroblasts. The inhibitory effect of Ac-SDKP on p44/42 MAPK phosphorylation was blocked in ET-1-treated RCFs after deletion of SHP-2, and NSC-87877 significantly blunted the inhibitory effect of Ac-SDKP on collagen production by cells stimulated with ET-1. These results suggest that in ET-1-stimulated cells, p44/42 MAPK activation positively regulates collagen production and Ac-SDKP inhibits this activation in part by stabilizing SHP-2 activity, thus reducing collagen production.
P44/42 MAPK could be activated by a variety of growth factors and cytokines. We previously reported that Ac-SDKP suppressed p44/p42 MAPK phosphorylation in TGF-1-stimulated human cardiac fibroblasts, associated with reduced fibroblast proliferation and myofibroblast differentiation and hence collagen production [19]. In addition, in aldosterone/salt-induced hypertension the antifibrotic effects of Ac-SDKP on the heart and kidney are accompanied by inhibition of p44/42 MAPK activation [18]. Therefore, we speculate that Ac-SDKP’s inhibitory effects on p44/42 MAPK activation may also be partially mediated through Ac-SDKP-preserved conventional PTP activity and hence a decrease in p44/42 MAPK phosphorylation.
Since MKP-3 is specific for dephosphorylation of p44/42 MAPK and resides in the cytoplasm [10], we measured MKP-3 activity by extracting MKP-3 by immunoprecipitation and performing an immune complex PTP assay. We found that MKP-3 was constitutively expressed in RCFs and its activity remained unchanged following stimulation with ET-1 for 30 minutes, suggesting that it is not involved in the early ET-1 signaling pathway in RCFs. Therefore, we believe our study shows for the first time that in cardiac fibroblasts, Ac-SDKP lowers collagen production in cardiac fibroblasts stimulated with ET-1 by preserving SHP-2 activity and thereby counteracting the marked increase in p44/42 activation.
These findings provide further evidence that Ac-SDKP is a potent inhibitor of collagen production in cardiac fibroblasts. When plasma and/or tissue levels of Ac-SDKP are elevated in patients treated with an ACEi, it may act as an endogenous antifibrotic factor. More importantly, design and synthesis of a new analogue of Ac-SDKP that is resistant to ACE or endopeptidases would be beneficial in treating or preventing fibrosis in patients with diabetes, hypertension and other cardiovascular diseases, and thus avoiding the undesirable side effects of ACEi.
Acknowledgments
This study was supported by National Institutes of Health grants HL 071806 (NER) and HL 028982 (OAC).
References
- 1.Ammarguellat F, Larouche I, Schiffrin EL. Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation. 2001;103:319–324. doi: 10.1161/01.cir.103.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. JCI. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brondello JM, Brunet A, Pouyssegur J, McKenzie FR. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Cheng TH, Lin H, Shih NL, Chen YL, Chen YS, Cheng CF, Lian WS, Meng TC, Chiu WT, Chen JJ. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol Pharmacol. 2006;69:1347–1355. doi: 10.1124/mol.105.017558. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 6.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol. 1986;18:283–290. doi: 10.1016/s0022-2828(86)80410-2. [DOI] [PubMed] [Google Scholar]
- 7.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer H, Schmenger K, Heinrich K, Horstmeyer A, Boning H, Breit A, Piiper A, Lundstrom K, Muller-Esterl W, Schroeder C. Coupling of endothelin receptors to the ERK/MAP kinase pathway. Roles of palmitoylation and G(alpha)q. Eur J Biochem. 2001;268:5449–5459. doi: 10.1046/j.0014-2956.2001.02486.x. [DOI] [PubMed] [Google Scholar]
- 9.Eghbali M, Tomek R, Sukhatme VP, Woods C, Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circulation Research. 1991;69:483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- 10.Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 11.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovascular Research. 1993;27:2130–2134. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 12.Hafizi S, Wharton J, Chester AH, Yacoub MH. Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem. 2004;14:285–292. doi: 10.1159/000080338. [DOI] [PubMed] [Google Scholar]
- 13.Haneda M, Sugimoto T, Kikkawa R. Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol. 1999;365:1–7. doi: 10.1016/s0014-2999(98)00857-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang WQ, Wang QR. Bone marrow endothelial cells secrete thymosin 4 and AcSDKP. Exp Hematol. 2001;29:12–18. doi: 10.1016/s0301-472x(00)00634-2. [DOI] [PubMed] [Google Scholar]
- 15.Matozaki T, Murata Y, Saito Y, Okazawa H, Ohnishi H. Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci. 2009;100:1786–1793. doi: 10.1111/j.1349-7006.2009.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s) AM J Physiol Cell Physiol. 2000;278:C154–C162. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 17.Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002;15:164–169. doi: 10.1016/s0895-7061(01)02291-9. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb N-E. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension. 2007;49:695–703. doi: 10.1161/01.HYP.0000258406.66954.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Carretero OA, Peterson EL, Rhaleb N-E. Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts. Am J Physiol Heart Circ Physiol. 2010;298:H1357–H1364. doi: 10.1152/ajpheart.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb N-E. Antifibrotic effects of N-acetyl-seryl-aspartyl-lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800. doi: 10.1161/01.hyp.37.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradelles P, Frobert Y, Creminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin beta 4 in mouse tissues. FEBS Lett. 1991;289:171–175. doi: 10.1016/0014-5793(91)81062-d. [DOI] [PubMed] [Google Scholar]
- 22.Pradelles P, Frobert Y, Creminon C, Liozon E, Masse A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochem Biophys Res Commun. 1990;170:986–993. doi: 10.1016/0006-291x(90)90489-a. [DOI] [PubMed] [Google Scholar]
- 23.Rhaleb N-E, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.hyp.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi ZQ, Yu DH, Park M, Marshall M, Feng GS. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidhu JS, Omiecinski CJ. An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J Pharmacol Exp Ther. 1997;282:1122–1129. [PubMed] [Google Scholar]
- 26.Song M, Park JE, Park SG, Lee dH, Choi HK, Park BC, Ryu SE, Kim JH, Cho S. NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26) Biochem Biophys Res Commun. 2009;381:491–495. doi: 10.1016/j.bbrc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Simonson MS, Pouyssegur J, Dunn MJ. Endothelin rapidly stimulates mitogen-activated protein kinase activity in rat mesangial cells. Biochem J. 1992;287(Pt 2):589–594. doi: 10.1042/bj2870589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 30.Warnecke C, Kaup D, Marienfeld U, Poller W, Yankah C, Grafe M, Fleck E, Regitz-Zagrosek V. Adenovirus-mediated overexpression and stimulation of the human angiotensin II type 2 receptor in porcine cardiac fibroblasts does not modulate proliferation, collagen I mRNA expression and ERK1/ERK2 activity, but inhibits protein tyrosine phosphatases. J Mol Med. 2001;79:510–521. doi: 10.1007/s001090100243. [DOI] [PubMed] [Google Scholar]
- 31.Wdzieczak-Bakala J, Fache MP, Lenfant M, Frindel E, Sainteny F. AcSDKP, an inhibitor of CFU-S proliferation is synthesized in mice under steady-state conditions and secreted by bone marrow in long term culture. Leukemia (Baltimore) 1990;4:235–237. [PubMed] [Google Scholar]
- 32.Zhan Y, Counelis GJ, O’Rourke DM. The protein tyrosine phosphatase SHP-2 is required for EGFRvIII oncogenic transformation in human glioblastoma cells. Exp Cell Res. 2009;315:2343–2357. doi: 10.1016/j.yexcr.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Tan Z, Diltz CD, You M, Fischer EH. Activation of mitogen-activated protein (MAP) kinase pathway by pervanadate, a potent inhibitor of tyrosine phosphatases. J Biol Chem. 1996;271:22251–22255. doi: 10.1074/jbc.271.36.22251. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo JL, Carretero OA, Peng H, Li XC, Regoli D, Neugebauer W, Rhaleb N-E. Characterization and localization of Ac-SDKP receptor binding sites using 125I-labeled Hpp-Aca-SDKP in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2007;292:H984–H993. doi: 10.1152/ajpheart.00776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]