Abstract
ADP is the cognate agonist of the P2Y1, P2Y12, and P2Y13 receptors. With the goal of identifying a high potency agonist that selectively activates the P2Y1 receptor, we examined the pharmacological selectivity of the conformationally constrained non-nucleotide analog (N)-methanocarba-2MeSADP [(1′S,2′R, 3′S,4′R,5′S)-4-[(6-amino-2-methylthio-9H-purin-9-yl)-1-diphosphoryloxymethyl]bicyclo[3.1.0]hexane-2,3-diol] among the three ADP-activated receptors. Each P2Y receptor was expressed transiently in COS-7 cells, and inositol lipid hydrolysis was quantified as a measure of receptor activity. In the case of the Gi-linked P2Y12 and P2Y13 receptors, a chimeric G protein, Gαq/i, was coexpressed to confer a capacity of these Gi-linked receptors to activate phospholipase C. 2MeSADP (2-methylthio-ADP) was a potent agonist at all three receptors exhibiting EC50 values in the sub to low nanomolar range. In contrast, whereas (N)-methanocarba-2MeSADP was an extremely potent (EC50 = 1.2 ± 0.2 nM) agonist at the P2Y1 receptor, this non-nucleotide analog exhibited no agonist activity at the P2Y12 receptor and very low activity at the P2Y13 receptor. (N)-Methanocarba-2MeSADP also failed to block the action of 2MeSADP at the P2Y12 and P2Y13 receptors, indicating that the (N)-methanocarba analog is not an antagonist at these receptors. The P2Y1 receptor selectivity of (N)-methanocarba-2MeSADP was confirmed in human platelets where it induced the shape change promoted by P2Y1 receptor activation without inducing the sustained platelet aggregation that requires simultaneous activation of the P2Y12 receptor. These results provide the first demonstration of a high-affinity agonist that discriminates among the three ADP-activated P2Y receptors, and therefore, introduce a potentially important new pharmacological tool for delineation of the relative biological action of these three signaling proteins.
The G protein-coupled P2Y receptor family is comprised of at least eight different human receptors that are activated by nucleoside diphosphates, nucleoside triphosphates, or nucleotide sugars to regulate a broad range of physiological responses including neurotransmission, muscle contraction, ion secretion, and platelet aggregation (Dubyak and El-Moatassim, 1993; Harden et al., 1998; Ralevic and Burnstock, 1998). A complex set of ectoenzymes that metabolize extracellular nucleotides has complicated the study of these receptors (Harden et al., 1997; Zimmermann, 2000). This difficulty is exacerbated by the lack of selective agonists or antagonists for most of the P2Y receptors.
ADP is the cognate agonist of the P2Y1, P2Y12, and P2Y13 receptors. Whereas the P2Y1 receptor is coupled to Gq and activates phospholipase C, the P2Y12 and P2Y13 receptors are coupled to Gi, inhibit adenylyl cyclase, and regulate ion channels (Harden et al., 1998; Hollopeter et al., 2001). ADP promotes platelet aggregation through a complex and incompletely defined interplay of cell signaling responses promoted by the P2Y1 and P2Y12 receptors (Kunapuli, 1998; Gachet, 2001). Although P2Y1 and P2Y12 receptor-selective antagonists exist (Gachet, 2003), molecules that selectively activate individual subtypes of the three member ADP receptor family have not been reported.
Our studies of bisphosphate antagonists of the P2Y1 receptor led to the discovery that the ribose moiety was dispensible in these molecules and could be replaced by a variety of carbocyclic or acyclic groups (Kim et al., 2000; Nandanan et al., 2000). Substitution of the ribose ring of bisphosphate molecules with a pseudoribose consisting of cyclopentane and cyclopropane rings fused in the methanocarba modification results in non-nucleotide analogs containing a pseudoribose fixed in either a Northern or Southern envelope conformation and in antagonist activity of sufficiently high selectivity and affinity (Kim et al., 2001; Boyer et al., 2002) to be utilized in a radioligand binding assay for the P2Y1 receptor (Waldo et al., 2002). (N)-Methanocarba-ATP and (N)-methanocarba-UTP are potent P2Y receptor agonists (Kim et al., 2002). Moreover, the high potency of (N)-methanocarba-2MeSADP observed at the human P2Y1 receptor in initial studies (Ravi et al., 2002) has prompted us to carry out a detailed comparison of the pharmacological activity of this analog at the P2Y1 receptor versus activity at the ADP-activated P2Y12 and P2Y13 receptors. Our results illustrate high selectivity of (N)-methanocarba-2MeSADP for the P2Y1 receptor. Furthermore, they demonstrate the usefulness of (N)-methanocarba-2MeSADP to, for example, effect independent activation of the platelet P2Y1 receptor under conditions that do not necessitate simultaneous blockade of the platelet P2Y12 receptor.
Materials and Methods
Transfection of COS-7 Cells and Quantification of P2Y Receptor-Stimulated Phospholipase C Activity
COS-7 cells were seeded in 12-well culture dishes at a density of approximately 60,000 cells per well and maintained in high glucose Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in an atmosphere of 90% air/10% CO2. The indicated DNA vectors (in pcDNA3.1) were transfected using Fugene 6 (Roche Diagnostics, Indianapolis, IN) transfection reagent (3 μl of Fugene/1 μg of DNA) according to the manufacturer’s protocol. The human P2Y12 and P2Y13 receptor DNA vectors (200 ng/well) were cotransfected with a DNA vector for Gαq/i (100 ng/well). Gαq/i is a chimeric construct of Gα subunits that confers to Gi-linked receptors a capacity to activate Gq and phospholipase C-β, and therefore, to promote inositol lipid hydrolysis in response to agonist-promoted receptor activation (Conklin et al., 1992). An expression vector for the human P2Y1 receptor, which couples to Gq and phospholipase C-β (Schachter et al., 1996; Waldo and Harden, 2004), was transfected (200 ng/well) in COS-7 cells in the absence of Gαq/i.
Approximately 24 h after transfection, the culture medium was changed to inositol-free Dulbecco’s modified Eagle’s medium (ICN Biomedicals Inc., Costa Mesa, CA) containing 1 μCi/well myo-[2-3H]inositol (American Radiolabeled Chemicals, St. Louis, MO), and metabolic labeling proceeded for 12 to 16 h. Agonist-promoted accumulation of [3H]inositol phosphates was quantified subsequent to addition of 10 mM LiCl to inhibit inositol phosphate phosphatases. The reaction was stopped after 60 min by aspiration of the medium and the addition of 50 mM formic acid followed by neutralization with 150 mM NH4OH. [3H]Inositol phosphates were quantified by Dowex chromatography as previously described (Brown et al., 1991).
Preparation and Assay of Washed Human Platelets
Blood was collected from healthy volunteers into syringes containing one-sixth final blood volume of anticoagulant (65 mM citric acid, 85 mM sodium citrate, and 110 mM dextrose). The blood was centrifuged at 180g for 15 min, and the supernatant (platelet rich plasma) was removed. The platelet rich plasma was centrifuged, and the platelets were resuspended in a buffer consisting of 137 mM NaCl, 2.7 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM HEPES (pH 7.4), and 0.2% bovine serum albumin. These centrifugations and washes were repeated twice followed by a final resuspension in the medium described above containing 0.25 U/ml apyrase. Platelet aggregation was measured using the optical mode of a Chrono-Log aggregometer (Chrono-Log Corporation, Havertown, PA). Four hundred and fifty microliters of platelet suspension containing 1 mg/ml fibrinogen were warmed to 37°C and stirred at 1000 rpm. Indicated concentrations of drugs were added to the sample, and aggregation was monitored for 8 min. Two modifications were included to obtain accurate estimations of platelet shape change. The offset mode of the Aggro/Link computer interface was applied, and the reference sample cuvette of the aggregometer included a platelet suspension equivalent to 50% of that present in cuvettes for drug testing.
Synthesis of (N)-Methanocarba-2MeSADP
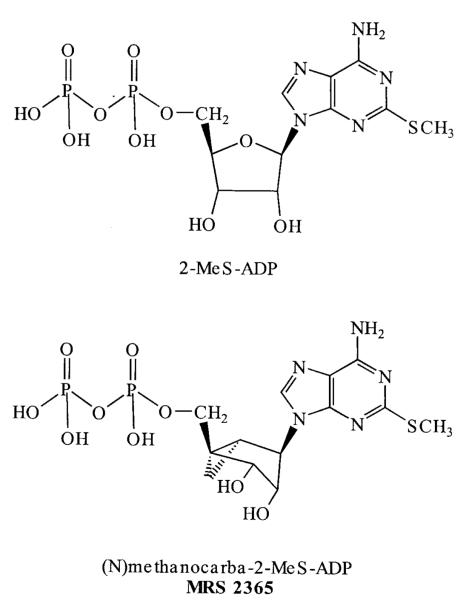
(N)-Methanocarba-2MeSADP (MRS2365; Fig. 1) was synthesized as previously described (Ravi et al., 2002). The chemical name of MRS2365 is (1′S,2′R, 3′S,4′R,5′S)-4-[(6-amino-2-methylthio-9H-purin-9-yl)-1-diphosphoryloxymethyl]bicyclo[3.1.0]hexane-2,3-diol.
Fig. 1.
Structure of 2MeSADP and (N)-methanocarba-2MeSADP.
Results
To compare the pharmacological selectivities of the ADP-activated human P2Y1, P2Y12, and P2Y13 receptors, we generated mammalian expression constructs for each of these receptors and transiently expressed these G protein-coupled receptors in COS-7 cells. The Gαq-coupled P2Y1 receptor was expressed alone, whereas the Gαi-coupled P2Y12 and P2Y13 receptors were coexpressed with a chimeric construct of Gαq, which incorporates the last five amino acids of Gαi1 at the carboxy terminus and consequently confers capacity of Gi-linked receptors to activate Gαq and phospholipase C-β (Conklin et al., 1992). Therefore, [3H]inositol phosphate accumulation was quantified as a measure of activation of each of the three different P2Y receptors.
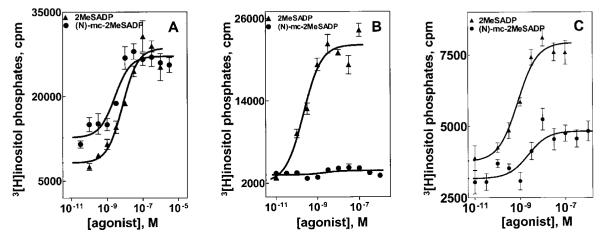
Although 2MeSADP exhibited little or no effect in empty vector-transfected COS-7 cells (data not shown), marked concentration-dependent stimulation of inositol lipid hydrolysis occurred in response to 2MeSADP in cells transiently expressing the P2Y1 receptor (Fig. 2A). We recently reported the synthesis and biological testing of a series of methanocarba analogs of adenine and uridine nucleotides (Ravi et al., 2002). These conformationally constrained non-nucleotide molecules retain, in several instances, a capacity to activate P2Y receptors. For example, (N)-methanocarba-2MeSADP activated the human P2Y1 receptor stably expressed in 1321N1 human astrocytoma cells with the highest potency (EC50 = 0.4 nM) yet reported for activation of this receptor. This high potency of (N)-methanocarba-2MeSADP also was observed after transient expression of the human P2Y1 receptor in COS-7 cells (Fig. 2A) where (N)-methanocarba-2MeSADP exhibited a potency (EC50 = 1.8 ± 1.0 nM; mean ± S.D. of n = 3 experiments) approximately 4-fold higher than that of 2MeSADP (EC50 = 6.6 ± 3.0 nM; mean ± S.D.; n = 3 experiments).
Fig. 2.
Agonist activities of 2MeSADP and (N)-methanocarba-2MeSADP at the human P2Y1, P2Y12, and P2Y13 receptors. COS-7 cells transfected with mammalian expression vectors for the human P2Y1, P2Y12, or P2Y13 receptors were preloaded with [3H]inositol, and agonist-promoted inositol lipid hydrolysis was quantified as described under Materials and Methods. Cells transfected with P2Y12 or P2Y13 receptor expression vectors also were cotransfected with an expression vector for Gαq/i, which confers capacity of these two Gi-coupled receptors to activate phospholipase C. Inositol phosphate responses were measured to the indicated concentrations of 2MeSADP (▲) or (N)-methanocarba-2MeSADP (●) in P2Y1 receptor expressing cells (A), P2Y12 receptor expressing cells (B), and P2Y13 receptor expressing cells (C). The data are presented as counts per minute (mean ± S.E.M.; n = 3) of [3H]inositol phosphate accumulation, and the results are representative of results obtained in at least three full concentration-effect curves generated for both agonists with each receptor.
Expression of the human P2Y12 receptor in COS-7 cells failed to confer an inositol phosphate response to 2MeSADP in these cells (data not shown). In contrast, coexpression of the P2Y12 receptor with Gαq/i resulted in robust 2MeSADP-promoted inositol lipid hydrolysis (Fig. 2B). The potency of 2MeSADP (0.3 ± 0.2 nM; mean ± S.D. of n = 3 experiments) was approximately 10-fold greater than that observed with the human P2Y1 receptor. In marked contrast to the activity observed at the human P2Y1 receptor, (N)-methanocarba-2MeSADP exhibited no agonist activity at the P2Y12 receptor (Fig. 2B). (N)-Methanocarba-2MeSADP also was not an antagonist at the P2Y12 receptor since a 10 μM concentration of this analog exhibited no effect on the capacity of 1 nM 2MeSADP to stimulate inositol phosphate accumulation (data not shown).
Expression of the human P2Y13 receptor in COS-7 cells failed to confer an inositol phosphate response to 2MeSADP in these cells (data not shown). As was observed with the P2Y12 receptor, coexpression of the P2Y13 receptor with Gαq/i conferred marked inositol lipid signaling responses to 2MeSADP (Fig. 2C). In contrast, little or no response to (N)-methanocarba-2MeSADP occurred. The results depicted in Fig. 2C represent the largest effect observed in four different experiments in which full concentration-effect curves were generated for activation of the P2Y13 receptor; essentially no effect was observed in the other three experiments. The stimulatory activity of 1 nM 2MeSADP also was not antagonized by 10 μM (N)-methanocarba-2MeSADP indicating that this analog is neither a partial agonist nor an antagonist at the P2Y13 receptor (data not shown).
To further confirm the large difference in response of the P2Y1 receptor versus the P2Y12 and P2Y13 receptors to (N)-methanocarba-2MeSADP, we carried out a series of experiments in which the response to this novel agonist was tested simultaneously at the three different ADP-activated P2Y receptors. Whereas the level of activation of the P2Y1 receptor observed with 10, 100, and 1000 nM concentrations of (N)-methanocarba-2MeSADP was essentially equivalent to that of a maximally effective concentration of 2MeSADP, no significant activation of the P2Y12 receptor or P2Y13 receptor was observed at these concentrations of (N)-methanocarba-2MeSADP (Fig. 3).
Fig. 3.
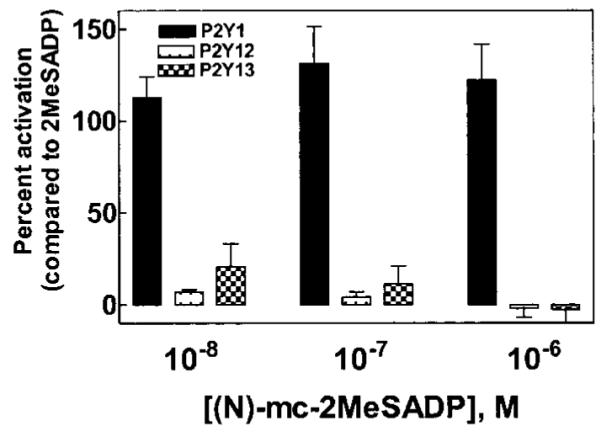
Comparative response of P2Y1, P2Y12, and P2Y13 receptors to (N)-methanocarba-2MeSADP. COS-7 cells expressing the P2Y1 receptor, the P2Y12 receptor + Gαq/i, or the P2Y13 receptor + Gαq/i were challenged with the indicated concentrations of (N)-methanocarba-2MeSADP. The response in each case is compared with that of a maximally effective concentration (100 nM) of 2MeSADP, which was assigned a value of 100%. The results are the mean ± S.E.M. of four independent experiments carried out in triplicate.
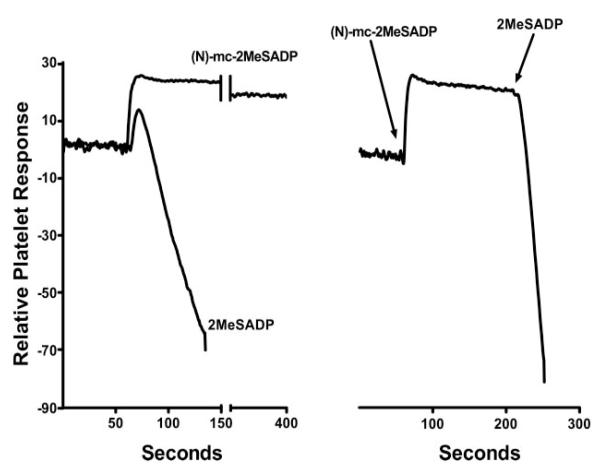
The physiological response of platelets to ADP involves the coordinated action of an ADP-activated P2Y1 receptor and an ADP-activated P2Y12 receptor. Thus, shape change and transient aggregation occurs upon activation of the P2Y1 receptor alone, but full and sustained aggregation requires activation of both the P2Y1 and P2Y12 receptors. Given the P2Y1 receptor-selective action of (N)-methanocarba-2MeSADP revealed in our studies with recombinant human receptors, we tested the action of this analog in studies with human platelets. Whereas the addition of 2MeSADP to washed human platelets caused the transient shape change and marked and sustained aggregation responses that have been widely reported to occur with ADP and 2MeSADP (Fig. 4), addition of (N)-methanocarba-2MeSADP only resulted in a shape change with no accompanying aggregation. The rapid shape change was sustained for at least 6 min of incubation with (N)-methanocarba-2MeSADP without any evidence of aggregation (Fig. 4). However, 2MeSADP-induced plateletaggregation still occurred if platelets were preincubated for 2 min with 1 μM (N)-methanocarba-2MeSADP prior to the addition of 2MeSADP (Fig. 4).
Fig. 4.
Induction of shape change of human platelets by (N)-methanocarba-2MeSADP. Washed human platelets were prepared, and drug-induced shape change (decrease in light transmission) and aggregation (increase in light transmission) were quantified in an aggregometer as described under Materials and Methods. Whereas addition of 30 nM 2MeSADP resulted in a transient shape change followed by platelet aggregation, addition of 1 or 10 μM (N)-methanocarba-2MeSADP only resulted in a rapidly occurring but sustained shape change. Platelets incubated with 1 μM (N)-methanocarba-2MeSADP for 3 min still responded with an aggregation response to 2MeSADP. These results are representative of results obtained in three different experiments.
The concentration dependence of (N)-methanocarba-2MeSADP for inducing shape change was determined (Fig. 5). Measurable effects were observed with 1 nM (N)-methanocarba-2MeSADP, and maximal shape change occurred with 10 to 30 nM (N)-methanocarba-2MeSADP. Platelet aggregation was not observed at any of the (N)-methanocarba-2MeSADP concentrations tested.
Fig. 5.
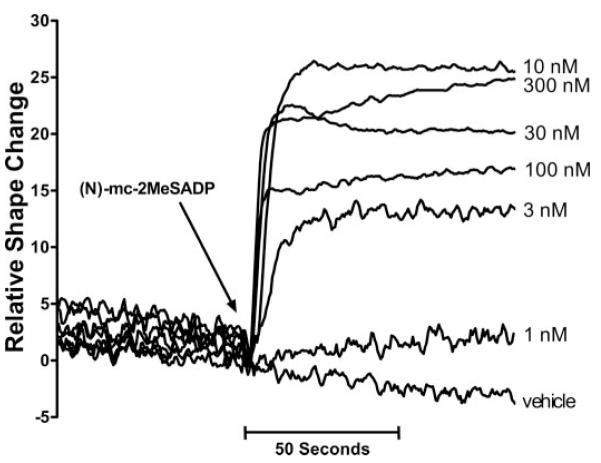
Concentration-dependent induction of platelet shape change by (N)-methanocarba-2MeSADP. Washed human platelets were prepared, and drug-induced shape change (decrease in light transmission) in response to the indicated concentrations of (N)-methanocarba-2MeSADP was quantified in an aggregometer as described under Materials and Methods. The results are representative of those obtained in three separate experiments.
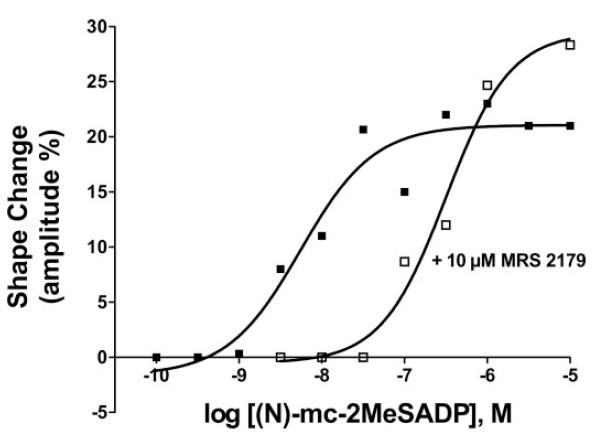
The P2Y1 receptor-selective action of (N)-methanocarba-2MeSADP on human platelets was confirmed using the P2Y1 receptor-selective antagonist MRS2179 (Boyer et al., 1998). Thus, although addition of 10 μM MRS2179 alone had no measurable effect on platelets, concentrations (1–100 nM) of (N)-methanocarba-2MeSADP that caused platelet shape change in the absence of antagonist had no effect in the presence of 10 μM MRS2179 (Fig. 6). The effects of MRS2179 were surmountable by higher concentrations of (N)-methanocarba-2MeSADP, and a maximal shape change was observed with 300 nM (N)-methanocarba-2MeSADP in the presence of 10 μM MRS2179. These primary response data were utilized to generate concentration-effect curves for (N)-methanocarba-2MeSADP (Fig. 7) and revealed an approximately two order of magnitude shift to the right of the concentration-effect curve of (N)-methanocarba-2MeSADP for induction of platelet shape change. Since these experiments were carried out with a concentration of MRS2179 that exceeds its KB by approximately 100-fold, the results are consistent with the expected effect of a competitive P2Y1 receptor antagonist blocking the action of the P2Y1 receptor agonist (N)-methanocarba-2MeSADP.
Fig. 6.
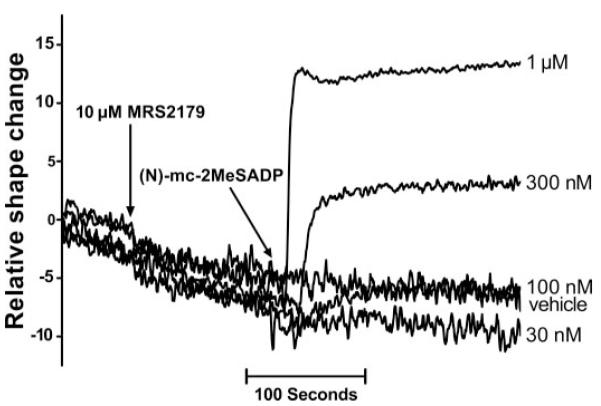
Antagonism of (N)-methanocarba-2MeSADP-induced platelet shape change by the P2Y1 receptor antagonist MRS2179. Washed human platelets were prepared, and drug-induced shape change (decrease in light transmission) and the capacity of the P2Y1 receptor antagonist MRS2179 to antagonize the shape change response to the indicated concentrations of (N)-methanocarba-2MeSADP was quantified in an aggregometer as described under Materials and Methods. In each case, 10 μM MRS2179 was added for 2 min prior to the addition of (N)-methanocarba-2MeSADP. The results are representative of those from three separate experiments.
Fig. 7.
Concentration-effect curve for (N)-methanocarba-2MeSADP in the absence or presence of MRS2179. The platelet shape change response was measured exactly as described in Fig. 5 for various concentrations of (N)-methanocarba-2MeSADP alone and in Fig. 6 for various concentrations of (N)-methanocarba-2MeSADP in the presence of 10 μM MRS2179. The resulting shape change tracings were transformed into concentration-effect curves by quantifying the relative maximal shape change occurring 1 min after agonist addition in each case. The results are presented as the average mean values (S.E.M. varied by less than 15% for each value) from three experiments.
Discussion
ADP is the cognate agonist of the P2Y1, P2Y12, and P2Y13 receptors, which also are potently activated by 2MeSADP (Harden et al., 1998; Ralevic and Burnstock, 1998; Communi et al., 2001; Hollopeter et al., 2001). We show here that whereas the P2Y1 receptor is activated by subnanomolar concentrations of (N)-methanocarba-2MeSADP, neither the P2Y12 nor the P2Y13 receptor is sensitive to activation by this non-nucleotide agonist. Therefore, a molecule has been identified that selectively binds and activates a member of the ADP-activated P2Y receptor family without exhibiting either agonist or antagonist action at the other two G protein-coupled receptors that comprise this subfamily of P2Y receptors. Our additional demonstration of activation of the platelet P2Y1 receptor without concurrent activation of the platelet P2Y12 receptor provides an initial glimpse of the potential usefulness of this conformationally constrained P2Y1 receptor-selective agonist for study of P2Y receptor-promoted signaling in mammalian tissues.
The molecular identification of the P2Y12 receptor (Hollopeter et al., 2001) and subsequent identification of the P2Y13 receptor (Communi et al., 2001), confirmed the previously held notion that activation of the P2Y1 receptor did not completely account for ADP-stimulated responses in glioma cells (Boyer et al., 1993, 1994; Schachter et al., 1997), in platelets (Fagura et al., 1998; Hechler et al., 1998; Jin and Kunapuli, 1998; Jin et al., 1998), and in other tissues. This was of particular importance to platelet biology since ADPpromotes platelet aggregation (Gachet and Cazenave, 1991), since human pathophysiologies related to platelet function may involve the ADP receptors or their downstream signaling cohorts (Cattaneo and Gachet, 1999), and since the platelet receptors for ADP are important drug targets (Cattaneo and Gachet, 1999; Gachet, 2003). Our discovery that bisphosphate nucleotide analogs were highly selective competitive antagonists of the P2Y1 receptor (Boyer et al., 1996, 1998) provided important reagents used to unravel the relative roles of the ADP-activated P2Y1 receptor and another ADP-activated receptor (now known to be the P2Y12 receptor) in the Gq- and Gi-mediated signaling responses, respectively, and in the shape change and aggregation responses of platelets to ADP (Fagura et al., 1998; Hechler et al., 1998; Jin and Kunapuli, 1998; Jin et al., 1998).
The identification of (N)-methanocarba-2MeSADP as an agonist that potently activates P2Y1 but not P2Y12 or P2Y13 receptors now provides a pharmacological approach to specifically activate the P2Y1 receptor in tissues that express both this receptor as well as the P2Y12 and/or P2Y13 receptor. This obviously is a goal in pharmacological studies of ADP-promoted responses of platelets, and our data with (N)-methanocarba-2MeSADP provide the first demonstration of independent activation of the P2Y1 receptor in a mammalian tissue expressing multiple ADP-activated P2Y receptors. This selective agonist also may prove important in studies in the central nervous system where both the P2Y1 (Webb and Barnard, 1999) and P2Y12 (Hollopeter et al., 2001) receptors are widely distributed.
Introduction of conformational constraints, in an otherwise freely flexible molecule, potentially provides a powerful approach in ligand development. Lock of the conformation of a biologically active molecule in its preferred geometry for association with a target receptor may enhance pharmacological potency and selectivity, and molecular insight into the conformational requirements of the binding site may be gleaned. In the case of P2Y receptor ligands, we have reported that the (N)-conformation of the ribose or pseudoribose moiety is accommodated or preferred at the P2Y1, P2Y2, P2Y4, or P2Y11 receptors, but not at the P2Y6 receptor. This conclusion was based on the synthesis and pharmacological characterization of both adenine and uracil nucleotide methanocarba derivatives. The present study extends this analysis to the Gi-linked P2Y12 and P2Y13 receptors, which do not recognize this analog locked in the (N)-conformation, thus placing these receptors in the same category as the P2Y6 receptor. The structural basis for this distinction of conformationally constrained analogs among the P2Y receptors is unclear, since recognition of (N)-methanocarba analogs does not correspond to any obvious protein sequence homology patterns among these receptors.
In the case of (N)-methanocarba-2MeSADP, we have illustrated that ring constrained (N)-methanocarba substitution combined with another functionality increases both agonist potency and, as illustrated here, receptor selectivity among ADP-activated P2Y receptors. Combined with our previous results illustrating that (N)-methanocarba-2MeSADP does not activate the Gq/phospholipase C-coupled P2Y2, P2Y4, P2Y6, and P2Y11 receptors (Ravi et al., 2002), the current results have significant implications for development of agonists of high potency and selectivity for other subfamilies of receptors within the P2Y receptor family.
Acknowledgments
We thank Savitri Maddileti for technical assistance and help with the figures and David Rinker for assistance in the construction of the manuscript.
This work was supported by U.S. Public Health Service Grants GM38213 and HL54889.
ABBREVIATIONS
- (N)-methanocarba-2MeSADP or MRS2365
(1′S,2′R,3′S,4′R,5′S)-4-[(6-amino-2-methylthio-9H-purin-9-yl)-1-diphosphoryloxymethyl]bicyclo[3.1.0]hexane-2,3-diol
- 2MeSADP
2-methylthio-ADP
- MRS2179
N6-methyl 2′-deoxyadenosine-3′,5′-bisphosphate
References
- Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′, 5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Lazarowski ER, Chen X-H, Harden TK. Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase but does not activate phospholipase C. J Pharmacol Exp Ther. 1993;267:1140–1146. [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Romero T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1-receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- Boyer JL, Zohn I, Jacobson KA, Harden TK. Differential effects of putative P2-purinoceptor antagonists on phospholipase C- and adenylyl cyclase-coupled P2Y-purinoceptors. Br J Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HA, Lazarowski ER, Boucher RC, Harden TK. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5′-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991;40:648–655. [PubMed] [Google Scholar]
- Cattaneo M, Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol. 1999;19:2281–2285. doi: 10.1161/01.atv.19.10.2281. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to G(i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Chabre O, Wong YH, Federman AD, Bourne HR. Mutational activation and coupling to receptors and recombinant Gaq: phospholipase C. J Biol Chem. 1992;267:31–34. [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Fagura MS, Dainty IA, McKay GD, Kirk IP, Humphries RG, Robertson MJ, Dougall IG, Leff P. P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y(ADP)-receptors. Br J Pharmacol. 1998;124:157–164. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemostasis. 2001;86:222–232. [PubMed] [Google Scholar]
- Gachet C. Platelet activation by ADP: the role of ADP antagonists. Ann Med. 2003;32:15–20. [PubMed] [Google Scholar]
- Gachet C, Cazenave JP. ADP-induced blood platelet activation: a review. Nouv Rev Fr Hematol. 1991;33:347–358. [PubMed] [Google Scholar]
- Harden TK, Barnard EA, Boeynaems JM, Burnstock G, Dubyak GR, Hourani SMO, Insel PA. P2Y receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media; London: 1998. pp. 209–217. [Google Scholar]
- Harden TK, Lazarowski ER, Boucher RC. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- Hechler B, Eckly A, Ohlmann P, Cazenave JP, Gachet C. The P2Y1 receptor, necessary but not sufficient to support full ADP-induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol. 1998;103:858–866. doi: 10.1046/j.1365-2141.1998.01056.x. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature (Lond) 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: structure-activity relationships and receptor docking. J Med Chem. 2001;44:3092–3108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Kavi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, Malmsjo M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: high potency of northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Gallo-Rodriguez C, Jang S-Y, Nandanan E, Adams M, Harden TK, Boyer JL, Jacobson KA. Acyclic analogues of deoxyadenosine 3′,5′-bisphosphates as P2Y1 receptor antagonists. J Med Chem. 2000;42:746–755. doi: 10.1021/jm9905211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP. Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol Sci. 1998;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, et al. Synthesis, biological activity and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter JB, Boyer JL, Li Q, Nicholas RA, Harden TK. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br J Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1 receptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji X, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding- and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Webb TE, Barnard EA. Molecular biology of P2Y receptors expressed in the nervous system. Prog Brain Res. 1999;120:23–31. doi: 10.1016/s0079-6123(08)63543-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]