Synopsis statement
Pediatric scleroderma includes two major groups of clinical entities, systemic sclerosis (SSc) and localized scleroderma (LS). Although both share a common pathophysiology, with an initial inflammatory phase associated with endothelial activation, and a later fibrotic phase evidenced by collagenization of tissue and appreciable skin thickness, their clinical manifestations differ. LS is typically confined to the skin and underlying subcutis, and though not fatal like SSc, up to a quarter of the patients may have extracutaneous disease manifestations, such as arthritis and uveitis. While any organ may be affected in SSc, vascular (Raynaud’s phenomenon), cutaneous (skin thickening), GI, pulmonary and musculoskeletal involvement are most commonly seen in children. Auto-antibody profiles in childhood onset SSc can assist in predicting internal organ involvement. Treatment for both forms of scleroderma targets the active inflammatory stage and halts disease progression; however, progress still needs to be made towards the development of a more effective anti-fibrotic therapy to help reverse disease damage.
Keywords: systemic sclerosis, localized scleroderma, morphea, pediatric rheumatology
Pediatric Scleroderma
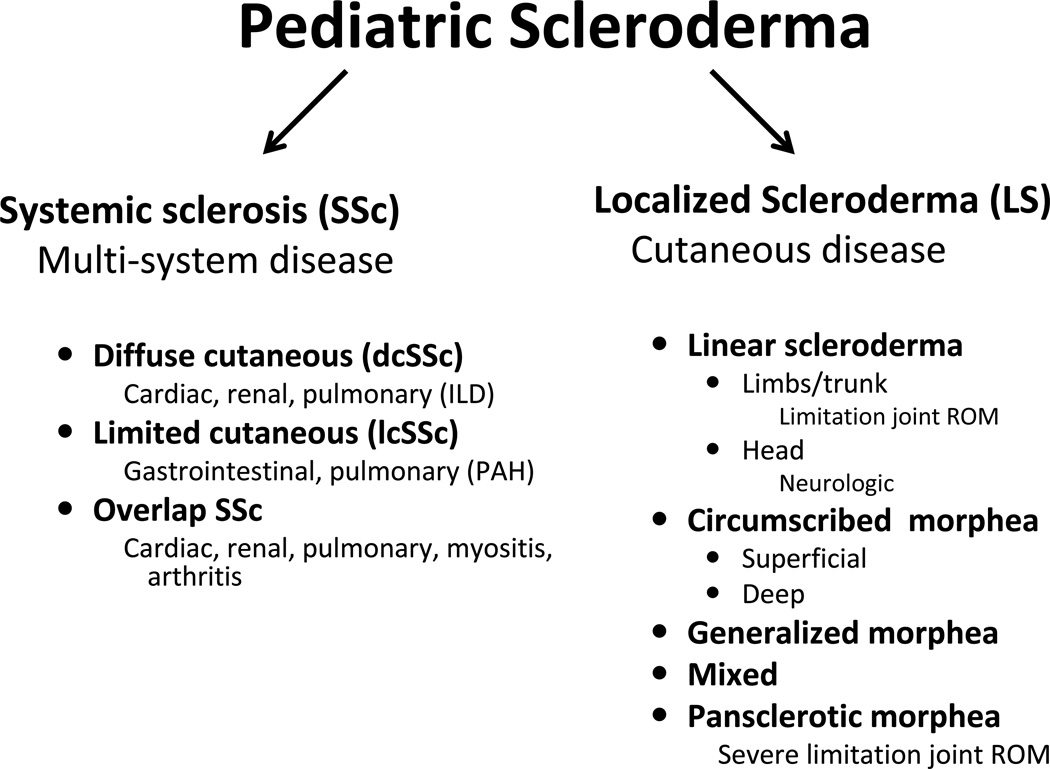
The term scleroderma literally means “skleros”, sclerosing or hardening, of the “derma”, skin. “Scleroderma” encompasses both forms of the disease: Systemic sclerosis (SSc), characterized by skin, vascular and visceral organ fibrosis, which more commonly affects adults, and localized scleroderma (LS), characterized by fibrosis of skin and underlying tissue without vascular or internal organ involvement, which more commonly affects children. Though they share a common underlying pathophysiology of excessive collagen deposition in an autoimmune setting, these two entities are clinically different with unique morbidities and prognoses (Fig 1). Both are uncommon in children with the estimated annual incidence of LS being 1–3 per 100,000 children [1] and SSc being 1 per million children [2]. The mean age of onset for both forms of pediatric scleroderma is between 7.3 and 8.8 years of age. Although less than 5% of all patients with SSc have pediatric onset, the majority of patients with LS have childhood onset [3–6]. Unfortunately, there is significant delay in diagnosis, with an average 1.9 to 2.8 years for SSc [3, 4] and 1.2 to 1.6 years for LS [5, 7]. For the few cases of congenital involvement of LS, the mean time to diagnosis is longer, 3.3 to 3.9 years [7, 8]. The approximate female to male ratio of pediatric SSc is 4:1 [3, 4] and for pediatric LS is 2:1 [5]. There is no clear evidence for racial predilection for either form of pediatric scleroderma.
Figure 1.
Pediatric scleroderma divided into systemic and localized disease, which is further differentiated into subtypes based on clinical findings of skin involvement. Clinical subsets are related, with particular morbidities as mentioned.
ILD = interstitial lung disease; PAH = pulmonary arterial hypertension; ROM = range of motion
Systemic Sclerosis
Categorization – clinical and serological
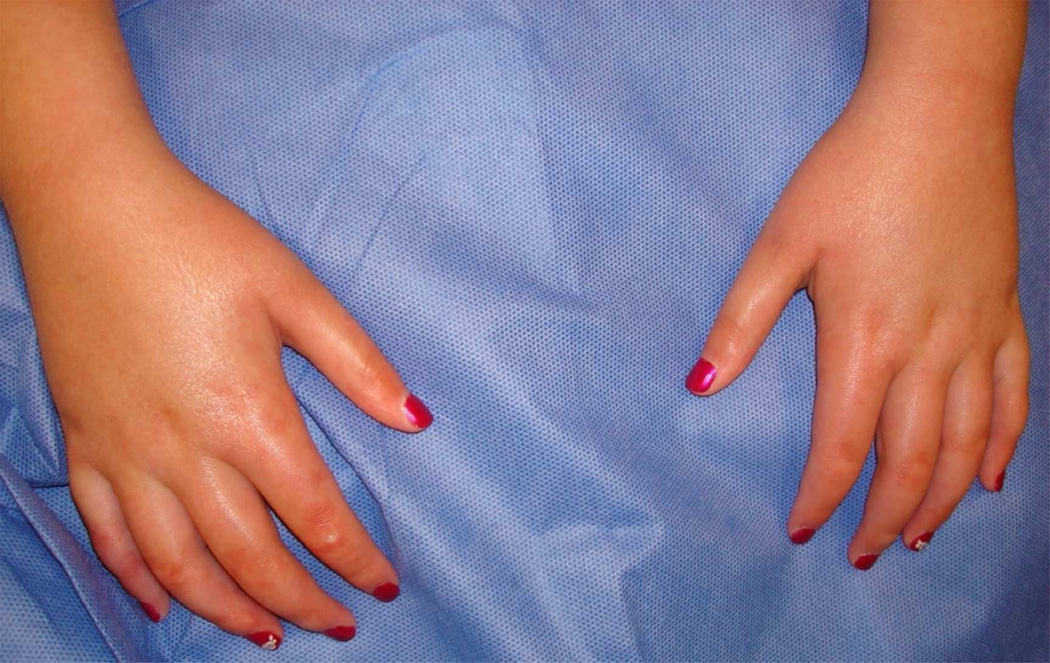
Systemic sclerosis (SSc) is a rare but potentially life threatening condition. There are three main clinical subtypes associated with different morbidities (Fig. 1): diffuse cutaneous (dc) SSc, characterized by widespread and rapidly progressive skin thickening (spreading proximal to elbows and knees) associated with early visceral disease (lung, heart, and kidney), limited cutaneous SSc (lcSSc), characterized by restricted and nonprogressive skin thickening (limited to distal extremities) associated with late visceral disease (pulmonary arterial hypertension, malabsorption), and overlap SSc, which can be dcSSc or lcSSc with features of another connective tissue disease, such as dermatomyositis or Systemic Lupus Erythematosus (SLE) [9]. Patients with CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) are considered to have lcSSc. Some patients with undifferentiated or mixed connective tissue disease may have shiny or full appearing skin of the distal fingers, but they must have the major criterion, ‘skin induration or sclerosis proximal to the metacarpal (MCP) or metatarsophalangeal (MTP)’, to be considered to have juvenile SSc (Fig. 2). There are an additional two minor criteria required for the diagnosis of SSc in children according to international consensus agreement (Table 1) [10]. In addition to the extent of skin thickening, particular scleroderma-related serum auto-antibody profiles assist in identifying subsets of SSc and predicting their organ involvement in both adult and childhood onset SSc [3, 4, 6, 11, 12]. For example, anti-topoisomerase antibody (Scl-70) would be expected in a patient with dcSSc, and would be worrisome for a rapid skin progression and the development of interstitial lung disease (ILD). [3, 4, 6]. The four most common antibodies in pediatric SSc are anti-topoisomerase antibody (Scl-70) (20–34%), typically associated with dcSSc and ILD as mentioned, followed by anti-centromere antibody (ACA) (2 – 16%), associated with lcSSc and pulmonary arterial hypertension (PAH), and anti-U1-RNP and Pm-Scl (polymyositis-scleroderma) antibodies (2 – 16%), both of which are commonly found in those with overlap syndromes of SSc and other connective tissue diseases with more prominent arthritis and myositis features, such as dermatomyositis or systemic lupus erythematosus [3, 4, 6]. The U1-RNP and Pm-Scl positive proportion of patients is likely higher than reported since some authors do not include overlap SSc or mixed connective tissue disease cases in their pediatric SSc cohorts. Overall, the frequency of these antibodies in children reflect the clinical subset frequencies of dcSSc, lcSSc and overlap SSc [3, 4, 6] Compared to adult SSc, overlap SSc is much more common in pediatric SSc (29% vs. 9%), as reflected by the increased frequency of U1-RNP and Pm-Scl antibodies, and is related to the higher percentage of myositis and arthritis observed in pediatric SSc [3]. There are several other SSc-related autoantibodies which associate with different organ manifestations; however they are much less common in childhood onset SSc. One of these is RNA polymerase III (POL3) antibody, which relates to severe renal disease in the form of scleroderma renal crisis. POL3 is rarely observed in childhood onset SSc, but can be found in up to 30% of adult onset SSc patients and reflects the clinically significant higher proportion of renal disease in adult SSc () [3, 6]. Although many scleroderma-related autoantibodies have been identified in adult SSc, there is a high proportion of pediatric patients, 20– 23%, who have a positive ANA without a specific autoantibody identified [3, 6].
Figure 2.
Typical shiny and thick skin of the hands with skin induration traveling up the forearm (past the metacarpal phalangeal joints) in a patient with systemic sclerosis.
Table 1.
Provisional guidelines for the classification criteria of juvenile systemic sclerosis *
| Major criterion (required) |
| Skin induration/thickening proximal to the MCP or MTP joints |
| Minor criterion (2 required) (scleroderma specific organ involvement) |
| Cutaneous |
| Sclerodactyly |
| Peripheral Vascular |
| Raynaud phenomenon |
| Nailfold capillary changes (megacapillaries and avascular areas) |
| Digital tip ulcers |
| Gastrointestinal |
| Dysphasia |
| Gastroesophageal reflux |
| Cardiac |
| Arrhythmias |
| Heart failure |
| Renal |
| Scleroderma renal crisis |
| New arterial hypertension |
| Respiratory |
| Pulmonary fibrosis (on chest radiograph or high resolution computed tomography) |
| Decreased DLCO |
| Pulmonary arterial hypertension (primary or secondary to ILD, assessed by echocardiography) |
| Neurologic |
| Neuropathy |
| Carpal tunnel syndrome |
| Musculoskeletal |
| Tendon friction rubs |
| Arthritis |
| Myositis |
| Serologic |
| Anti-nuclear antibody |
| SSc-selective autoantibodies |
| Anti-topoisomerase 1 (Scl-70), anti-centromere, anti-RNA polymerase I or III, anti-PM-Scl, anti-fibrillin |
Modified from Zulian et al Arthritis Rheum 2007; 57(2):203–212
Sensitivity of 90% and specificity of 96% when the major and two minor criteria present in clinical scenarios
Age of onset of the disease must be less than 16 years
Abbreviations: MCP = metacarpal phalangeal, MTP = metatarsophalangeal, DLCO =diffusing capacity for carbon monoxide, ILD = interstitial lung disease
Clinical manifestations
Major clinical manifestations of pediatric SSc
Vascular
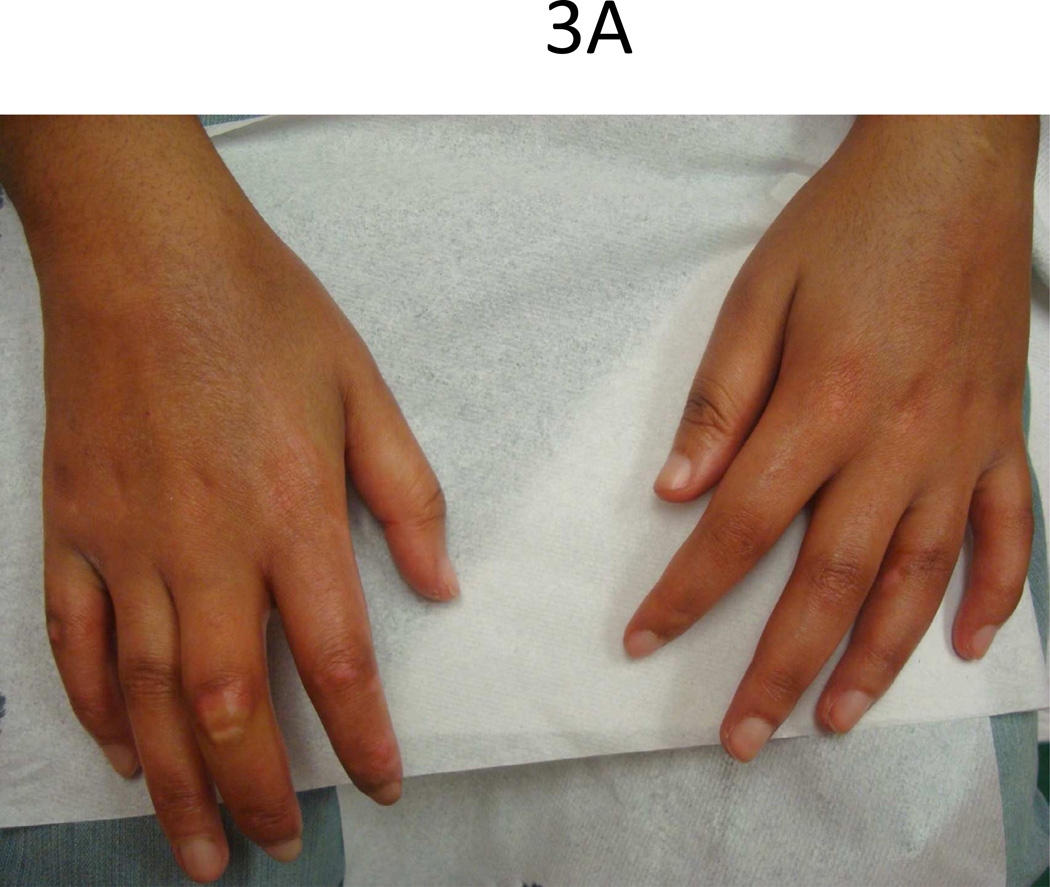
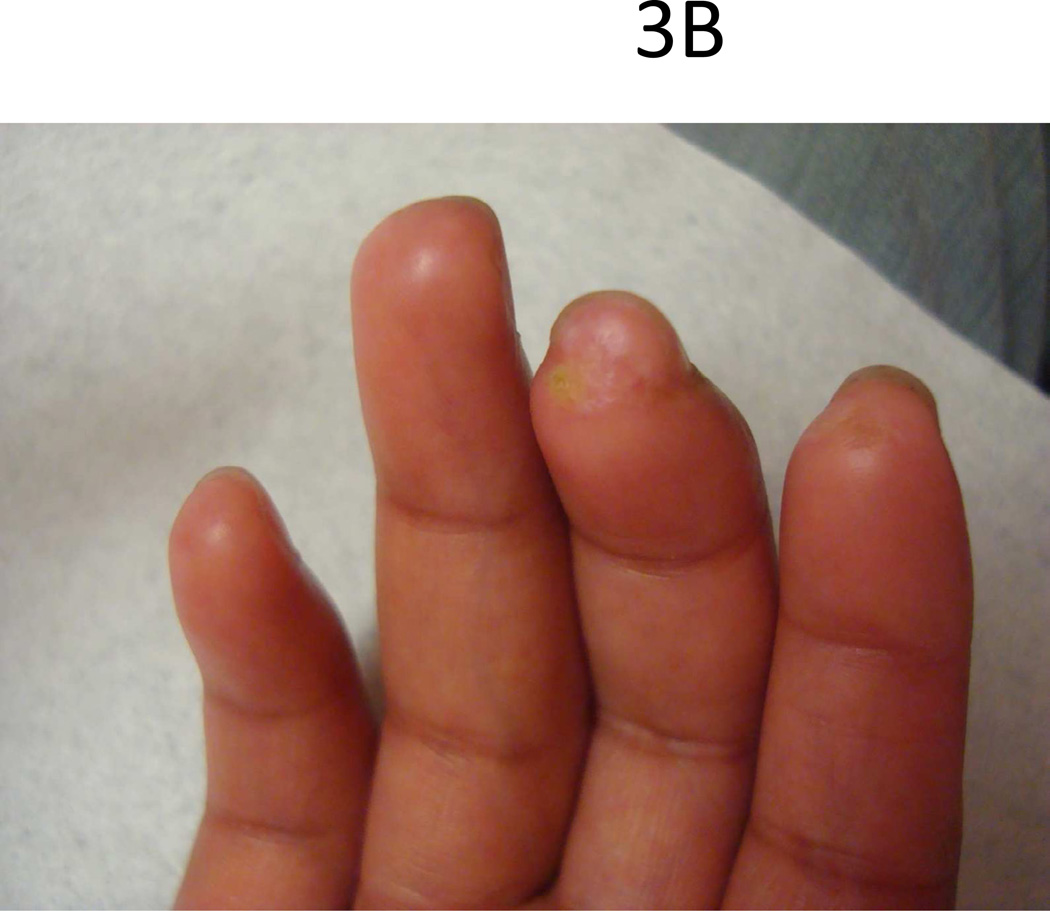
Common features at the onset of pediatric SSc are Raynaud’s phenomenon (RP) (70%) and skin changes of hands (60%) including edema, sclerodactyly and induration proximal to the MCPs [4]. In the largest cohort of pediatric SSc (153 patients) studied, 53% presented with both skin induration of hands and RP, and 10% of those presenting with RP also had digital infarcts (Fig. 3) [4]. Throughout the course of the disease, almost all will have RP (97%) and skin changes of the hands (96%) [3].
Figure 3.
Teenage female with typical (A) sclerodactyly and (B) digital pitting and ulcers.
Raynaud’s phenomenon is a vasospastic response leading to a triphasic color change of the hands (first pallor because of vasoconstriction, then blue secondary to cyanosis, and finally red because of reperfusion with subsequent swelling and pain) associated with a sensation of numbness and tingling. RP can also occur in toes and other acral areas including the ears, tip of nose, or tongue. In SSc, arterioles are more narrow and stiff due to marked intimal hyperplasia and fibrosis from endothelial dysfunction, which leads to more pronounced RP with resultant tissue damage/ischemia [13]. These vasculopathic changes are identifiable in the nailfold capillary beds of these patients by capillary microscopy, demonstrating dilatation, tortuosity, hemorrhage, drop-off (avascularization) and later arborization of the capillaries [14]. The prevalence of nailfold capillary changes in pediatric SSc is reported to be 50% [3, 4], but is likely higher if standardized microscopy was performed. Poor perfusion of the fingers eventually leads to digital tip pitting, ulceration (Fig. 3b), and in more severe cases auto-amputation. Healthy individuals can also experience Raynaud’s phenomenon without being at risk for developing an underlying connective tissue disease (primary Raynaud’s). These individuals typically never develop digital tip ulcers or have nailfold capillary changes since they do not have an underlying vasculopathy. Features that help the clinician differentiate primary Raynaud’s phenomenon from that secondary to a connective tissue disease (CTD) in both children and adults are the following: lack of nailfold capillary changes, digital tip pitting and/or ulceration, and a negative ANA [15, 16].
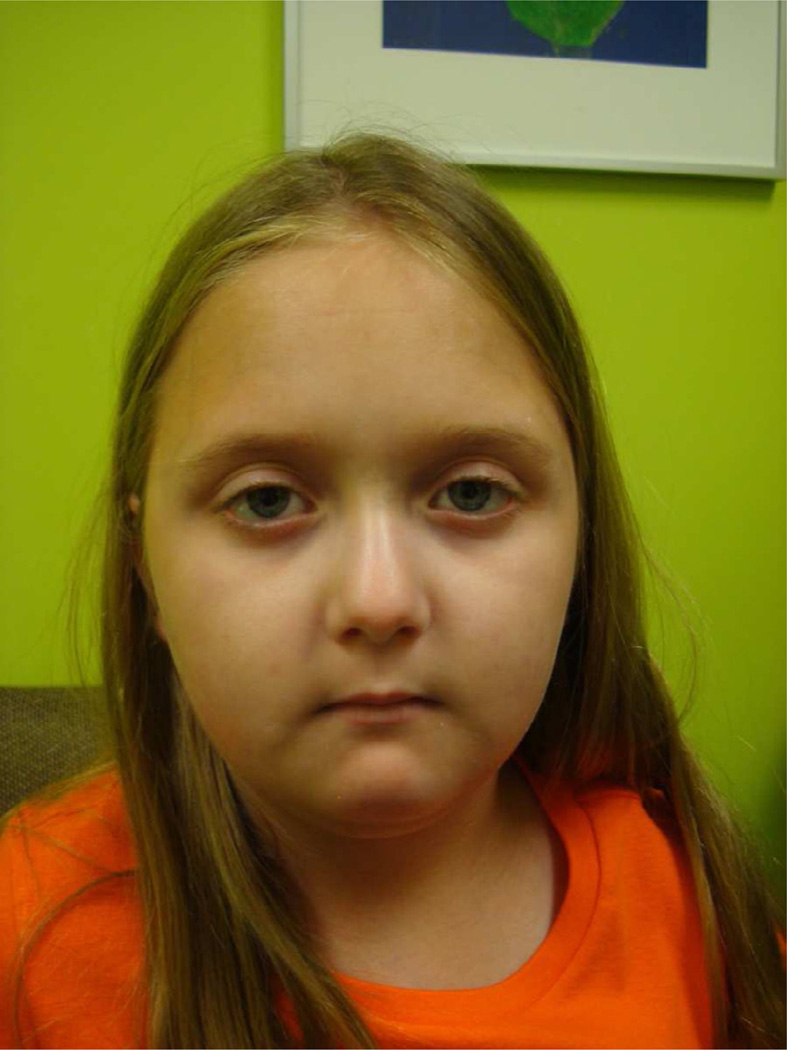
Cutaneous manifestations frequently bring children to medical attention, but because of the insidious and subtle onset of skin changes, there is often a delay in diagnosis with a mean time of 1.9 and 2.8 years between first sign of disease and diagnosis [3, 4]. Early in the clinical course, the skin is edematous with particular predilection for the distal extremities; rarely, more proximal limb, face, and trunk involvement is present. The induration phase, for which scleroderma is named, is characterized by loss of the natural pliability of the skin and the presence of a palpable skin thickness. The skin takes on a shiny, tense appearance with distal tapering of the fingers (Figs. 2 and 3a). Over time as skin thickens and underlying structures, such as tendons, become affected and shortened, the finger joints start to lose range of motion both in extension and flexion, and in more severe cases a ‘claw hand’ deformity results with great impact on performing activities of daily living (ADL). The typical scleroderma facies of tight skin and skin atrophy produces a pinched nose, thin pursed lips, small mouth, prominent teeth, and an expressionless appearance (Fig. 4). Skin thickness is measured as mild-moderate-severe throughout the body and a cumulative score is obtained using the modified Rodnan skin score (mRSS) [17]. The mRSS is obtained over longitudinal visits and a skin thickness progression rate (STPR) can be calculated and assist in predicting timing of internal organ involvement in those with dcSSc. A high STPR is associated with scleroderma renal crisis [18].
Figure 4.
Expressionless facies of scleroderma. Pre-pubertal female with decreased oral aperture and ‘pursed lips’.
Other skin findings in SSc include subcutaneous calcium deposits (calcinosis cutis) which may occur at pressure points, typically found on extensor surfaces of hand joints in SSc, and may occasionally extrude through the skin in a fashion similar to dermatomyositis. These lesions may be painful and ulcerate. However, they are more typically associated with chronic disease and are rarely present at time of disease onset and diagnosis. Telangiectasias are also a manifestation of SSc, most typically found in the lcSSc variant (previously known as CREST syndrome) and are commonly distributed on the face and upper extremities.
Other organ manifestations in SSc (in order of decreasing frequency) include gastrointestinal, pulmonary, musculoskeletal, cardiac, renal and neurological symptoms. Gastrointestinal symptoms occur in approximately half of the children, although more detailed investigation often indicates the presence of abnormalities in a larger percentage [3]. Esophageal dysmotility and gastroesophageal reflux often leads to dysphagia and esophagitis. Distal dysphagia, especially with solids, causing a sensation of ‘food getting stuck’ mid-chest is typical and represents dysfunction of the distal smooth muscle of the esophagus. Gastroparesis with delayed gastric emptying in addition to esophageal dysfunction can lead to increased reflux symptoms. Typically patients will complain of night time cough when lying prone due to silent aspiration and a brackish taste in their mouth upon wakening due to silent reflux. Chronic reflux may lead to esophageal strictures. Evaluation of the GI tract using manometry and intra-esophageal 24-hour pH monitoring have been reported as sensitive indicators of lowered esophageal tone and reflux [19]. However, these modalities are used less often in pediatrics. Instead, a swallow study to evaluate for aspiration and dysmotility, followed by an upper GI study with small bowel follow through, is typically employed. If the small bowel is involved, cramps, diarrhea, and constipation may result from peristaltic dysfunction. Episodes of pseudo-obstruction with post-prandial abdominal distension, pain, and nausea can occur due to a functional ileus. Bacterial overgrowth, steatorrhea, weight loss, volvulus, and even perforation can occur. Colonic disease occurs in the form of wide-mouth diverticula and a loss of the normal colonic architecture. Another complication of GI involvement with SSc is acute GI bleeding associated with gastric antral venular ectasia (GAVE) requiring photocoagulation. This condition is uncommon in children and is associated with early dc SSc, RNA-polymerase III positive patients [20].
Pulmonary involvement in pediatric SSc ranges from 30–70% in the literature and includes interstitial lung disease (ILD), pulmonary arterial hypertension (PAH) (either primary, which results from vasculopathy, or secondary from ILD), and abnormal pulmonary function tests (PFTs). PFT abnormalities especially of concern are decreased forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) [21]. Interstitial lung disease (ILD) typically begins with an inflammatory phase, aveolitis, characterized by a mixed cellular infiltrate in the lung interstitium that spills over into the alveolar spaces. Bronchoaveolar lavage (BAL) demonstrates increased neutrophils and/or eosinophils in addition to alveolar macrophages [22]. The presence of aveolitis is also suggested by ground-glass opacities on high resolution computerized tomography (HRCT) scans of the lungs. As ILD progresses, inflammation subsides, as there is a transition towards fibrosis with the thickening of alveolar walls and remodeling of the lung. Progressive pulmonary fibrosis may result in moderate to severe restrictive lung disease with significant decrease in FVC and DLCO. Clinically, ILD typically presents as slowly progressive dyspnea with exertion over years. In comparison, PAH presents with rapid progression of dyspnea on exertion over months. In the setting of PAH, PFTs demonstrate a decreased DLCO and abnormal echocardiogram findings with estimated Pulmonary Artery (PA) or Right Ventricular (RV) systolic pressure > 40mmHg, verified by right heart catheterization with mean PA pressure > 25mmHg. For most children with SSc or suspected SSc, initial assessment includes chest x-ray, pulmonary function tests, including DLCO, and an echocardiogram. Additional cardiopulmonary testing is pursued if abnormalities are detected or the patient is too young to appropriately complete testing (i.e. PFTs). Abnormal PFTs prompt evaluation with HRCT of chest, and possibly BAL, if further evidence of inflammation (aveolitis) is required or evaluation of infection is needed before starting therapy [21, 23]. Evidence of PAH on echocardiogram is more formally assessed by right-heart catheterization.
Compared to adult onset SSc, musculoskeletal involvement is more common in pediatric SSc. Approximately 30 – 40% experience inflammatory arthritis (joint effusions) in addition to the typical ‘dry synovitis’ of scleroderma, reflected by the fibrosis of tendons transversing the joints, which limits their range of motion (ROM). Early in the disease process, especially in those with dcSSc, tenosynovitis/bursitis causes palpable tendon friction rubs (a ‘leathery crepitus’) when the joint is extended or flexed [24]. When the patient has myopathy, typically there is a symmetrical proximal weakness, especially of the shoulder girdle and humeral muscles, sometimes with pronounced atrophy. Myositis is also reflected by elevated muscle enzymes and changes on MRI of muscle groups similar to that of juvenile dermatomyositis (JDM). The muscle biopsy of SSc differs in that of JDM by having more fibrosis and the presence of thickened capillaries [25]. Children with dcSSc and myositis are particularly at risk for severe cardiac involvement, including myocardial perfusion deficits and dilated cardiomyopathy[26].
Cardiac involvement, although infrequent, is the major cause of scleroderma-related mortality in children with SSc, and is evidenced by 10 out of 15 deaths in Martini et al cohort [4] and 5 out of 32 in Scalapino et al cohort [3]. Cardiac manifestations result from the combination of myocardial fibrosis, vascular insufficiency, and inflammation and include heart block, arrhythmia, congestive heart failure, cardiomyopathy, and pericarditis. In contrast to pediatric SSc, scleroderma-related mortality in adults is primarily caused by pulmonary involvement, both ILD and PAH [27].
Mild renal dysfunction is not uncommon in SSc and is secondary to vasculopathy instead of glomerulonephritis, which is observed in systemic lupus erythematosus. This is supported by pathologic findings of intimal proliferation of the interlobular arteries of the kidney and lack of active urinary sediment (i.e. red blood cell casts). In contrast, a more severe and morbid form of renal disease, scleroderma renal crisis (SRC), only occurs in approximately 15% of adult patients with SSc and even less commonly in children. SRC is characterized by accelerated arterial hypertension, renal insufficiency, microangiopathic hemolytic anemia, and thrombocytopenia. In adult SSc, SRC previously had a 20% survival rate at 1 year, but now has an 80% survival rate due to the advent of angiotensin converting enzyme inhibitors (ACEI) [27]. SRC is most frequently seen early in disease course of dcSSc RNA – polymerase III positive patients and is thought to be augmented by higher doses of corticosteroids. Renal crisis is rare in children with SSc, with less than 5% affected and only 5 cases reported (2 of whom died from renal disease) among the three large published cohorts [3, 4, 28].
Outcome
Despite all the potential organ involvement in pediatric SSc, it has a far more favorable prognosis at 5, 10, and 15 years compared to adult SSc with a lower frequency of severe organ involvement. Foeldevari and colleagues [28] recently reported Kaplan Meier survival rates at 10 years for children to be 98% versus 75% in adults. Treatment of SSc is dependent on organ involvement but general measures such as antacid medication for GI protection, prokinetic agents for dysmotility, rotating antibiotics for malabsorption, as well as vasodilators for Raynaud’s phenomenon (RP) (may also help with PAH), non-steroidal anti-Inflammatory drugs (NSAID) and physical therapy (PT) for arthritis or tendonitis are generally recommended. Corticosteroids for myositis, arthritis and other inflammatory clinical features must be used with caution as they can prompt scleroderma renal crisis. More specific treatment recommendations for different organ manifestations have been agreed upon by a consensus conference of international scleroderma experts and were published in 2009 [29]. It is notable that there has been a shift in both pediatric and adult SSc treatment of early diffuse skin disease from the use of D-penicillamine to mycophenolate mofetil (MMF) due to potential antiproliferative effects, although not included in the treatment recommendations previously discussed [30].
Localized Scleroderma
Classification
Localized scleroderma, also known as ‘morphea’, has a different pattern of skin involvement than systemic sclerosis (SSc) and encompasses several subtypes classified by depth and pattern of the lesion(s). The group of disorders is characterized by fibrosis that is mainly confined to the skin and subcutaneous tissue; however, deeper forms also involve the fascia, muscle, tendon and joint capsule. The traditional classification delineated by Peterson et al. [31] described five subclassifications including plaque morphea, generalized morphea, bullous morphea, linear morphea, deep and pansclerotic morphea (Table 2a). However, more recently a consensus of experts made modifications to provide more clinically applicable classifications, titled the ‘Padua criteria’, which include a ‘mixed morphea’ subtype, given the prevalence of this category to be 15% (Table 2b) [32].
Table 2.
| a: LS° Subtypes: Mayo Classification‡ |
|---|
| Plaque morphea† |
| Morphea en plaque† |
| Guttate morphea |
| Atrophoderma of Pasini and Pierini |
| Generalized morphea† |
| Bullous morphea |
| Linear morphea† |
| Linear morphea (linear scleroderma)† |
| En coupe de sabre† |
| Progressive hemifacial atrophy |
| Deep morphea† |
| Subcutaneous morphea† |
| Eosinophilic fasciitis |
| Morphea profunda† |
| Disabling pansclerotic morphea of children |
| b: Proposed subtypes for Juvenile LS°: Padua Preliminary Classification‡ | |
|---|---|
| Circumscribed Morphea | Oval/round lesions
|
| Linear Scleroderma | Linear lesions can involve dermis, subcutaneous tissue, muscle, bone
|
| Generalized morphea | ≥ 4 large plaques (>3cm) on at least 2 of 7 anatomic areas: head/neck, R upper extremity, LUE, RLE, LLE, anterior trunk, posterior trunk |
| Pansclerotic morphea | Circumferential involvement of limb (spares fingertips/toes) All depths skin/subcutaneous tissue/muscle/bone |
| Mixed morphea | Combination of two or more of above subtypes |
LS = localized scleroderma
most prevalent subtypes in pediatric and adult LS
modified from Peterson et al Mayo Clin Proc 1995; 70:1068–76
modified from Laxer & Zulian Curr Opin Rheumatol 2006;18:606
Disease activity and damage features (clinical and histological findings)
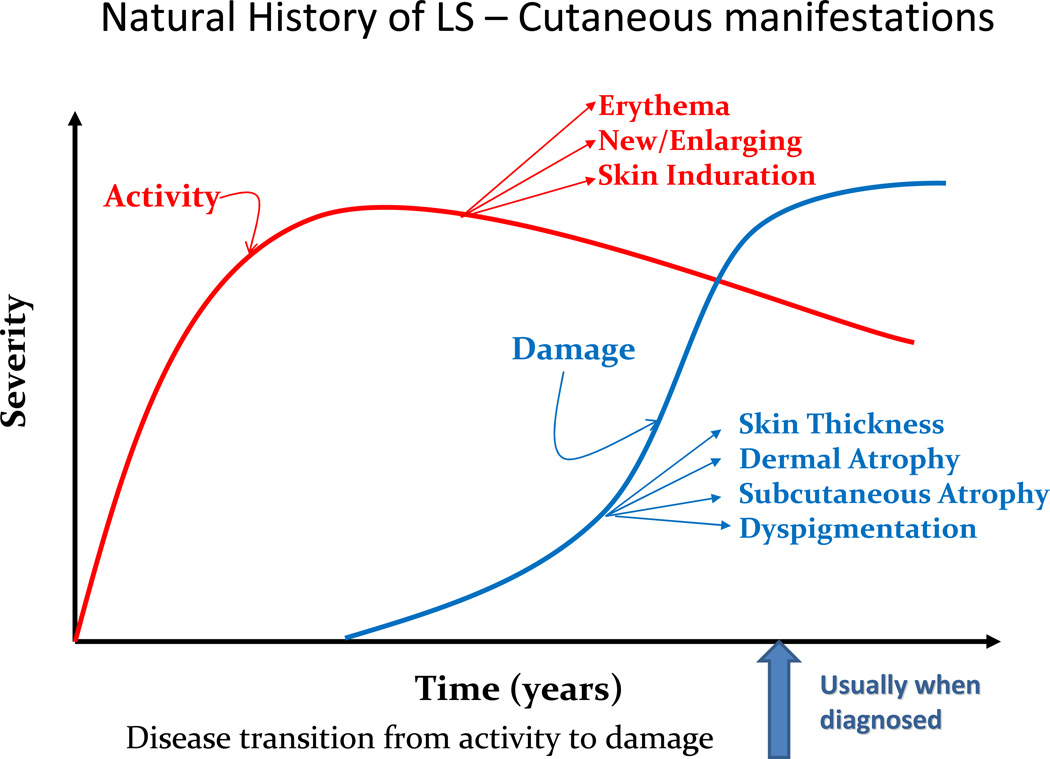
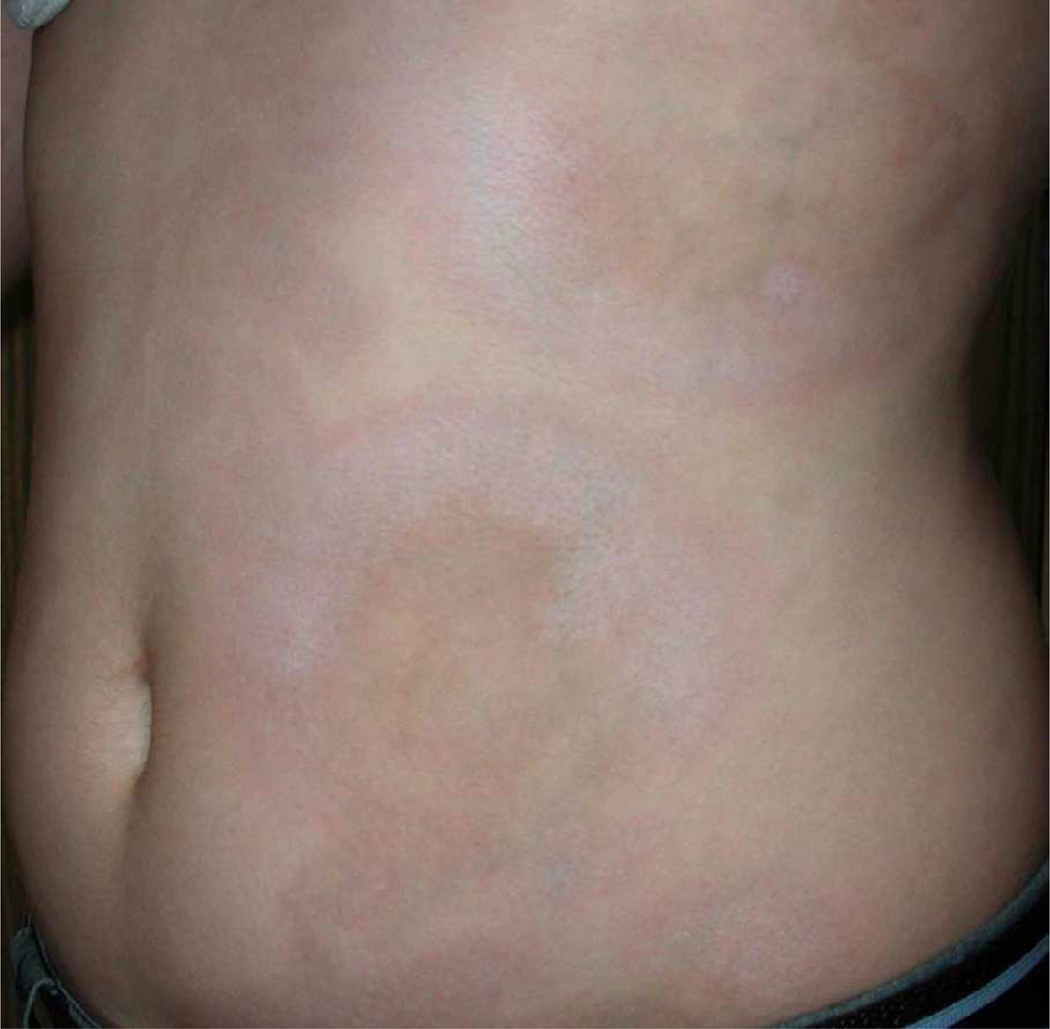
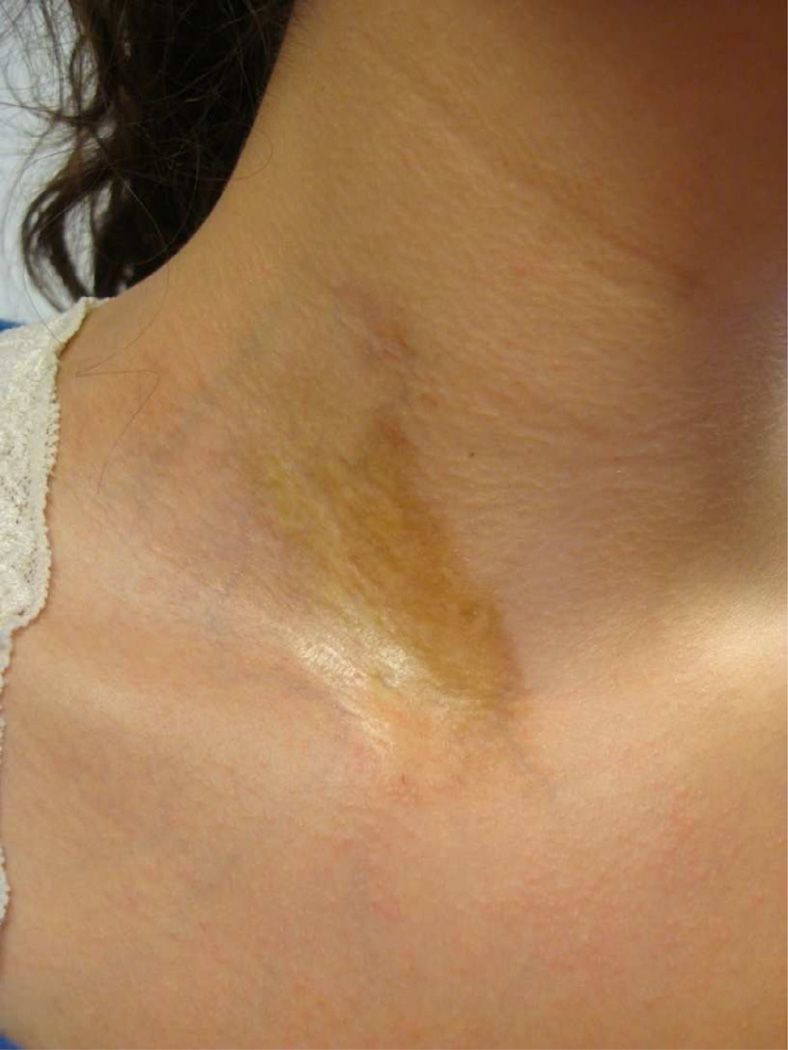
Similar to SSc both clinically and histologically, LS exhibits an earlier more active phase of disease and a later more fibrotic phase (Fig. 5). Early active lesions in LS are characterized by a violaceous inflammatory border or ‘lilac ring’ (Fig. 6) and skin induration throughout the lesion, including the border. During this active state, lesions may also expand and new lesions can accumulate [33]. Histological findings in the early stages of disease consist of a perivascular infiltrate of predominantly lymphocytes with admixed rare plasma cells and eosinophils in the deep reticular dermis and subcutis, accompanied by thickened collagen bundles, decreased elastic fibers and swollen endothelial cells [34, 35]. Over time, disease damage accumulates and is represented by an increase in skin thickness, especially at the center of the lesion, sometimes leaving an ivory-colored sclerotic center (Fig. 7). Histological evaluation of the skin reveals thickened hypocellular (homogenized) collagen which replaced the previous inflammatory infiltrate around the dermal appendages, leaving behind atrophic eccrine glands and hair follicles. In addition, capillaries are decreased in number with a fibrotic wall and narrowed lumen [34, 35]. The clinical outcome of these findings is reflected in dermal and subcutaneous atrophy. On physical examination, when the dermis is atrophic, there is lack of hair growth at the site of the lesion, visible veins, and slight ‘cliff drop’ appearance to the skin (Fig. 8). Subcutaneous atrophy exists on a continuum and can be as mild as a flattening of the skin, to a more ‘scooped out’ concave appearance of the adipose tissue in more moderate lesions, and in the most severe instances, to the point one can see the muscles moving underneath the skin. Post-inflammatory hyper- and hypo-pigmentation also are a result of previous inflammation and are often the features which bring the lesion to the attention of a medical provider. Often families will miss the early active phase and consider the erythema/violaceous color more of a bruise or injury, but it is the fact that the ‘bruise that does not go away’ (post-inflammatory hyperpigmentation) that often prompts the family to seek medical attention (Fig. 5).
Figure 5.
Phases of localized scleroderma and associated features. Active disease features, erythema, skin induration/edema and new or enlarging lesions, occur first and give way to disease damage features, dyspigmentation, atrophy and skin thickening.
Figure 6.
Typical ‘lilac ring’ observed at the border of multiple plaque lesions of the trunk indicating active disease, courtesy of Elena Pope at Children’s Sick Kids, Toronto.
Figure 7.
Waxy yellow center of plaque lesion remaining on right side of the neck years after the onset of localized scleroderma.
Figure 8.
Dermal atrophy of LS, demonstrated by ‘cliff drop’ lesions in a teenage female with generalized morphea.
Pathogenesis (shared between LS and SSc)
The shared histological findings between the skin of those with SSc and LS exemplify their common pathophysiology despite different clinical disease features. Both are autoimmune diseases with an inflammatory, fibrotic and vascular component. Mononuclear cell populations identified in both SSc and LS skin specimens are predominately T cells, both CD8 and CD4 positive, with a higher percentage of CD4 cells (T-helper) [36, 37]. Th-cell associated cytokines and chemokines such as interleukins [IL] -2, -4, -6, -8, -13 and TNF-a, have been identified in the circulating sera and stimulated PBMCs for LS and SSc patients [38–43]. Of note, some serum cytokines have been found in higher concentrations in LS subjects compared to SSc, which is interesting since LS is termed a ‘localized disease’; however, this may support the high frequency of extracutaneous manifestations (approximately 25%) demonstrated in LS [44]. There have been several correlations between clinical variables of disease severity in LS and cytokines and their soluble receptors, such as number of plaque and linear lesions (IL-13, TNF-a,sIL-2r, sIL-6r), generalized morphea and linear scleroderma subsets (IL-2,-4,-6,-8,-13,TNF-a, sIL-2r, sIL-6r), and antibody correlation, anti-histone and/or ss-DNA antibodies (IL-4, -6, TNF-a, sIL-2r, sIL-6r) [38–40, 45–47]. These T-cell associated cytokines in turn, further stimulate fibroblasts and endothelial cells to produce transforming growth factor Beta (TGF-β) and connective tissue growth factor (CTGF), which are thought to be the main stimulators of tissue fibrosis (via increased collagen production) and endothelial cell damage (via influence of adhesion molecules ICAM-1 and VCAM-1) [48–51].
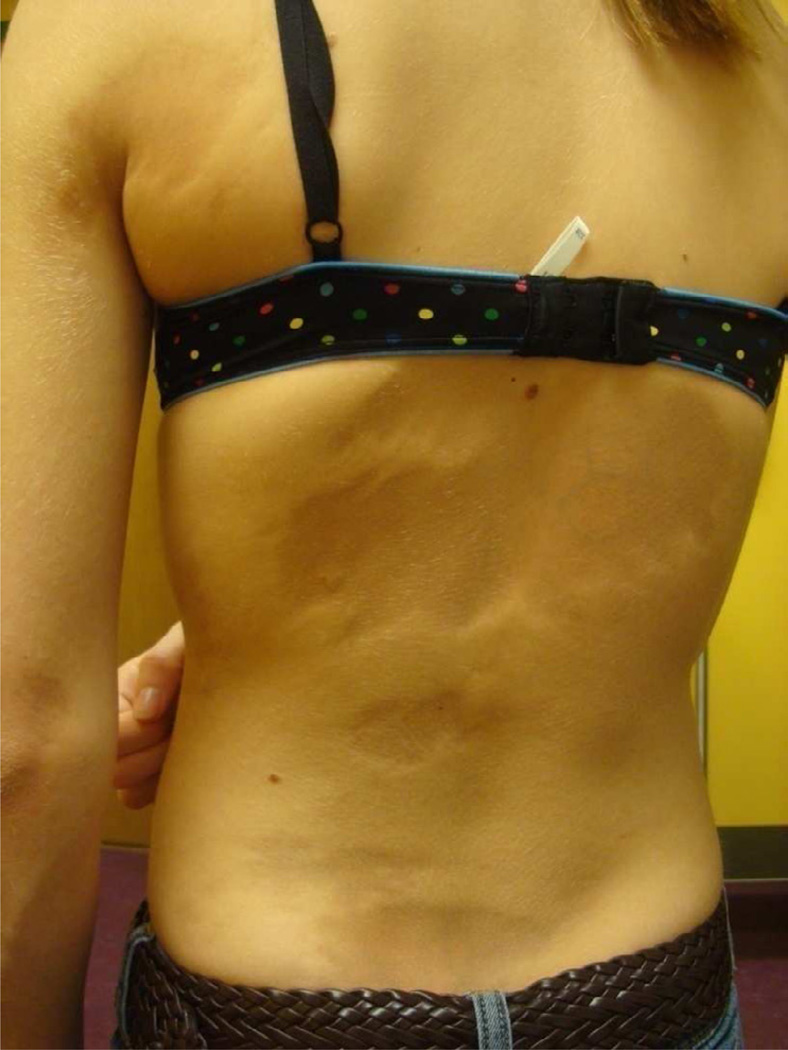
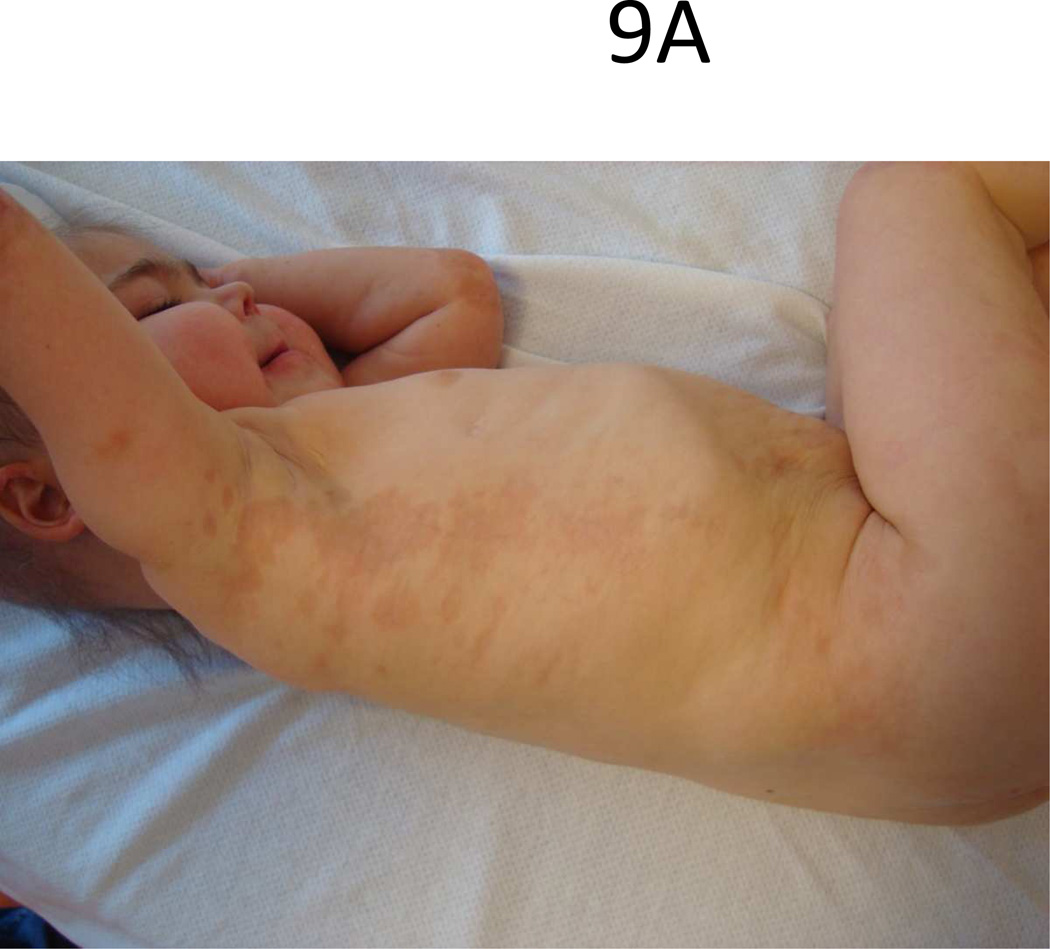
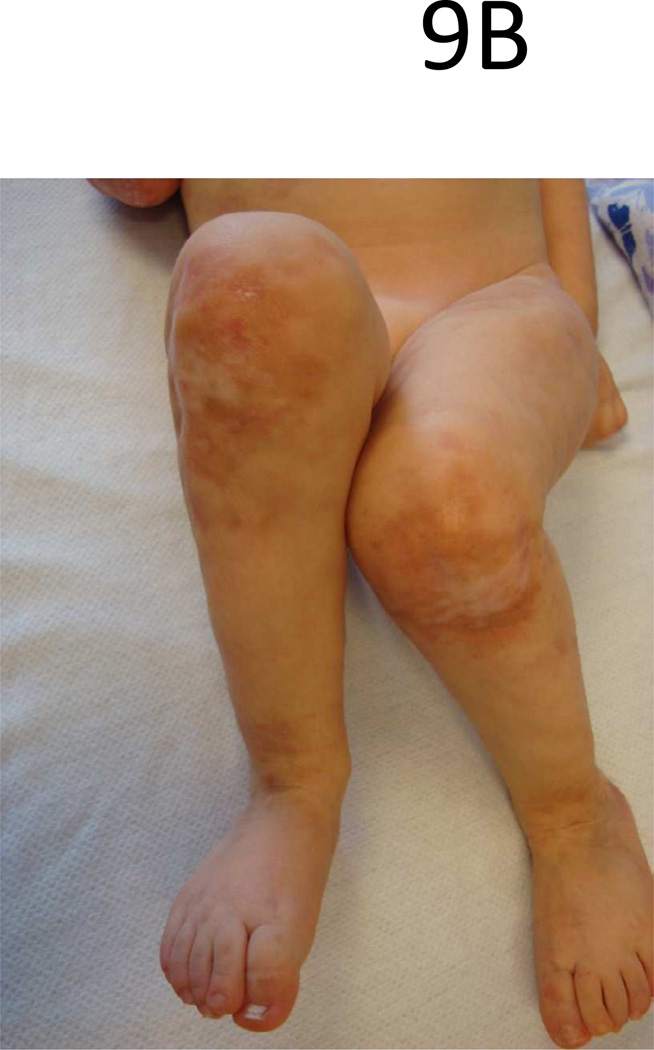
Microchimerism is another area of interest being investigated regarding a shared pathogenesis between LS and SSc. Clinically and histologically, LS and SSc share characteristics with chronic graft versus host disease (cGVHD) (Fig 9), a type of microchimerism which plays a role in pathogenesis. Chimeric cells have been identified in patients with LS and SSc using real time PCR techniques [52, 53].
Figure 9.
Linear lesion down trunk (A) and legs (B) of a toddler with sclerodermatous chronic graft versus host disease.
Autoimmunity, as one of the components propagating LS, is supported by the auto-antibody profile, concurrent associated autoimmune diseases, and family history of autoimmune disease observed in patients with LS. Autoantibodies are commonly positive in LS, including anti-nuclear antibody (ANA), anti-histone antibody (AHA), and anti-single stranded DNA antibody (ss-DNA Ab) [54]. ANA is a classic serologic marker of autoimmune disease and is found in a high frequency of LS patients, with a reported range between 42 and 73% [5, 55]. It is thought that higher titers are associated with early disease [55] and increased risk for extracutaneous manifestations [44, 56]. The prevalence of ss-DNA in LS is approximately 50% [57] and is associated with linear scleroderma (especially those with joint contractures), deep muscle involvement, and increased number of anatomic sites; all reflecting an increase in disease severity [56, 58]. In a sub-group of LS patients, ss-DNA Ab has been shown to reflect disease activity status with titers decreasing as the skin lesions improve clinically [54, 56]. AHA is found in a similar frequency in LS (47%) [59] and is also correlated with indicators of disease severity; including total surface area of body involved, number of lesions, linear disease and the presence of joint contractures [54, 56]. In a study focusing on linear scleroderma, the combination of positive ss-DNA and AHA was found in 23% of patients (adult and pediatric LS) and was significantly correlated with the presence of joint contractures [56]. The aforementioned antibodies indicate disease severity and may help in stratifying patients with early diagnosed disease, suggesting the possible initiation of more aggressive therapy and increased surveillance.
Concomitant and familial rheumatic and autoimmune disease is more prevalent in LS (pediatric and adult) compared to the general population [60]. Concomitant disease was reported in 5–10% of children [7, 44, 60] and 30% of adults with LS [60]. The most common disorders present were dermatologic autoimmune disease (psoriasis, vitiligo, alopecia areata), followed by rheumatic conditions (juvenile idiopathic arthritis, rheumatoid arthritis (RA), systemic lupus erythematosus(SLE) and Sjogren syndrome) [7, 60]. When evaluated by LS subtype, the frequency of concomitant autoimmune disease was significantly higher in patients with generalized morphea [60]. Familial rheumatic diseases and other autoimmune disorders were reported in 12–25% of all patients (both pediatric and adult), with the most common being psoriasis, followed by RA, SLE, SSc, LS, multiple sclerosis, and thyroiditis [5, 7, 60]. Again, an association between generalized morphea and increased frequency of these diseases was demonstrated [5].
Clinical manifestations
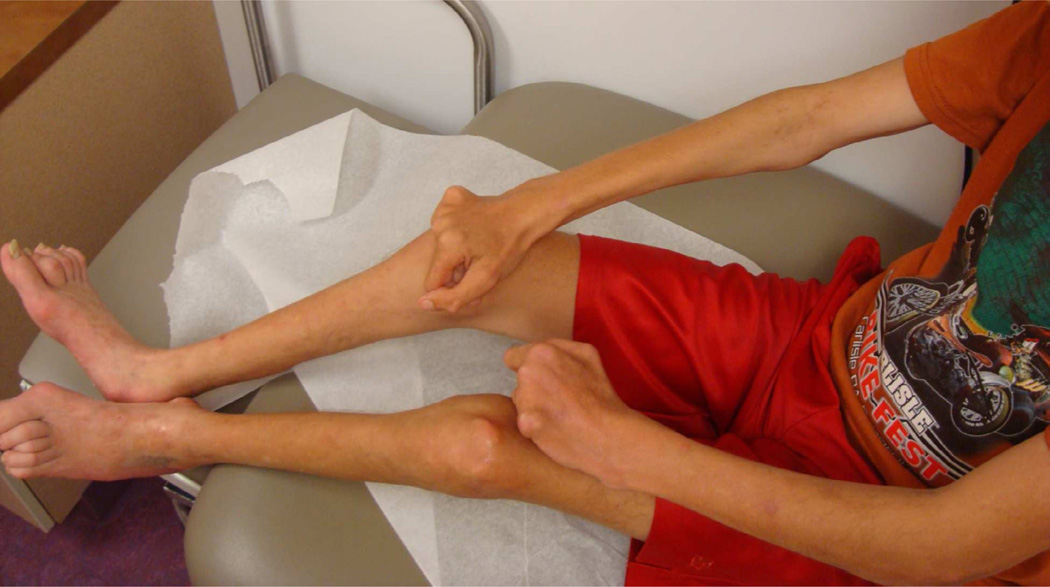
Although the term localized scleroderma or ‘morphea’ encompasses all of the subtypes into a single category, their cutaneous/subcutaneous findings and extra cutaneous associations are quite unique. Linear scleroderma (LiScl) is the most common subtype occurring in 50–60% of children with LS, and is characterized by a linear streak or band involving the dermis, subcutis, and often, the underlying muscle, tendons and bone. LiScl typically occurs as a single, linear unilateral band on the extremities, trunk or head (Fig 10) [5, 7]. Legs are the most commonly involved sites, followed by arms, frontal head and trunk. Lesions of LiScl affecting the extremities are most worrisome when they transverse a joint, since there is the potential for sclerosis and tightening of the underlying muscle, tendon and joint capsule, resulting in joint contracture and associated limb dysfunction. Of particular concern in pediatric patients with LiScl is involvement of the epiphyseal growth plate, which can lead to permanent shortening or atrophy of the limb. Orthopedic complications are reported in approximately 30–50% of patients with LiScl of the limb(s) [7, 58, 61]. Peterson et al reported mild to moderate disability at follow-up in those with LiScl of the extremity in their cohort (mean follow-up time of 9.2 years) [1].
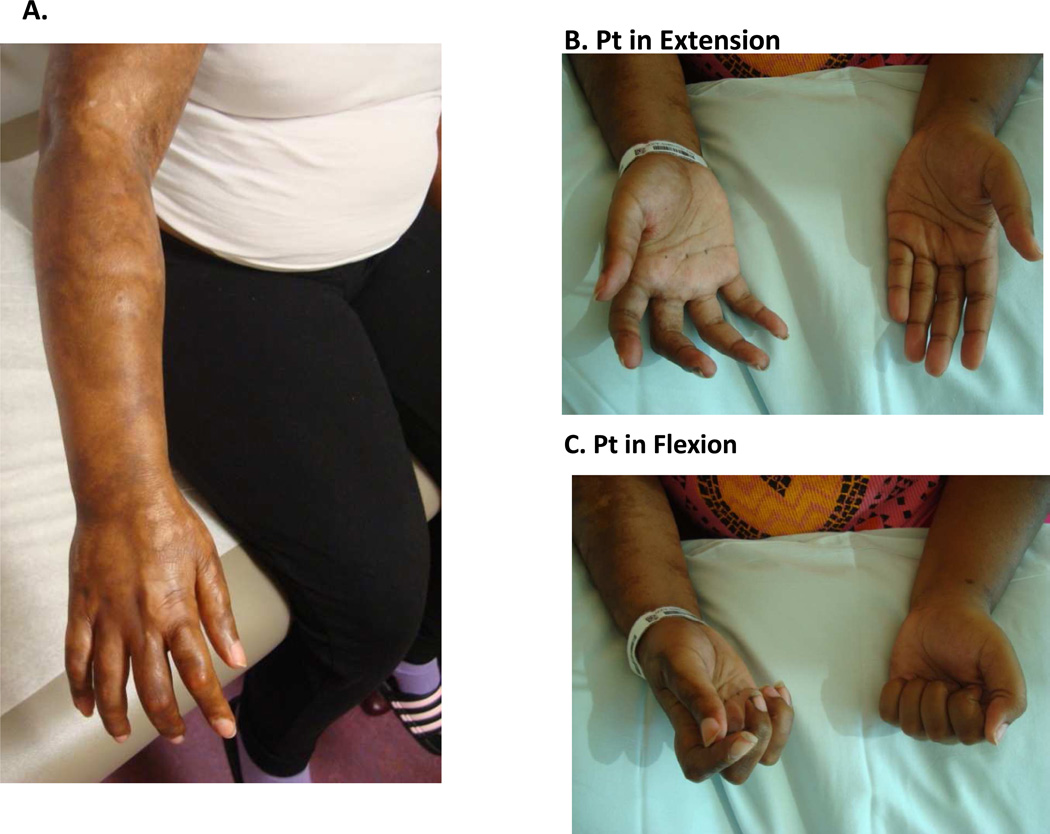
Figure 10.
Linear scleroderma lesion affecting unilateral right upper extremity (A). This young woman has associated joint contractures of the wrist, metacarpal and phalangeal joints. Joint contractures of the hand are grossly apparent compared to the contralateral side during active extension (B) and flexion (C).
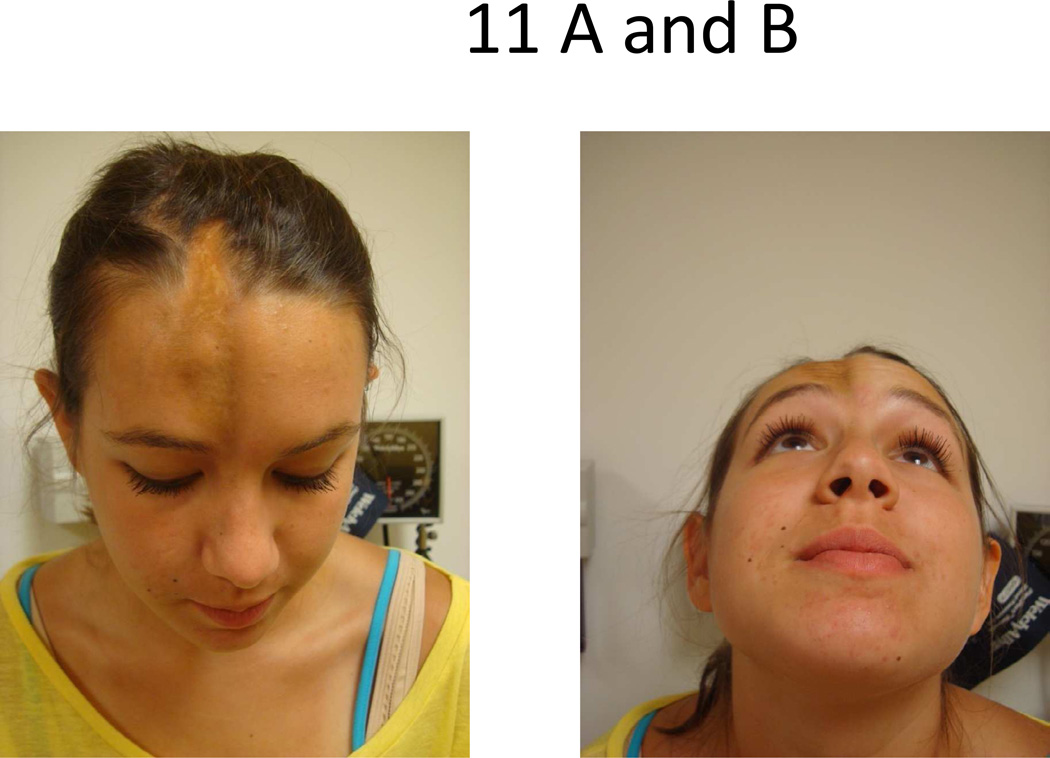
Approximately 25% of those with LiScl have scalp/head involvement [5]. Linear lesions involving the scalp and forehead are termed “en coup de sabre” (ECDS) because the linear depression resembles a saber wound scar (Fig 11). Progressive hemifacial atrophy, also known as Parry Romberg syndrome (PRS) is another linear variant of LS causing hemi-atrophy of the face, including mandible, maxilla, tongue, subcutaneous and muscle tissue (Fig 12). Typically, ECDS is associated with cutaneous findings of dyspigmentation and skin thickness, whereas PRS is not and usually has more pronounced subcutaneous atrophy. These disorders can occur together, either at the same location (side of face) or opposing sides [62]. Though controversial, ECDS and PRS are likely different spectra of the same condition, supported by similar manifestations and prevalence of neurological symptoms [62, 63] and cellular infiltrates (lymphocytic infiltrate of neurovascular bundles) of the skin [64] and brain tissue [65]. PRS and ECDS both carry the same risks for neurological involvement, with 8–20% of the patients with these conditions demonstrating central nervous involvement expressed as seizures, chronic headaches, optic neuritis, and less frequently, neuropsychiatric changes or ischemic stroke [62, 63]. Orthodontic and ophthalmologic abnormalities also have been reported in similar prevalence in both conditions [63]. If the onset of the disease (ECDS or PRS) occurs before 10 years of age, there is a particular risk for skeletal hypoplasia of the affected side of the face, due to the immaturity of the skeleton, and dental and cosmetic abnormalities result [66].
Figure 11.
En coup de sabre (ECDS) lesion affecting scalp and head in a unilateral fashion (A) causing moderate amount of subcutaneous tissue loss and skeletal deformity (B).
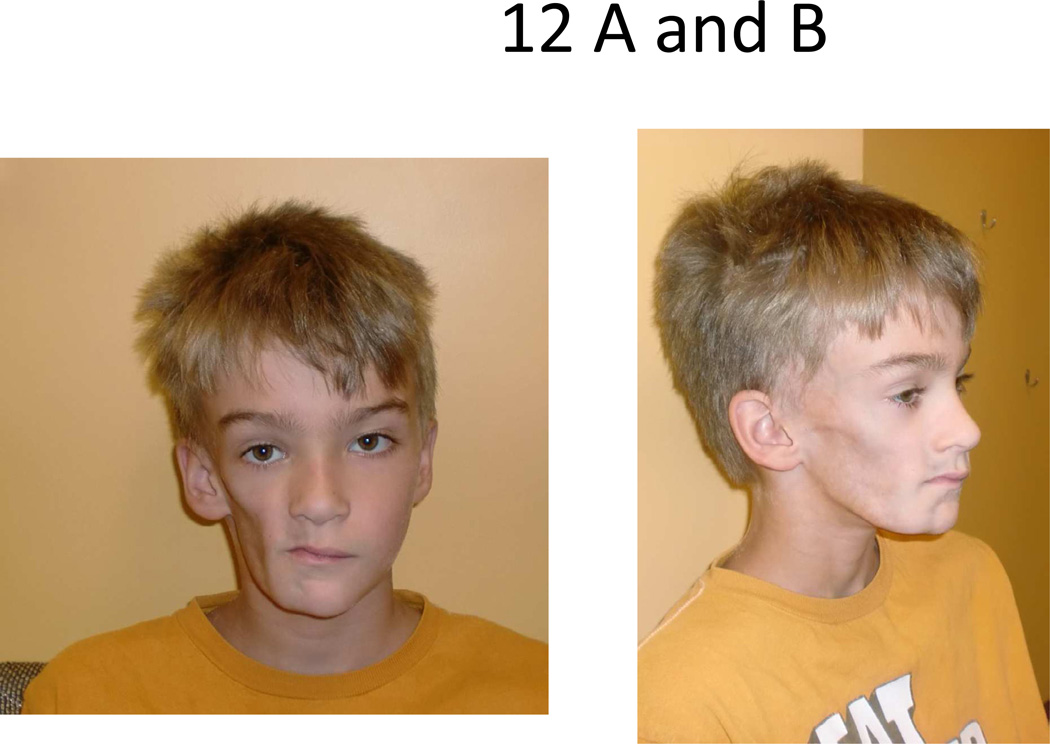
Figure 12.
Severe example of Parry Romberg Syndrome/ hemifacial atrophy of the right face.
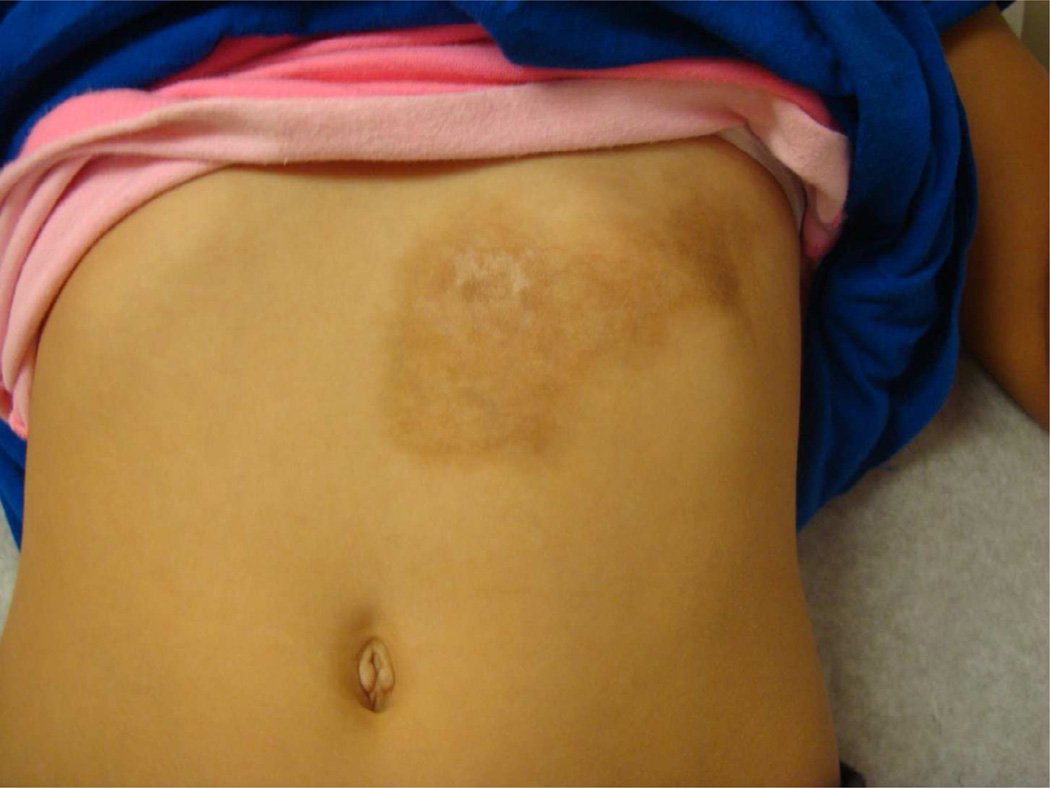
Superficial circumscribed morphea, previously termed plaque morphea per Peterson classification, is the most common presentation of LS in adults (60%) and accounts for approximately one-quarter of LS in children [5, 60]. It is characterized by one or few oval or rounded areas of induration with an ivory waxy center and peripheral violaceous halo, ranging from 1 to 30 cm in diameter. These lesions typically arise on the trunk or proximal extremities (Fig 13). The superficial variant is limited to the epidermis and dermis. Deep circumscribed morphea, previously referred to as subcutaneous morphea, morphea profunda or deep morphea, presents as a round/oval lesion deeper in the tissue. These lesions affect the dermis and subcutis with variable penetration into the fascia and muscle. Typically there are only subtle cutaneous changes (such as slight erythema and thinning of the dermis) but a great deal of inflammatory infiltrate in deep subcutaneous tissue and fascia is present, causing a depression in the adipose tissue (‘scooped out’ appearance) and thickened, bound-down skin.
Figure 13.
Typical isolated oval lesion on trunk consistent with superficial circumscribed morphea.
Generalized morphea (GM) is defined by more than 3 plaque lesions that are each greater than 3 cm wide. Typically the plaques become confluent and affect several anatomic areas, the trunk being most common. GM is an uncommon subtype, occurring in less than 10% of LS patients [5, 7]. As mentioned previously, this subtype is often associated with several autoimmune phenomena, including ss-DNA and AHA antibodies and concomitant and family history of autoimmune disorders. Systemic symptoms, such as myalgia, arthralgia and fatigue, are more common in GM [7, 60].
Pansclerotic morphea is a rare subset of LS which only affects 1–2% of patients and is typically disabling. It initially involves the extremities, but later may migrate to the trunk, only sparing the face and distal areas of fingers and toes (Fig 14). There is a rapidly progressive fibrosis of the deep dermis, subcutis, fascia, and muscles, with occasional bone involvement. This leads to significant contracture of joints, muscle atrophy, cutaneous ulcerations and sometimes restrictive pattern respiratory insufficiency if the truck is involved. The development of squamous cell carcinoma is an additional complication in pansclerotic morphea, especially in areas of chronic wounds [67].
Figure 14.
Pansclerotic morphea affecting limbs circumferentially and sparing distal toes and fingers.
Mixed morphea is the final subtype considered from the Padua consensus and is defined by the presence of more than one clinical subtype in a single LS patient. Mixed morphea is rather common and is seen in approximately 15% of the pediatric LS population. The most frequent presentation of mixed morphea is a combination of linear and plaque morphea [5].
Bullous morphea is sometimes considered a rare subtype of LS. However, many experts agree that the bullous lesions are secondary to aggressive linear or deep LS disease and likely reflect disruption of the lymphatic drainage. Eosinophilic fasciitis (EF), once considered a type of deep morphea by Peterson criteria, is not considered a subtype of LS by Padua criteria. It often behaves as a mixture of deep morphea and linear scleroderma, resulting in deep infiltration and joint contractures [31].)
Extracutaneous manifestations
Extracutaneous manifestations (ECM) are not uncommon in LS and occur in approximately one-quarter of the patients throughout the course of disease [44]. According to a large international study of 750 children with LS, ECM are more frequent in patients with LiScl and consist essentially of orthopaedic complications (19% entire group, 50% in those with LiScl of the extremity), neurologic or ocular findings (5% entire group, 40% in those with LiScl of the head), Raynaud’s phenomenon (2.1% of entire group) and other autoimmune conditions such as thyroiditis (2% all subtypes) [44]. Less common ECM are gastrointestinal, respiratory and renal, all which occurred in less than 2% of the 750 patients examined [44]. These estimates may be lower than the true percentage of ECM, since only symptomatic patients were included in analysis. Another limitation with the study was a lack of a control population to evaluate the prevalence of these manifestations in a comparable pediatric population. In another series, which screened for GI manifestations in LS patients (such as gastroespohageal reflux) and neurological imaging changes in ECDS/PRS patients, investigators found a higher occurrence of ECM; 20% and 60% respectively [63, 68]. Interestingly, in Zulian et al study of 750 children with LS, of the 168 patients with ECM, 25% had articular, neurologic and ocular manifestations unrelated to the site of skin lesions, i.e. arthritis of a peripheral joint with uninvolved overlying skin and subcutaneous tissue, further supporting the hypothesis that ‘localized scleroderma’ is more of a systemic process [44]. Twenty-four of the 750 children (3.2%) had significant ocular involvement, most of whom had linear scleroderma of the head (67%) [69]. These findings support routine screening of LS patients by ophthalmology for inflammatory eye findings, especially for those with linear scleroderma of the head. This benign screening tool can detect clinically ‘silent’ disease and treatment can prevent long term visual sequela.
Evaluation
No laboratory test is diagnostic of LS. Although a ‘localized’ disease, there have been several autoantibodies associated with LS that can be supportive of diagnosis and indicate more severe/involved disease (please refer to autoimmunity section). Specifically, ANA, AHA and ss-DNA positivity have been detected at a relatively high frequency (40–50%). In addition, rheumatoid factor is present in one third of patients, particularly those who have GM, and also correlates to disease severity (number of lesions) [5]. General markers of inflammation, including erythrocyte sedimentation rate and serum immunoglobulin levels, may be useful markers of disease activity in select patients with LS, particularly those with deep or eosinophilic-like variants. However, the majority of patients presenting with active LS lesions do not have elevation of these markers. Diagnosis of LS is usually made by clinical examination, and if there is uncertainty, a skin and subcutaneous biopsy can aid in the diagnosis. Differential diagnosis is limited and includes two skin conditions with well defined plaque lesions: lichen sclerosus et atrophicus and atrophoderma of Pasini and Pierini. Both are superficial dermal and epidermal inflammatory conditions which are thought by some to be on the milder spectrum of localized scleroderma. Cutaneous T-cell lymphoma, especially if there is a deeper purple skin discoloration, and collagenoma, usually presenting with induration of the skin and subcutis without pigmentary changes from birth or early childhood, are included in the differential. Other possible conditions are associated with certain exposures and generally have more wide-spread lesions, such as graft versus host disease (status-post transplant), nephrogenic fibrosing dermatopathy (gadolinium administered in renal compromised state), and porphyria cutanea tarda (sun exposure)
Several tools have been employed for the assessment of skin and subcutaneous involvement in LS, such as clinical skin scores, measurement tools and imaging. Clinical skin scores are commonly used by physicians and include the modified Rodnan Skin Score [17] and the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT), which combines disease activity and damage parameters [33, 70]. A computerized skin score (CSS) can be used to determine the size of lesion in an objective manner [71]. Measurement tools, including a durometer, which measures the degree of skin hardness [72] and a cutometer, which measures skin elasticity [73], have also been proposed as useful. In addition, several types of imaging can be employed for diagnostic or assessment purposes, such as thermography [74, 75], laser doppler flowmetry [76], ultrasound [77, 78], and MRI [79]. Some of these techniques have been validated in LS, including the LoSCAT, the CSS, ultrasound using higher frequencies (10–25 MHz), and MRI. However, currently standard measures are not widely used and assessment depends on center-specific modalities which rely on the investigator’s experience.
Recent efforts through the Childhood Arthritis and Rheumatology Research Alliance (CARRA) have focused on collaborative projects among pediatric rheumatology and dermatology investigators in North America. Their goal is to establish specific clinical measures to assess activity and damage, which will assist in monitoring progression and disease state in LS. These projects are in the process of assessing clinical measures like the LoSCAT and identifying others through multi-centered studies with the Localized Scleroderma Clinical and Ultrasound Study group (LOCUS). The LOCUS group is composed of experts in LS, both pediatric rheumatologists and dermatologists, who were asked to rank clinical variables which most strongly reflected disease activity and damage in LS. There was >75% consensus agreement and high content validity (modified kappa statistic 0.88 – 1.00) regarding four activity parameters; the appearance of new lesions, expansion of current lesions, erythematous/violaceous hue at the border and skin induration at the border of a lesion [33]. Cutaneous disease damage parameters ranked highest amongst the group were post-inflammatory hyperpigmentation and hypopigmentation, dermal atrophy and subcutaneous atrophy (88% agreement and Inter-Item Content Validity Index of 0.88 – 1.0) [70].
Once systemic immunosuppressive therapy is initiated in patients with moderate to severe LS, the signs of disease activity typically resolve within months and some of the damage features mildly to moderately improve [80–85]. It is after this initial improvement that the detection of subclinical disease is important, as to not wean off of immunosupression too early, potentially resulting in relapse [80, 83, 84]. A recent study in pediatric LS found a 28% relapse rate after a mean duration of treatment with MTX of 2.4 years. Linear extremity subtype and older age at disease onset were found to be significant predictors of relapse, and although not significant, there was a noticeable difference in duration of MTX treatment between relapsers and nonrelapsers [85]. However, it remains difficult to detect subclinical disease which is why multiple imaging and thermal modalities are being pursued.
Treatment
Approach to the treatment of LS initially depends on the severity of the disease which is characterized by the subtype of LS, potential involvement of deeper tissue and structures (such as joints), location of LS and disease activity state. The length of disease duration does have an impact on disease activity state, with most superficial circumscribed or plaque lesions ‘burning out’ in three to five years. Any remaining central sclerosis may soften over time without intervention. If only disease damage parameters remain and there is no expansion, erythema or other clinical disease activity parameters present, simply monitoring the lesion is acceptable. However, if there is any sign of active disease, both pediatric rheumatologists and dermatologists agree that a single superficial circumscribed lesion in a non-cosmetically concerning location can be treated either topically with corticosteroids, calcineurin inhibitors, imiquimod, vitamin D, or via recurrent phototherapy with ultraviolet (UV) light. All modalities have had clinical success in case series or uncontrolled trials, exhibited by lightening of hyperpigmentation and decreased skin thickness. Many dermatologists prescribe these medications in combination, and recommend using occlusive dressing to increase absorption of topical therapies [86]. UV treatment has several modalities; broad band UVA, narrow band UVB, and UVA-1. UVA-1 was successful in two randomized control trials, especially in regards to early inflammatory and superficial dermal lesions [87, 88]. However, access to UV therapy is limited and treatment is time consuming (often requiring three 30 to 60 minute sessions per week), making this a difficult option for many. In addition, the risk for potential long-term effects such as skin aging and carcinogenesis are unknown in children.
There is growing consensus among both rheumatologists and dermatologists that systemic therapy is warranted for more moderate to severe LS. Features which might indicate moderate to severe disease include deep involvement of the subcutis, fascia, and muscle, that transverse a joint and potentially cause functional impairment, linear or atrophic lesions affecting the face/scalp, rapidly progressive or widespread active disease, and those who have failed topical or UV therapy [86, 89]. The most commonly employed systemic therapy is methotrexate (MTX) in conjunction with corticosteroids (CS), confirmed by a recent survey of 158 North American pediatric rheumatologists by Li et al. [89]. Clinical vignettes were distributed to clinicians describing patients with moderate-severe LS, and clinicians were asked to respond with their treatment recommendations. The clinicians’ response as to initial therapy was overwhelmingly MTX combined with CS (over 80% would use both). Although there was excellent agreement as to the choice of medications, treatment regimens varied broadly regarding administration (oral vs. subcutaneous vs. intravenous) as well as corticosteroid taper and length of therapy [89].
A subcommittee of CARRA is deriving best consensus treatment protocols for those with moderate to severe LS to help address this issue and narrow down a handful of regimens that would be useful to the clinician. These protocols have four treatment plans for induction of therapy. Three protocols involve subcutaneous MTX (1mg/kg, with a maximum dose 25mg per week) and the fourth was derived for those who either fail MTX or are intolerant to MTX. The protocols are divided into MTX alone, MTX with oral CS and taper, MTX with IV CS, and mycophenolate mofetil (MMF) in addition to either oral or IV CS. These protocols are currently being submitted for publication. MMF has been shown to be effective in severe MTX-resistant pediatric LS, as exemplified by a case series of 10 patients (two with pansclerotic morphea, three with GM, two with LiScl extremities and 3 with LiScl head). All cases improved with MMF and were able to wean CS dose [90].
The first double-blind randomized control trial in children with LS using objective measures was performed by Zulian et al comparing MTX and oral CS vs. placebo and oral CS. MTX was found to be effective and safe, confirming the community’s support for the use of MTX in LS. In addition, those treated with MTX significantly improved and had significantly less relapse compared to those on CS alone [84].
Other therapies
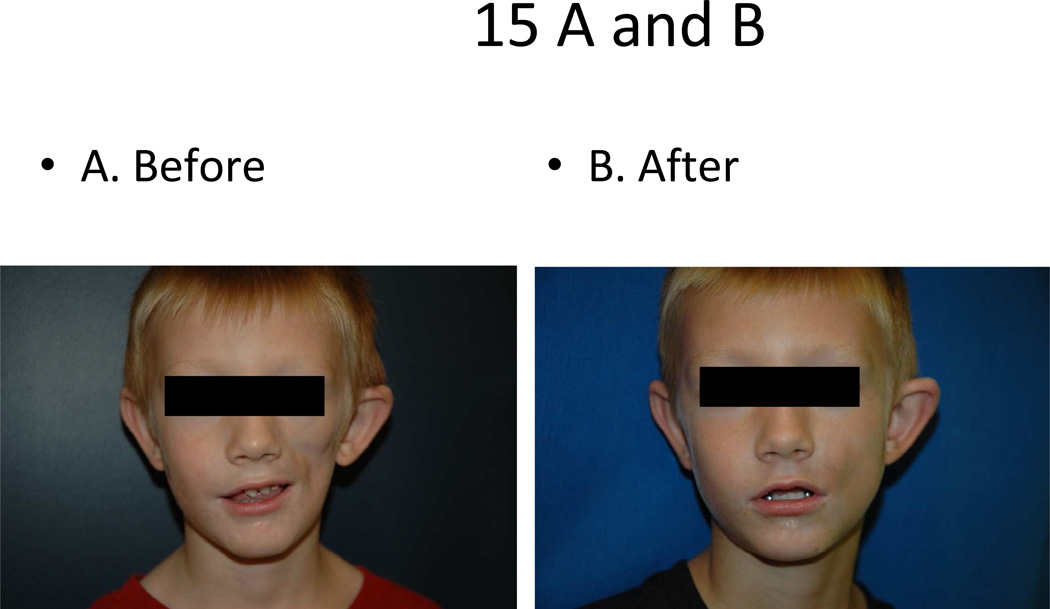
Intensive physical and occupational therapy in conjunction with systemic immunosuppressive therapy is recommended for those with linear or deep scleroderma of the extremity to help avoid and or minimize joint contractures. Also, for those with ECDS and/or PRS, plastic surgery intervention is felt to be reasonable after the disease has been in remission without any signs of clinical activity. Augmentation includes fat grating with micro droplets from the patient’s abdominal subcutis to assist in filling in some of the subcutaneous atrophy to yield a more symmetric facial contour (Fig 15). For more severe disease, fasciocutaneous flaps and facial bone reconstruction are also options, though are typically reserved for the late teen age years, after the skeleton has matured [91]. One center reports that of 10 teenagers with ECDS and/or PRS who underwent surgical intervention to correct facial deformity, 9 reported satisfaction with the results of the procedure(s) and all would recommend surgical intervention to others with this condition, signifying a potential great impact on quality of life for these patients [92].
Figure 15.
Parry Romberg Syndrome affecting left face before (A) and after (B) subcutaneous fat grafting.
Acknowledgments
Source of support: None
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has nothing to disclose.
References
- 1.Peterson LS, Nelson AM, Su WP, Mason T, O'Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24(1):73–80. [PubMed] [Google Scholar]
- 2.Pelkonen PM, Jalanko HJ, Lantto RK, Makela AL, Pietikainen MA, Savolainen HA, Verronen PM. Incidence of systemic connective tissue diseases in children: a nationwide prospective study in Finland. J Rheumatol. 1994;21(11):2143–2146. [PubMed] [Google Scholar]
- 3.Scalapino K, Arkachaisri T, Lucas M, Fertig N, Helfrich DJ, Londino AV, Jr, Steen VD, Medsger TA., Jr Childhood onset systemic sclerosis: classification, clinical and serologic features, and survival in comparison with adult onset disease. J Rheumatol. 2006;33(5):1004–1013. [PubMed] [Google Scholar]
- 4.Martini G, Foeldvari I, Russo R, Cuttica R, Eberhard A, Ravelli A, Lehman TJ, de Oliveira SK, Susic G, Lyskina G, et al. Systemic sclerosis in childhood: clinical and immunologic features of 153 patients in an international database. Arthritis Rheum. 2006;54(12):3971–3978. doi: 10.1002/art.22207. [DOI] [PubMed] [Google Scholar]
- 5.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, Cuttica R, Higgins GC, Van Suijlekom-Smit LW, Moore TL, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford) 2006;45(5):614–620. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 6.Foeldvari I, Zhavania M, Birdi N, Cuttica RJ, de Oliveira SH, Dent PB, Elborgh R, Falcini F, Ganser G, Girschick H, et al. Favourable outcome in 135 children with juvenile systemic sclerosis: results of a multi-national survey. Rheumatology (Oxford) 2000;39(5):556–559. doi: 10.1093/rheumatology/39.5.556. [DOI] [PubMed] [Google Scholar]
- 7.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385–396. doi: 10.1016/j.jaad.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Zulian F, Vallongo C, de Oliveira SK, Punaro MG, Ros J, Mazur-Zielinska H, Galea P, Da Dalt L, Eichenfield LF. Congenital localized scleroderma. J Pediatr. 2006;149(2):248–251. doi: 10.1016/j.jpeds.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29(2):255–273. doi: 10.1016/s0889-857x(03)00023-1. vi. [DOI] [PubMed] [Google Scholar]
- 10.Zulian F, Woo P, Athreya BH, Laxer RM, Medsger TA, Jr, Lehman TJ, Cerinic MM, Martini G, Ravelli A, Russo R, et al. The Pediatric Rheumatology European Society/American College of Rheumatology/European League against Rheumatism provisional classification criteria for juvenile systemic sclerosis. Arthritis Rheum. 2007;57(2):203–212. doi: 10.1002/art.22551. [DOI] [PubMed] [Google Scholar]
- 11.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–205. [PubMed] [Google Scholar]
- 12.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34(1):1–15. doi: 10.1016/j.rdc.2007.12.001. v. [DOI] [PubMed] [Google Scholar]
- 13.Rodnan GP, Myerowitz RL, Justh GO. Morphologic changes in the digital arteries of patients with progressive systemic sclerosis (scleroderma) and Raynaud phenomenon. Medicine (Baltimore) 1980;59(6):393–408. doi: 10.1097/00005792-198011000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Maricq HR. Wide-field capillary microscopy. Arthritis Rheum. 1981;24(9):1159–1165. doi: 10.1002/art.1780240907. [DOI] [PubMed] [Google Scholar]
- 15.LeRoy EC, Medsger TA., Jr Raynaud's phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10(5):485–488. [PubMed] [Google Scholar]
- 16.Duffy CM, Laxer RM, Lee P, Ramsay C, Fritzler M, Silverman ED. Raynaud syndrome in childhood. J Pediatr. 1989;114(1):73–78. doi: 10.1016/s0022-3476(89)80604-3. [DOI] [PubMed] [Google Scholar]
- 17.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, Weinstein A, Weisman M, Mayes M, Collier D, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–1285. [PubMed] [Google Scholar]
- 18.Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger TA., Jr Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70(1):104–109. doi: 10.1136/ard.2009.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber P, Ganser G, Frosch M, Roth J, Hulskamp G, Zimmer KP. Twenty-four hour intraesophageal pH monitoring in children and adolescents with scleroderma and mixed connective tissue disease. J Rheumatol. 2000;27(11):2692–2695. [PubMed] [Google Scholar]
- 20.Ingraham KM, O'Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol. 2010;37(3):603–607. doi: 10.3899/jrheum.090600. [DOI] [PubMed] [Google Scholar]
- 21.Panigada S, Ravelli A, Silvestri M, Granata C, Magni-Manzoni S, Cerveri I, Dore R, Toma P, Martini A, Rossi GA, et al. HRCT and pulmonary function tests in monitoring of lung involvement in juvenile systemic sclerosis. Pediatr Pulmonol. 2009;44(12):1226–1234. doi: 10.1002/ppul.21141. [DOI] [PubMed] [Google Scholar]
- 22.Silver RM, Miller KS, Kinsella MB, Smith EA, Schabel SI. Evaluation and management of scleroderma lung disease using bronchoalveolar lavage. Am J Med. 1990;88(5):470–476. doi: 10.1016/0002-9343(90)90425-d. [DOI] [PubMed] [Google Scholar]
- 23.Rabinovich CE. Challenges in the diagnosis and treatment of juvenile systemic sclerosis. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.148. [DOI] [PubMed] [Google Scholar]
- 24.Rodnan GP, Medsger TA. The rheumatic manifestaions of progressive systemic sclerosis (scleroderma) Clin Orthop Relat Res. 1968;57:81–93. [PubMed] [Google Scholar]
- 25.Ringel RA, Brick JE, Brick JF, Gutmann L, Riggs JE. Muscle involvement in the scleroderma syndromes. Arch Intern Med. 1990;150(12):2550–2552. [PubMed] [Google Scholar]
- 26.Quartier P, Bonnet D, Fournet JC, Bodemer C, Acar P, Ouachee-Chardin M, Le Bidois J, Prieur AM. Severe cardiac involvement in children with systemic sclerosis and myositis. J Rheumatol. 2002;29(8):1767–1773. [PubMed] [Google Scholar]
- 27.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66(7):940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foeldvari I, Nihtyanova SI, Wierk A, Denton CP. Characteristics of patients with juvenile onset systemic sclerosis in an adult single-center cohort. J Rheumatol. 2010;37(11):2422–2426. doi: 10.3899/jrheum.100001. [DOI] [PubMed] [Google Scholar]
- 29.Kowal-Bielecka O, Landewe R, Avouac J, Chwiesko S, Miniati I, Czirjak L, Clements P, Denton C, Farge D, Fligelstone K, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68(5):620–628. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 30.Nihtyanova SI, Denton CP. Current approaches to the management of early active diffuse scleroderma skin disease. Rheum Dis Clin North Am. 2008;34(1):161–179. doi: 10.1016/j.rdc.2007.11.005. viii. [DOI] [PubMed] [Google Scholar]
- 31.Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70(11):1068–1076. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 32.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–613. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 33.Arkachaisri T, Vilaiyuk S, Li S, O'Neil KM, Pope E, Higgins GC, Punaro M, Rabinovich EC, Rosenkranz M, Kietz DA, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36(12):2819–2829. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lever WF, Elder DE, Elenitsas R, Jaworsky C, Johnson BL. Lever's Histopathology of the skin. 8th edn. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 35.McKee PH. Pathology of the skin : with clinical correlations. 2nd. edn. London: Mosby-Wolfe; 1996. [Google Scholar]
- 36.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 37.Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. Am J Dermatopathol. 1998;20(3):242–245. doi: 10.1097/00000372-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch Dermatol Res. 1995;287(2):193–197. doi: 10.1007/BF01262331. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa M, Sato S, Nagaoka T, Fujimoto M, Takehara K. Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology. 2003;207(2):141–147. doi: 10.1159/000071783. [DOI] [PubMed] [Google Scholar]
- 40.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin 8 in serum samples of patients with localized scleroderma. Arch Dermatol. 1994;130(10):1327–1328. [PubMed] [Google Scholar]
- 41.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24(2):328–332. [PubMed] [Google Scholar]
- 42.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35(1):67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa M, Sato S, Ihn H, Takehara K. Enhanced production of interleukin-6 (IL-6), oncostatin M and soluble IL-6 receptor by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Rheumatology (Oxford) 1999;38(7):612–617. doi: 10.1093/rheumatology/38.7.612. [DOI] [PubMed] [Google Scholar]
- 44.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, Espada G, Corona F, Mukamel M, Vesely R, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873–2881. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 45.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Clinical significance of serum levels of soluble interleukin-2 receptor in patients with localized scleroderma. Br J Dermatol. 1996;134(5):843–847. [PubMed] [Google Scholar]
- 46.Uziel Y, Krafchik BR, Feldman B, Silverman ED, Rubin LA, Laxer RM. Serum levels of soluble interleukin-2 receptor. A marker of disease activity in localized scleroderma. Arthritis Rheum. 1994;37(6):898–901. doi: 10.1002/art.1780370618. [DOI] [PubMed] [Google Scholar]
- 47.Nagaoka T, Sato S, Hasegawa M, Ihn H, Takehara K. Serum levels of soluble interleukin 6 receptor and soluble gp130 are elevated in patients with localized scleroderma. J Rheumatol. 2000;27(8):1917–1921. [PubMed] [Google Scholar]
- 48.Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gore-Hyer E, Pannu J, Smith EA, Grotendorst G, Trojanowska M. Selective stimulation of collagen synthesis in the presence of costimulatory insulin signaling by connective tissue growth factor in scleroderma fibroblasts. Arthritis Rheum. 2003;48(3):798–806. doi: 10.1002/art.10953. [DOI] [PubMed] [Google Scholar]
- 50.Uziel Y, Feldman BM, Krafchik BR, Laxer RM, Yeung RS. Increased serum levels of TGFbeta1 in children with localized scleroderma. Pediatr Rheumatol Online J. 2007;5:22. doi: 10.1186/1546-0096-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higley H, Persichitte K, Chu S, Waegell W, Vancheeswaran R, Black C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud's phenomenon. Arthritis Rheum. 1994;37(2):278–288. doi: 10.1002/art.1780370218. [DOI] [PubMed] [Google Scholar]
- 52.Lambert NC, Pang JM, Yan Z, Erickson TD, Stevens AM, Furst DE, Nelson JL. Male microchimerism in women with systemic sclerosis and healthy women who have never given birth to a son. Ann Rheum Dis. 2005;64(6):845–848. doi: 10.1136/ard.2004.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rak JM, Pagni PP, Tiev K, Allanore Y, Farge D, Harle JR, Launay D, Hachulla E, Didelot R, Cabane J, et al. Male microchimerism and HLA compatibility in French women with sclerodema: a different profile in limited and diffuse subset. Rheumatology (Oxford) 2009;48(4):363–366. doi: 10.1093/rheumatology/ken505. [DOI] [PubMed] [Google Scholar]
- 54.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 2005;44(3):274–279. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]
- 55.Takehara K, Moroi Y, Nakabayashi Y, Ishibashi Y. Antinuclear antibodies in localized scleroderma. Arthritis Rheum. 1983;26(5):612–616. doi: 10.1002/art.1780260506. [DOI] [PubMed] [Google Scholar]
- 56.Arkachaisri T, Fertig N, Pino S, Medsger TA., Jr Serum autoantibodies and their clinical associations in patients with childhood- and adult-onset linear scleroderma. A single-center study. J Rheumatol. 2008;35(12):2439–2444. doi: 10.3899/jrheum.080098. [DOI] [PubMed] [Google Scholar]
- 57.Falanga V, Medsger TA, Jr, Reichlin M. Antinuclear and anti-single-stranded DNA antibodies in morphea and generalized morphea. Arch Dermatol. 1987;123(3):350–353. [PubMed] [Google Scholar]
- 58.Falanga V, Medsger TA, Jr, Reichlin M, Rodnan GP. Linear scleroderma. Clinical spectrum, prognosis, and laboratory abnormalities. Ann Intern Med. 1986;104(6):849–857. doi: 10.7326/0003-4819-104-6-849. [DOI] [PubMed] [Google Scholar]
- 59.Sato S, Fujimoto M, Ihn H, Kikuchi K, Takehara K. Clinical characteristics associated with antihistone antibodies in patients with localized scleroderma. J Am Acad Dermatol. 1994;31(4):567–571. doi: 10.1016/s0190-9622(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 60.Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145(5):545–550. doi: 10.1001/archdermatol.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marzano AV, Menni S, Parodi A, Borghi A, Fuligni A, Fabbri P, Caputo R. Localized scleroderma in adults and children. Clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13(2):171–176. [PubMed] [Google Scholar]
- 62.Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56(2):257–263. doi: 10.1016/j.jaad.2006.10.959. [DOI] [PubMed] [Google Scholar]
- 63.Kister I, Inglese M, Laxer RM, Herbert J. Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology. 2008;71(19):1538–1545. doi: 10.1212/01.wnl.0000334474.88923.e3. [DOI] [PubMed] [Google Scholar]
- 64.Pensler JM, Murphy GF, Mulliken JB. Clinical and ultrastructural studies of Romberg's hemifacial atrophy. Plast Reconstr Surg. 1990;85(5):669–674. discussion 675-666. [PubMed] [Google Scholar]
- 65.Holland KE, Steffes B, Nocton JJ, Schwabe MJ, Jacobson RD, Drolet BA. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117(1):e132–e136. doi: 10.1542/peds.2005-0470. [DOI] [PubMed] [Google Scholar]
- 66.Thorne C, Grabb WC, Smith JW. Grabb and Smith's plastic surgery. 6th edn. Philadelphia: Lippincott Williams &Wilkins; 2007. [Google Scholar]
- 67.Wollina U, Buslau M, Heinig B, Petrov I, Unger E, Kyriopoulou E, Koch A, Kostler E, Schonlebe J, Haroske G, et al. Disabling pansclerotic morphea of childhood poses a high risk of chronic ulceration of the skin and squamous cell carcinoma. Int J Low Extrem Wounds. 2007;6(4):291–298. doi: 10.1177/1534734607308731. [DOI] [PubMed] [Google Scholar]
- 68.Dehen L, Roujeau JC, Cosnes A, Revuz J. Internal involvement in localized scleroderma. Medicine (Baltimore) 1994;73(5):241–245. doi: 10.1097/00005792-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Zannin ME, Martini G, Athreya BH, Russo R, Higgins G, Vittadello F, Alpigiani MG, Alessio M, Paradisi M, Woo P, et al. Ocular involvement in children with localised scleroderma: a multi-centre study. Br J Ophthalmol. 2007;91(10):1311–1314. doi: 10.1136/bjo.2007.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA., Jr Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford) 49(2):373–381. doi: 10.1093/rheumatology/kep361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zulian F, Meneghesso D, Grisan E, Vittadello F, Belloni Fortina A, Pigozzi B, Frigo AC, Martini G, Ruggeri A. A new computerized method for the assessment of skin lesions in localized scleroderma. Rheumatology (Oxford) 2007;46(5):856–860. doi: 10.1093/rheumatology/kel446. [DOI] [PubMed] [Google Scholar]
- 72.Seyger MM, van den Hoogen FH, de Boo T, de Jong EM. Reliability of two methods to assess morphea: skin scoring and the use of a durometer. J Am Acad Dermatol. 1997;37(5 Pt 1):793–796. doi: 10.1016/s0190-9622(97)70121-x. [DOI] [PubMed] [Google Scholar]
- 73.de Rie MA, Enomoto DN, de Vries HJ, Bos JD. Evaluation of medium-dose UVA1 phototherapy in localized scleroderma with the cutometer and fast Fourier transform method. Dermatology. 2003;207(3):298–301. doi: 10.1159/000073093. [DOI] [PubMed] [Google Scholar]
- 74.Birdi N, Shore A, Rush P, Laxer RM, Silverman ED, Krafchik B. Childhood linear scleroderma: a possible role of thermography for evaluation. J Rheumatol. 1992;19(6):968–973. [PubMed] [Google Scholar]
- 75.Martini G, Murray KJ, Howell KJ, Harper J, Atherton D, Woo P, Zulian F, Black CM. Juvenileonset localized scleroderma activity detection by infrared thermography. Rheumatology (Oxford) 2002;41(10):1178–1182. doi: 10.1093/rheumatology/41.10.1178. [DOI] [PubMed] [Google Scholar]
- 76.Weibel L, Howell KJ, Visentin MT, Rudiger A, Denton CP, Zulian F, Woo P, Harper JI. Laser Doppler flowmetry for assessing localized scleroderma in children. Arthritis Rheum. 2007;56(10):3489–3495. doi: 10.1002/art.22920. [DOI] [PubMed] [Google Scholar]
- 77.Hoffmann K, Gerbaulet U, el-Gammal S, Altmeyer P. 20-MHz B-mode ultrasound in monitoring the course of localized scleroderma (morphea) Acta Derm Venereol Suppl (Stockh) 1991;164:3–16. [PubMed] [Google Scholar]
- 78.Li SC, Liebling MS, Haines KA, Weiss JE, Prann A. Initial evaluation of an ultrasound measure for assessing the activity of skin lesions in juvenile localized scleroderma. Arthritis Care Res (Hoboken) 2011;63(5):735–742. doi: 10.1002/acr.20407. [DOI] [PubMed] [Google Scholar]
- 79.Horger M, Fierlbeck G, Kuemmerle-Deschner J, Tzaribachev N, Wehrmann M, Claussen CD, Fritz J. MRI findings in deep and generalized morphea (localized scleroderma) AJR Am J Roentgenol. 2008;190(1):32–39. doi: 10.2214/AJR.07.2163. [DOI] [PubMed] [Google Scholar]
- 80.Cox D, G OR, Collins S, Byrne A, Irvine A, Watson R. Juvenile localised scleroderma: a retrospective review of response to systemic treatment. Ir J Med Sci. 2008;177(4):343–346. doi: 10.1007/s11845-008-0217-0. [DOI] [PubMed] [Google Scholar]
- 81.Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91–95. doi: 10.1016/s0022-3476(00)90056-8. [DOI] [PubMed] [Google Scholar]
- 82.Fitch PG, Rettig P, Burnham JM, Finkel TH, Yan AC, Akin E, Cron RQ. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol. 2006;33(3):609–614. [PubMed] [Google Scholar]
- 83.Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–1020. doi: 10.1111/j.1365-2133.2006.07497.x. [DOI] [PubMed] [Google Scholar]
- 84.Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, Alessio M, Torre FL, Podda RA, Gerloni V, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006. doi: 10.1002/art.30264. [DOI] [PubMed] [Google Scholar]
- 85.Mirsky L, Chakkittakandiyil A, Laxer RM, O'Brien C, Pope E. Relapse after systemic treatment in paediatric morphoea. Br J Dermatol. 2011 doi: 10.1111/j.1365-2133.2011.10535.x. [DOI] [PubMed] [Google Scholar]
- 86.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65(5):925–941. doi: 10.1016/j.jaad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54(3):440–447. doi: 10.1016/j.jaad.2005.11.1063. [DOI] [PubMed] [Google Scholar]
- 88.Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium-dose is more effective than low-dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20-MHz ultrasound assessment. J Am Acad Dermatol. 2009;60(5):786–791. doi: 10.1016/j.jaad.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 89.Li SC, Feldman BM, Higgins GC, Haines KA, Punaro MG, O'Neil KM. Treatment of pediatric localized scleroderma: results of a survey of North American pediatric rheumatologists. J Rheumatol. 2010;37(1):175–181. doi: 10.3899/jrheum.090708. [DOI] [PubMed] [Google Scholar]
- 90.Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford) 2009;48(11):1410–1413. doi: 10.1093/rheumatology/kep244. [DOI] [PubMed] [Google Scholar]
- 91.Asai S, Kamei Y, Nishibori K, Katoh T, Torii S. Reconstruction of Romberg disease defects by omental flap. Ann Plast Surg. 2006;57(2):154–158. doi: 10.1097/01.sap.0000216243.91162.c2. [DOI] [PubMed] [Google Scholar]
- 92.Palmero ML, Uziel Y, Laxer RM, Forrest CR, Pope E. En coup de sabre scleroderma and Parry-Romberg syndrome in adolescents: surgical options and patient-related outcomes. J Rheumatol. 2010;37(10):2174–2179. doi: 10.3899/jrheum.100062. [DOI] [PubMed] [Google Scholar]