Abstract
Photodynamic Therapy (PDT) uses a photosensitizing drug in combination with visible light to kill cancer cells. PDT has an advantage over surgery or ionizing radiation because PDT can eliminate tumors without causing fibrosis or scarring. Disadvantages include the dual need for drug and light, and a generally lower efficacy for PDT versus surgery. This minireview describes basic principles of PDT, photosensitizers available, and aspects of tumor biology that may provide further opportunities for treatment optimization. An emerging biomodulatory approach, using methotrexate or Vitamin D in combination with aminolevulinate-based PDT, is described. Finally, current clinical uses of PDT for solid malignancies are reviewed.
Keywords: ALA, PDT, Vitamin D, differentiation, porphyrin
1. Introduction
The concept of photodynamic therapy (PDT) is over 100 years old, since Raab and Von Tappeiner discovered that certain dyes, such as acridine orange and eosin, could cause cell death in the presence of light (described in [1]). Beginning with more recent clinical studies of PDT for malignant lesions in humans in 1977 [2], PDT has evolved more rapidly and is becoming an increasingly accepted therapeutic modality, either alone or in combination with other treatments for various malignant and nonmalignant conditions [3–6]. The purpose of this Minireview is to describe the current uses of PDT for treatment of solid tumors, and to highlight ongoing research approaches that offer promise for improving PDT efficacy. After an introduction to the general principles of PDT, the use of PDT in clinical settings will be summarized, and ongoing research approaches to improve PDT efficacy will be described.
2. General Overview of PDT
PDT a combination treatment in which a photosensitizer (PS) and light, in the presence of oxygen, is used to kill tumor cells (reviewed in [5; 6]). Although none of the components of PDT is toxic on their own, the light-induced activation of PS causes the production of reactive oxygen species (ROS), including singlet oxygen (1O2), which trigger the destruction of tumor cells. 1O2 has a short lifetime (approximately 1–320 nanoseconds) and a limited diffusion distance (10 – 55 nm) inside a cell. Therefore, photodynamic damage is highly dependent upon the subcellular localization of the PS, which can target bcl-2 (an anti-apoptotic protein) in the mitochondria and endoplasmic reticulum. This triggers cell death through activation of apoptosis, a process that selectively takes apart the cell through the action of proteolytic enzymes [7]. Another specific cell death pathway, autophagy, described as cellular auto-digestion within double-walled cytoplasmic vacuoles, can also commonly occur after PDT [7]. Necrosis (the least specific form of cell death) is rarely observed [7].
PDT is a two-step procedure. First a PS is administered; then, the region where the tumor is located is irradiated with visible light at a wavelength(s) that matches the absorption spectrum of the PS. These two steps provide dual selectivity. First, cancer cells accumulate and retain PS to a greater extent than cells in the surrounding normal tissue. Second, tumor-specificity is further enhanced by focusing the light source only on the tumor; this activates the PS and releases free radicals, damaging tumor cells and triggering cell death. In a clinical setting, the PS is often administered intravenously and distributes throughout the body, often accumulating preferentially in tumors relative to normal surrounding tissues. After an optimal period for selective PS accumulation (a function of the particular PS used), the tumor is illuminated and the PS is photoactivated. A high-intensity light source is used to induce photochemical reactions that kill cells. As another option, low intensity light can be used to stimulate the PS to emit fluorescent light for diagnostic purposes. Fluorescence diagnosis can be used to evaluate the location, depth and size of tumors, or to estimate the amount of PS present [6]. This information can also be used to adjust the light delivery so as to optimize the therapeutic outcome [8].
Based upon these underlying principles of PDT, many different regimens have been developed by experimenting with the following variables: (i) the photosensitizer, including the choice of molecule, route, and dose of administration; (ii) the light source, including wavelength, overall duration, and whether illumination is continuous or intermittent. The drug-light interval, defined as the time between PS administration and exposure to light, is also important because it determines PS concentration and sensitivity (ref. [5; 9]); (iii) the oxygen concentration within tissues, a potential problem within large hypoxic tumors; (iv) the biological responses of the tumor. Differences in cell physiology between various tumors play a large role in the effectiveness of PDT. Biomodulation (defined below), before and during PDT, offers another avenue for optimization of photodynamic therapy.
3. Photosensitizers (PS) used in PDT
A large number of PS molecules that vary in structure, size, and charge have been tested in vitro and in vivo as PDT agents. The first PS used clinically for PDT was a water-soluble mixture of porphyrins called hematoporphyrin derivative (HPD), and later, a purified version called porfimer sodium (Photofrin). Although Photofrin is still commonly used for PDT, prolonged phototoxicity (6–10 weeks) and a relatively low absorbance at 630 nm are considered potential disadvantages. Taking into account the limitations of the first generation of PS, a 2nd generation PS were developed such as BPD-MA, m-THPC, and Pc4 (see TABLE 1 for more information) that do not cause prolonged photosensitivity and are activated by longer wavelengths of light (660–690 nm) that penetrate more deeply into tissue. TABLE 1 summarizes PS that have been approved for PDT treatments [4; 10]. Newer PS, belonging to a 3rd generation not yet approved for clinical use, include padeliporfin (WST-11) and antibody-conjugated PS that have absorption maxima at 700–800 nm, favoring deeper and more tumor-specific delivery [11–13].
TABLE 1.
Photosensitizers for Photodynamic Therapy
| Trade name | Photosensitizer * | Structure | Excitation λ (nm) | Cancer Indication (Approvals) * |
|---|---|---|---|---|
| I. CLINICALLY APPROVED PHOTOSENSITIZERS | ||||
| Levulan | 5-Aminolevulinic acid (ALA) | Porphyrin precursor | 635 |
|
| Metvix, Metvixia | Methyl ester of 5-ALA | Porphyrin precursor | 635 |
|
| Photofrin | Porfimer sodium; also called Hematoporphyrin derivative (HPD) | Porphyrin | 630 |
|
| Foscan | Meta-tetrahydroxyphenylchlorin (m-THPC) | Chlorin | 652 |
|
| Laserphyrin | Taporfin sodium (Talaporfin), mono-(L)-aspartylchlorin-e6 (MACE, NPe6, LS11) | Chlorin | 664 |
|
| Fotolon | Chlorin e6 + polyvinypyrrolidone | Chlorin | 660 |
|
| Fotoditazin | Chlorin e6 + chlorin p6 | Chlorin | 660 |
|
| Photosense | Aluminum sulphonated phthalocyanines | Phthalocyanine | 675 |
|
| II. PHOTOSENSITIZERS IN CLINICAL TRIALS ** | Ongoing Cancer Studies ** | |||
| Hexvix; Benzvix | ALA esters (hexyl-ALA/benzyl-ALA) | Porphyrin precursor | 635 |
|
| Verteporfin | Benzoporphyrin derivative- monoacid ring A (BPD-MA) | Porphyrin | 690 |
|
| Photochlor | 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-alpha (HPPH) | Chlorin | 665 |
|
| Purlytin | Tin ethyl etiopurpurin (SnEt2) | Chlorin | 664 |
|
| Lutex | Lutetium texaphyrin/Motexafin lutetium | Texaphyrin | 732 |
|
| Pc4 | Silicon phthalocyanine 4 (Pc4) | Phthalocyanine | 675 |
|
| Tookad | Padoporfin | Bacteriochlorin | 762 |
|
| WST-11 | Padeliporfin | Baceriochlorin | 753 |
|
Compounds, diseases, and countries in which clinical uses for each disease indication are fully approved. EU, European Union (including the U.K.)
Compounds in the lower table are still investigational. Also, note that many clinically-approved PS in the upper table are being investigated beyond their originally approved indications.
A new approach to PDT using -aminolevulinic acid (ALA) was introduced in 1990 [14]. ALA is a precursor to porphyrins that are made within all cells; the intracellular porphyrins serve as the actual PS [14]. ALA, or one of its esters such as methyl-ALA, is administered as a prodrug (given topically or orally) which is then transported into cells and actively converted into protoporphyrin IX (PpIX) via heme biosynthetic enzymes present within mitochondria and the cytoplasm. Both the uptake of ALA, and the conversion to PpIX, often occur at higher rates in neoplastic cells than in cells of normal surrounding tissues. Topical ALA-based PDT has become a great success in dermatology, primarily because of the small and soluble nature of ALA and its esters, which favors permeability through the stratum corneum that overlies skin tumors. Also, local topical delivery significantly reduces the risk of prolonged, generalized photosensitivity [15].
4. Light sources for PDT
The wavelength of the light source must correspond to a maximal absorption peak of the PS in order to achieve good cell killing. For the tetrapyrrol-based compounds such as the porphyrins, the biggest absorption peak is nearly always in the Soret-band region around 400 nm. A light source with a high fluence rate, capable of delivering a large dose of light in a short time, is also important. Thus, lasers are often used for PDT but are not absolutely necessary (see below). The nature of light absorption within tissues also matters. Blue light (~400 nm) has relatively low penetration in tissue (only absorbed in the superficial epidermis of skin, for example), whereas red light (600 – 700 nm) penetrates more deeply. Infrared wavelengths, however, especially those greater than 800 nm wavelengths, provide too little energy to initiate photodynamic reactions by production of ROS [16]. Several light sources have been successfully used for PDT, including fluorescent tubes, incandescent filament lamps, broadband red light from high-pressure lamps, pulsed dyed lasers and light emitting diodes (LEDs). LEDs are inexpensive alternatives and have several advantages including portability, high fluence rates and relatively narrow spectral bandwidths [17].
The success of PDT can depend heavily upon the use of light dosimetry to measure and plan for adequate and uniform illumination of the tumor. This becomes especially important when treating deep internal malignancies using light delivered via optical fibers. Dosimetry enables one to determine an optimal fluence rate, total light dose, and exposure time for any given tumor, while sparing normal tissues [8].
5. Oxygen
The presence of oxygen in a tumor is critical for the outcome of PDT, since O2 is required for photochemical reactions to occur; hypoxic regions tend to be relatively unresponsive [18]. Oxygen pressure (pO2) can vary greatly between tumor regions, and is dependent upon the tumor vasculature and oxygen diffusion rates [19]. Oxygen availability is mainly a problem in deep solid tumors, whereas O2 levels in superficial skin tumors may not be rate-limiting. The rate of O2 consumption during PDT illumination is another important consideration. Under a high-intensity light source, O2 may be consumed faster than the rate of diffusion through tissue, resulting in transient O2 depletion in the tumor [20]. Careful manipulation of O2 levels in the tumor microenvironment, either by using fractionated light to minimize O2 depletion during treatment, or conversely, by delivering PS and light to intentionally target vessels to shut off the tumor's blood supply (starve the tumor), is important when designing an effective PDT regimen [21].
6. Biomodulation of PpIX levels and cell death pathways within tumors
As described above, the importance of three factors (drug, light, and oxygen) for a successful PDT outcome is supported by numerous studies, in vitro and in vivo, which have provided insights into detailed mechanisms (reviewed in [10; 22; 23]). At this point, however, efforts to optimize PDT through manipulation of PS, light, and oxygen have probably reached a plateau, and opportunities for further improvements may lie elsewhere. In that regard, a new frontier is opening up in the area of biomodulation for PDT, which emphasizes the importance of the physiological state of the target tumor. Biomodulation is a term that encompasses biological mechanisms by which cells metabolize PS prior to PDT, and respond to the treatment afterwards.
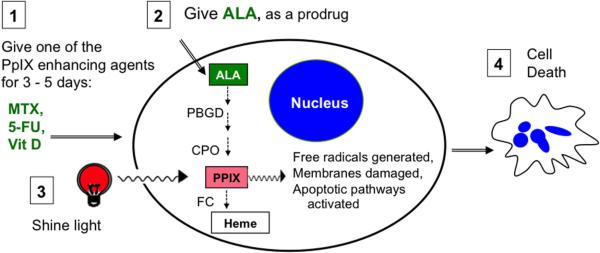
Much activity in this area has centered around ALA-based PDT. Several approaches involve the manipulation of enzymatic pathways by which 5-ALA is converted to heme. For example, the coadministration of ALA with an iron chelator has proven to be effective in both preclinical and clinical studies [24; 25]. Since iron is required for the formation of heme from PpIX, removal of iron blocks this rate-limiting step, yielding higher PpIX levels that are available for PDT targeting. The first iron chelator explored for this purpose, desferrioxamine (DFO), increased PpIX levels in cell lines but failed to elevate the level of PpIX in skin tumors as compared to healthy skin [26]. Another iron chelator, 1,2-diethyl-3-hydroxypyridin-4-one (CP94), has now been shown to selectively increase PpIX levels in preclinical and clinical studies [24; 25]. Another approach that originated in our laboratory involves the use of agents capable of promoting cellular differentiation (e.g. methotrexate, MTX; 5-fluorouracil, 5-FU; or vitamin D, Vit D), in combination regimens with PDT. Tumors are comprised of poorly-differentiated and rapidly proliferating cells that fail to follow normal physiological and developmental programs. We found that when cancer cells are pushed toward a more differentiated state, higher PS concentrations within the cells can be achieved and they become more susceptible to PDT [27–30]. This concept is illustrated in Figure 1 and described more fully in the section on Skin Cancer.
Fig. 1. Photodynamic therapy with ALA.
Pretreatment with an agent such as Vit D (step 1) leads to increased activity of the heme synthesis pathway. When ALA is given (step 2), more PpIX is produced. Light exposure (step 3) excites PpIX, generates free radicals, and initiates apoptosis (step 4). ALA, 5-aminolevulinic acid. Enzymes of the heme pathway: PBGD, porphobilinogen deaminase; CPO, coproporphyrinogen oxidase; FC, ferrochelatase. PpIX, protoporphyrin IX.
To date, PDT has been utilized for pre-neoplastic and neoplastic diseases in a wide variety of organ systems, including: skin, genitourinary, esophagus, prostate, bile duct, pancreas, head and neck, and brain. Below, the use of PDT is described in different organs, starting with a brief summary of current clinical status and followed by some relevant preclinical research. Clinical synopses were drawn from an evidence-based review of PDT in oncology, performed by an expert consensus panel for the Department of Health of the U.K. in 2008, which focused upon randomized controlled trials (RCTs) [31]. We also used information from some observational studies and uncontrolled clinical trials, because the latter studies, while imperfect, help to give a more complete picture of the current state of knowledge about this emerging technology [32].
7. Skin cancer and precancer
In terms of numbers of patients treated, PDT is used more frequently for skin cancers and precancers than for any other solid tumor. The success of PDT in dermatology relates to easy accessibility; skin tumors are highly amenable to topical drug and light delivery.
7.1. Clinical
Although the early development of PDT prior to 1990 focused mainly upon treating internal malignancies with PS delivered systemically and light delivered through optical fibers [33], the evolution of PDT into a popular treatment for skin tumors began with Kennedy and Pottier's description of a very simple regimen that uses topical ALA cream and external exposure to light [14; 34]. ALA or its esters (methyl-ALA, MAL; hexyl-ALA, HAL) can be applied topically, producing PpIX in the tumor cells which is then activated using a simple broadband light source. ALA-PDT and MAL-PDT are rapidly becoming preferred techniques for treating actinic keratoses (preneoplastic lesions of the skin), and MAL-PDT is effective for basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) in many settings. Advantages of PDT over surgery or ionizing radiation include excellent cosmetic results (scarless healing) and repeatability (little or no risk of genetic mutations with multiple PDT treatments). Most nonmelanoma skin cancers are amenable to PDT.
Actinic keratoses (AK)
These scaly red lesions on sun-exposed areas such as the face and hands, histologically show squamous dysplasia and carry an increased risk of progression to invasive squamous cell carcinoma (SCC). The risk of AK progression to full-blown skin cancer (~5% in normal patients) rises dramatically (10–50%) in immunosuppressed organ transplant recipients (OTRs), a group for whom aggressive SCC outstrips all other causes of death [35–37]. PDT represents a broad field-treatment for AK that can significantly reduce the risk of progression to SCC. Good clinical evidence exists in the form of 28 randomized control trials (RCTs) that have examined ALA-PDT and MAL-PDT for AK [31]. The overall conclusion is that PDT is at least as effective as cryotherapy or 5-fluorouracil cream (5-FU) in terms of lesion clearance (except for thick, hyperkeratotic lesions), and is clearly superior in terms of cosmetic result and patient tolerability. For AKs in immunosuppressed OTRs, MAL-PDT appears to offer better clearance than 5-FU in this high risk population [38; 39]. An NIH-sponsored trial in the U.S. to ask whether a combination of 5-FU plus PDT might be better than PDT alone for AKs in OTRs, is currently underway (ClinicalTrials.gov identifier NCT01525329).
Bowen's disease
This is squamous cell carcinoma in-situ, a more advanced form of AK. Seven RCTs have been completed [31], comparing ALA-PDT or MAL-PDT to cryotherapy, 5-FU, or placebo, and aggregated data indicate better rates of complete response (86–93%) and better cosmetic results with PDT than with the other treatments [40].
Squamous cell carcinoma
For invasive SCC, PDT is not currently recommended as first-line therapy; surgery gives better results. However, PDT can substantially reduce the size of large SCC tumors, and a recent clinical study suggests that neoadjuvant treatment in which topical PDT is used to shrink SCC prior to surgical excision, can reduce morbidity and increase overall curative response [41].
Basal cell carcinoma
BCC is the most common form of skin cancer. Thirteen RCTs have been reported, comparing PDT with surgical excision, cryotherapy, or placebo [31]. For superficial BCC, the outcome after PDT appears similar to surgery or cryotherapy, but the cosmesis after PDT is better. For nodular (deep) BCC, PDT is less effective than surgery for lesion clearance. A standard protocol for MAL-PDT of BCC used in Europe, but that regimen may not yet be optimized.
In summary, ALA-based PDT is useful for many nonmelanoma skin cancers and precancers, providing good clearance with excellent cosmetic results (scarless healing). Remaining problems include poor efficacy for deep nodular BCC and invasive SCC, and PDT-related pain experienced by patients during illumination. The pain can only be ameliorated with vigorous cooling of the skin or by regional nerve blocks in severe cases [42].
7.2. Preclinical
While a myriad of studies have examined PDT effects in cultured cells, preclinical (in vivo) models are essential for meaningful study of the complex responses of solid tumors to PDT. In this regard, preclinical models in the skin have been most helpful. We will first describe the cutaneous tumor models available for studying PDT responses, then describe studies that led to the concept of “differentiation-enhancing” pretreatments for ALA-PDT.
Several mouse models for preclinical testing of PDT conditions have been developed in the last two decades. These models include: (1) Chemical induction of superficial tumors in mouse skin. These are generated by the topical application of 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol-13-acetate (DMBA/TPA), which induce premalignant papillomas through mutation of Ha-Ras, with subsequent transformation into SCC [43]. (2) UVB induction of tumors, by prolonged and repeated exposure to ultraviolet B radiation. UVB is both a tumor initiator and promoter, and reliably generates squamous cell carcinomas (SCC). (3) Intradermal or subcutaneous implantation of transformed carcinoma cells beneath the skin of mice, to model invasive tumors or metastases.
The chemical carcinogenesis model has been used successfully in PDT studies by several groups, including ours. Tumor regression studies involving Pc4-PDT [44; 45], and studies involving methotrexate or vitamin D combined with ALA-PDT have been recently reported [27; 28]. UVB-induced tumors were employed in studies examining tumor responses to PDT utilizing different PS such as hypericin, Pc4, AlClPc, photofrin, HAL, and MAL [46–49]. Subcutaneous models were created by implantation of carcinoma cells such as RIF-1 radiation-induced fibrosarcoma cells, EMT6 mouse mammary cells, MDA-MB231 breast cancer cells, and A431 human squamous carcinoma cells for use in PDT studies. Such models were used to investigate the apoptotic response of tumors by PARP-cleavage, overexpression of COX-2, and tumor regression following PDT using photofrin, Pc4, or methylene blue as the PS [50–53]. In our laboratory, the subcutaneous SCC model with implantation of human A431 cells was used to study tumor responses to combinations of differentiation-inducing pretreatment regimens prior to ALA-PDT, which elucidated the role of TNF and caspase induction during apoptosis following ALA-PDT [27; 28].
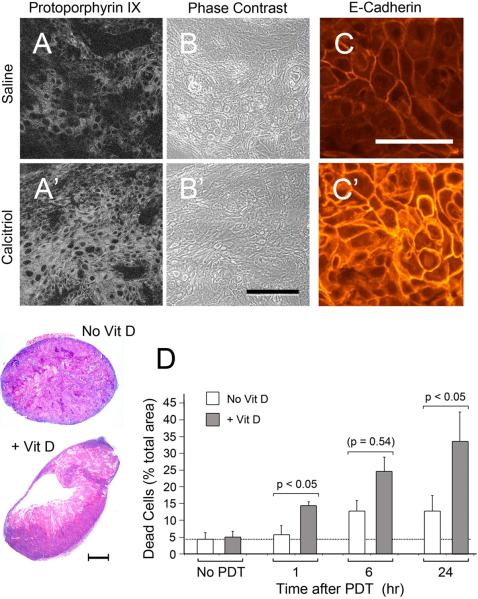
As a salient example of PDT research using preclinical skin models, the discovery of new combinations of “differentiation-promoting” agents and ALA-PDT will now be described. Because ALA-PDT alone is suboptimal for deep tumors, any approach that significantly increases the amount of PS (PpIX) in such tumors would be welcome. Various approaches to try to increase ALA penetration into the epidermis have been tried with only partial success [54; 55]. Our approach was to try to enhance PpIX production by pharmacological interventions that increase the synthesis of PpIX within cells (Figure 2). We have identified three agents (MTX, 5-FU, and Vit D ), each of which can change the expression of heme synthetic enzymes in tumors, thereby elevating PpIX production prior to ALA-PDT. This is illustrated for pretreatment with Vit D vs. saline control (Fig. 2A' vs. 2A). Coincidentally, these agents also promote tumor cell differentiation (Fig. 2C' vs. 2C).
Figure 2. Examples of the biomodulatory effects of a differentiation-promoting agent (1,25 dihydroxy-vitamin D3; calcitriol) upon a solid tumor.
The A431 squamous cell carcinoma line was implanted subcutaneously in nude mice. Mice were preconditioned with daily i.p. injections of calcitriol (1 μg/kg body weight) for 3 days, and on the 4th day, ALA was administered for 4 hr prior to tumor harvest (A–C) or illumination with 635 nm light (D). Compare to inert saline controls (A–C), the calcitriol preconditioned tumors (A' – C') exhibited higher PpIX levels by confocal microscopy (A, A') in sections with equal cellularity (B, B'), and also showed stronger staining of a differentiation-associated molecule, ECadherin (C, C'). Scale bars, 50 μm. At various times after 635 nm illumination, histological H&E staining revealed significantly more cell death in the calcitriol-pretreated tumors (D). Scale bar, 250 μm. Adapted from ref. [28], with permission.
A link between enhanced differentiation and PpIX accumulation was first suggested by the discovery that skin keratinocytes, forced to undergo growth arrest and terminal differentiation, became more sensitive to ALA-PDT due to enhanced accumulation of PpIX [29]. A combination regimen (cPDT) was proposed, in which pretreatment with the differentiation-promoting agents would be followed by ALA-PDT, thereby enhancing PDT efficacy. Subsequent work has shown that benefits of cPDT are twofold: (1) increased levels of the PpIX target, especially in neoplastic cells; (2) enhanced activity of cell-death mechanisms. With cPDT, a differentiation-inducing pretreatment prior to PDT redirects cancer cells toward normal processes of terminal differentiation and apoptosis, thereby increasing cell death post-PDT (Fig. 2D). Note that cPDT differs from other forms of differentiation therapy, including the use of retinoids in promyelocytic leukemia, in the following ways: (1) cPDT utilizes the prodrug ALA, exploiting the fact that PpIX is preferentially produced in target tumor cells compared to surrounding normal tissue; (2) the differentiation pretreatment is administered over a relatively short time (generally 3 to 5 days), thereby reducing potential toxicity.
The cPDT concept has been successfully tested in various preclinical models (cultured cells in vitro and mouse tumors in vivo), using each of the three pro-differentiating agents (MTX, Vit D, or 5FU) as a 3-day pretreatment regimen. In each case, the pretreatment given prior to ALA-PDT led to increased tumor cell killing due to higher concentrations of PpIX achieved. Remarkably, these agents exert these effects at a concentration several fold (10- to 1000-fold) below their normal clinical dose [27–30]. The mechanisms behind these selective increases in PpIX were shown to involve altered regulation of key enzymes of the heme biosynthetic pathway. Regulation of heme synthetic enzymes during PDT has been characterized in different model systems [56–58]. During pretreatment with MTX or Vit D, levels of coproporphyrinogen oxidase (CPO) and ferrochelatase (FC) are altered in a manner that increases the level of PpIX in tumors [27; 28; 30]. Studies to combine MTX, Vit D, or 5-FU pretreatment with CP94 (an iron chelator), to further elevate PpIX, are being contemplated.
8. PDT for genital and perineal cancers (mucosal intraepithelial neoplasia)
8.1. Clinical
By analogy to the well-established benefit of PDT for actinic keratoses of the skin, PDT is now being used as another option for precancerous conditions that occur in other mucocutaneous epithelia, such as the cervix, vulva, and perianal region [59]. Each of these dysplasias is considered a precursor to invasive carcinoma, and a nondisfiguring therapy to prevent cancer progression would be valuable. For cervical intraepithelial neoplasia (CIN), PDT offers a nonscarring alternative to cone biopsy, as described in a case series of 112 patients whose CIN was treated using PDT with chlorin e6 (Fotolon), with 92% clearance [60]. In another study of 25 patients with CIN treated with hexyl-ALA PDT, complete response and fibrosis-free healing was confirmed histologically [61]. For vulvar intraepithelial neoplasia (VIN), use of Foscan [62] or ALA [63] as the PS may ameliorate the need for radical mutilating surgery. In one interesting study, 25 women with high-grade papillomavirus-associated VIN were treated with topical imiquimod (an immunomodulatory agent) for 8 weeks, then PDT with topical MAL plus red light at weeks 12 and 16. At the end of 1 year of follow-up, the overall response rate was 55% with complete responses in 24% [64]. Note that the alternative to PDT in this cohort with advanced VIN disease was surgical ablation [64]. Similarly, penile intraepithelial neoplasia (PIN) [65] and anal intraepithelial neoplasia (AIN) [66] have been treated with aminolevulinate-based PDT, sometimes with complete clearance. Extramammary Paget's disease (perineal intraepithelial neoplasia in older adults) responds to PDT with porfimer sodium or ALA [67; 68]. In summary, PDT is a viable alternative to surgery or radiotherapy for managing intraepithelial neoplastic conditions because it avoids scarring and can be repeated many times. Relative disadvantages include pain during light delivery (which may require general anesthesia). Randomized trials are still needed to document superiority over other treatments, particularly topical imiquimod alone.
8.2. Preclinical
As with squamous neoplasia of the skin, a combination approach using biomodulators such as 5FU or Vitamin D to enhance the 5-ALA mediated accumulation of target protoporphyrins should be considered in future clinical trials. Also worth considering are future studies to examine mechanisms by which imiquimod, a Toll-like receptor agonist, affects the inflammatory (immunologic) responses to tumor cell-associated antigens when administered before or after PDT.
9. PDT for esophageal cancer and precancer (Barrett's)
9.1. Clinical
In the distal esophagus, squamous-to-columnar conversion of the distal esophagus can occur due to chronic reflux of gastric acid. This condition (called Barrett's esophagus) predisposes to development of high-grade dysplasia, which may progress to invasive adenocarcinoma if not treated. As an alternative to surgical esophagectomy, PDT with porfimer sodium (Table 1), or with 5-ALA in some studies, with light delivered endoscopically, has been used with considerable success [69]. There have been 11 RCTs performed of patients treated with PDT for Barrett's esophagus. One large multi-center study of 208 patients, followed for 5 years, showed that porfimer sodium PDT plus omeprazole (an acid-lowering agent) was significantly more effective than omeprazole alone for eliminating high-grade dysplasia (HGD) in Barrett's patients (77% vs. 39% without HGD), and for reducing progression to cancer (15% vs. 29% with new esophageal cancers) [70].
Another endoscopic technique called radiofrequency ablation (RFA) appears to be equally effective to PDT for elimination of high grade dysplasia, and because RFA does not require any additional drug, may be gaining favor among clinicians [71]. However, both PDT and RFA represent good alternatives to esophagectomy.
Advanced esophageal cancers have been treated with PDT, mainly as a palliative measure (tumor debulking), but not as a preferred primary modality.
9.2. Preclinical
For Barrett's and esophageal cancer, most research activity has centered on efforts to identify tissue biomarkers that might identify which patients will respond to PDT of Barrett's esophagus [72].
10. PDT for head & neck cancer
10.1. Clinical
This group of diseases refers to carcinoma of the mouth, pharynx, larynx, salivary glands, and middle ear, with most of the tumors arising in mucosal linings. PDT has the potential to control cancers in these locations with less loss of function (swallowing, taste, speech, etc) as compared to surgery or radiation therapy [73]. By 2010, over 1500 patients have been treated with PDT for head and neck cancers, mostly for squamous carcinoma but also other cancer pathologies [74]. Porfimer sodium (Photofrin), 5-ALA, Foscan, and Photochlor have all been used by systemic delivery; Foscan in particular is licensed in the EU for head and neck indications (Table 1). For early oral cancer, two large multicenter European studies showed very good response rates (71–80% CR) [75; 76]. For early cancers of the larynx (true vocal cord), PDT with porfimer sodium was shown to be 91% effective at 5 years and 100% effective after retreatment of recurrences, making PDT a much less mutilating treatment than laryngectomy [77].
10.2. Preclinical
Although yet to be applied to SCC of the head and neck, the pro-differentiation approaches described in section 6. might be applicable to ALA-PDT of head and neck cancers. Other interesting combination approaches have been described, such as the use of sphingolipid analogs to enhance Foscan-mediated PDT of head and neck SCC in mice; these compounds may also represent biomarkers for predicting treatment response [78].
11. PDT for lung cancer
11.1. Clinical
PDT is increasingly being used to treat cancers of the airways and other tumors in the thoracic cavity [79]. PDT using systemic Photofrin and light delivered via a bronchoscope appears to be quite effective for early SCC of the airways, with efficacy similar to localized electrocautery, cryotherapy, or laser therapy. Another interesting niche for PDT is mesothelioma; PDT delivered in an open-chest setting (by filling the inter-pleural space with a translucent fluid to distribute light to all tumors studded across the pleural surface) is proving an effective way to treat this otherwise intractable cancer [80].
11.2. Preclinical
An orthotopic model of disseminated non-small cell lung carcinoma in mice, in which PDT is administered via diffusion of light throughout the thoracic cavity to treat widespread tumor nodules, could prove very useful for preclinical investigations [80].
12. PDT for prostate cancer
12.1. Clinical
PDT is one of several minimally-invasive alternatives to surgery or radiotherapy for prostate cancer that is being tried (the others being cryotherapy, high-intensity ultrasound, and thermal laser ablation). All of these alternatives reduce the risk of the post-surgical side effects of incontinence and impotence. Foscan, Tookad, and Lutex have been used in observational studies of prostate cancer treatment (Table 1). The photosensitizer is injected intravenously, and optical fibers for light delivery are inserted into the prostate gland under transrectal ultrasound guidance. Several Phase 3 randomized controlled trials to compare PDT with active clinical surveillance, are now underway in Europe.
12.2. Preclinical
Studies in which prostate cancer cell lines were implanted in mice, to create in vivo tumor models, have offered valuable insights about PDT mechanisms of action. For example, experimental in vivo tumor models were used to show the following: (i), prostate cancers are more highly vascularized when grown orthotopically as compared to subcutaneously [18]; (ii), VEGF plays a very important role in the response to PDT [81]; (iii), microheterogeneity of the vascular bed within tumors can greatly affect photosensitizer delivery and efficacy [82]. Other studies conducted with prostate cancer cell lines suggest that pro-differentiation approaches using MTX and Vit D could have potential, if 5-ALA were employed for PDT of prostate cancer [30; 83].
13. PDT for bile duct and pancreas
13.1. Clinical
Biliary tract cancer (carcinoma of the gall bladder and/or bile ducts) has a low survival rate. Patients usually die of unrelieved biliary obstruction, with a median survival of only 9 months. For patients with inoperable tumors that cause obstruction, standard practice is to place a tube (stent) within the biliary drainage system. In a systematic review of two RCTs and four longitudinal prospective studies totalling 170 patients treated with porfimer sodium PDT plus stenting, palliative treatment of cholangiocarcinoma with PDT was associated with increased survival benefit, improved biliary drainage, and quality of life compared with stenting alone, but the quality of this evidence was considered to be low [84]. The preliminary results of a large, multicentre randomised study comparing stenting alone with porfimer sodium PDT plus stenting have also been presented, which showed a significantly shorter survival time in the PDT group [85]. Therefore, there are currently conflicting data on whether PDT improves outcome in patients with cholangiocarcinoma.
For patients with operable biliary tract tumors, one small study suggested that neoadjuvant PDT (given prior to surgery, to shrink the tumor) could increase the probability of surgical resection margins that are free of tumor (R0 resection), prompting calls for a further trial of neoadjuvant PDT and surgery for biliary cancer [86].
Pancreatic cancer is another highly lethal disease, with a median survival of 6–10 months (3–6 months if the disease is metastatic). At the time of presentation, most patients have advanced disease and are unsuitable for surgery. PDT represents a minimally invasive procedure for tumor debulking, and is performed with transabdominal light fibers (inserted percutaneously through thin needles placed directly into the pancreas under CT or MR image guidance). A preliminary study in 2002 using Foscan looked promising [87], and a new Phase 1 study using verteporfin as the systemic photosensitizer is currently underway in the UK [88].
13.2. Preclinical
Studies in a pig model showed that ablation of pancreatic tissue under image guidance is safe [89]. Other preclinical studies indicate a rationale for combining verteporfin-PDT and gemcitabine; gemcitabine is an approved chemotherapeutic drug for pancreatic cancer, so the addition of PDT would be logical [90; 91].
14. Other Uses for PDT in Cancer
There are many other proposed uses for PDT in oncology, still anecdotal but interesting and potentially promising. One to especially highlight is the use of 5-ALA for fluorescence guided surgical resection of glioma multiforme of the brain. Protoporphyrin IX accumulates specifically in the neoplastic tissue, helping neurosurgeons to identify fluorescent tumor margins in brain tissue (which lacks anatomical landmarks) [92]. Such new uses for photosensitizing drugs as theranostics (the combination of photodiagnosis with surgery), or as neoadjuvants (PDT performed prior to surgical resection), should offer many new therapeutic options in oncology in the future.
Acknowledgements
The authors would like to acknowledge grant P01-CA84203 from the National Cancer Institute/NIH, which supported much of the work they performed as reviewed in this article.
Financial support: NIH grant P01-CA84203
Abbreviations used
- ALA
-aminolevulinic acid
- calcitriol
1 ,25-dihydroxy-vitamin D3
- CPO
coproporphyrinogen oxidase
- E-cad
E-cadherin
- FC
ferrochelatase
- HAL
hexyl-ALA
- MAL
methyl-ALA
- PBGD
porphobilinogen deaminase
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
- PS
photosensitizer
- TNF
tumor necrosis factor alpha
- Vit D
vitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest None declared.
REFERENCES
- [1].Moan J, Peng Q. An outline of the history of PDT. In: Patrice T, editor. Photodynamic Therapy, The Royal Society of Chemistry. Cambridge, UK: 2003. pp. 3–15. www.rsc.org. [Google Scholar]
- [2].Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- [3].Pass HI. Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst. 1993;85:443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- [4].Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- [5].Hasan T, Ortel B, Solban N, Pogue BW. Photodynamic therapy of cancer. In: Kufe D, Bast R, Hait W, Hong W, Pollock R, Weichselbaum R, Holland J, Frei E, editors. Cancer Medicine. 7th edition BC Decker, Inc.; Hamilton, Ontario: 2006. pp. 537–548. [Google Scholar]
- [6].Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kessel D. Death pathways associated with photodynamic therapy. Med Laser Appl. 2006;21:219–224. doi: 10.1016/j.mla.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pogue BW, Samkoe KS, Gibbs-Strauss SL, Davis SC. Fluorescent molecular imaging and dosimetry tools in photodynamic therapy. Methods Mol Biol. 2010;635:207–222. doi: 10.1007/978-1-60761-697-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ortel B, Calzavara-Pinton P. Advances in photodynamic therapy. A review. G Ital Dermatol Venereol. 2010;145:461–475. [PubMed] [Google Scholar]
- [10].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Josefsen LB, Boyle RW. Photodynamic therapy: novel third-generation photosensitizers one step closer? Br J Pharmacol. 2008;154:1–3. doi: 10.1038/bjp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moore CM, Pendse D, Emberton M. Photodynamic therapy for prostate cancer--a review of current status and future promise. Nat Clin Pract Urol. 2009;6:18–30. doi: 10.1038/ncpuro1274. [DOI] [PubMed] [Google Scholar]
- [13].Zhao B, He YY. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev Anticancer Ther. 2010;10:1797–1809. doi: 10.1586/era.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6:143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- [15].De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]
- [16].Juzeniene A, Nielsen KP, Moan J. Biophysical aspects of photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:7–28. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.20. [DOI] [PubMed] [Google Scholar]
- [17].Juzeniene A, Juzenas P, Ma LW, Iani V, Moan J. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med Sci. 2004;19:139–149. doi: 10.1007/s10103-004-0314-x. [DOI] [PubMed] [Google Scholar]
- [18].Chen B, Pogue BW, Zhou X, O'Hara JA, Solban N, Demidenko E, Hoopes PJ, Hasan T. Effect of tumor host microenvironment on photodynamic therapy in a rat prostate tumor model. Clin Cancer Res. 2005;11:720–727. [PubMed] [Google Scholar]
- [19].Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- [20].Pogue BW, Sheng C, Benevides J, Forcione D, Puricelli B, Nishioka N, Hasan T. Protoporphyrin IX fluorescence photobleaching increases with the use of fractionated irradiation in the esophagus. J Biomed Opt. 2008;13:034009. doi: 10.1117/1.2937476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–305. doi: 10.1615/critreveukargeneexpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- [22].Ortel B, Shea CR, Calzavara-Pinton P. Molecular mechanisms of photodynamic therapy. Front Biosci. 2009;14:4157–4172. doi: 10.2741/3520. [DOI] [PubMed] [Google Scholar]
- [23].Solban N, Ortel B, Pogue B, Hasan T. Targeted optical imaging and photodynamic therapy. Ernst Schering Res Found Workshop. 2005:229–258. doi: 10.1007/3-540-26809-x_12. [DOI] [PubMed] [Google Scholar]
- [24].Campbell SM, Morton CA, Alyahya R, Horton S, Pye A, Curnow A. Clinical investigation of the novel iron-chelating agent, CP94, to enhance topical photodynamic therapy of nodular basal cell carcinoma. Br J Dermatol. 2008;159:387–393. doi: 10.1111/j.1365-2133.2008.08668.x. [DOI] [PubMed] [Google Scholar]
- [25].Curnow A, McIlroy BW, Postle-Hacon MJ, Porter JB, MacRobert AJ, Bown SG. Enhancement of 5-aminolaevulinic acid-induced photodynamic therapy in normal rat colon using hydroxypyridinone iron-chelating agents. Br J Cancer. 1998;78:1278–1282. doi: 10.1038/bjc.1998.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Choudry K, Brooke RC, Farrar W, Rhodes LE. The effect of an iron chelating agent on protoporphyrin IX levels and phototoxicity in topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 2003;149:124–130. doi: 10.1046/j.1365-2133.2003.05351.x. [DOI] [PubMed] [Google Scholar]
- [27].Anand S, Honari G, Hasan T, Elson P, Maytin EV. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res. 2009;15:3333–3343. doi: 10.1158/1078-0432.CCR-08-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71:6040–6050. doi: 10.1158/0008-5472.CAN-11-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ortel B, Chen N, Brissette J, Dotto GP, Maytin E, Hasan T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br J Cancer. 1998;77:1744–1751. doi: 10.1038/bjc.1998.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sinha AK, Anand S, Ortel BJ, Chang Y, Mai Z, Hasan T, Maytin EV. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br J Cancer. 2006;95:485–495. doi: 10.1038/sj.bjc.6603273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fayter D, Corbett M, Heirs M, Fox D, Eastwood A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol Assess. 2010;14:1–129. doi: 10.3310/hta14370. [DOI] [PubMed] [Google Scholar]
- [32].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dougherty TJ. Photosensitization of malignant tumors. Semin Surg Oncol. 1986;2:24–37. doi: 10.1002/ssu.2980020104. [DOI] [PubMed] [Google Scholar]
- [34].Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- [35].Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, Fauchald P, Simonsen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- [36].Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- [37].Lampros TD, Cobanoglu A, Parker F, Ratkovec R, Norman DJ, Hershberger R. Squamous and basal cell carcinoma in heart transplant recipients. J Heart Lung Transplant. 1998;17:586–591. [PubMed] [Google Scholar]
- [38].Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, Burg G, Binswanger U, Kempf W. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen's disease in transplant recipients. Transplantation. 2004;77:115–121. doi: 10.1097/01.TP.0000107284.04969.5C. [DOI] [PubMed] [Google Scholar]
- [39].Perrett CM, McGregor JM, Warwick J, Karran P, Leigh IM, Proby CM, Harwood CA. Treatment of post-transplant premalignant skin disease: a randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol. 2007;156:320–328. doi: 10.1111/j.1365-2133.2006.07616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Morton CA, Brown SB, Collins S, Ibbotson S, Jenkinson H, Kurwa H, Langmack K, McKenna K, Moseley H, Pearse AD, Stringer M, Taylor DK, Wong G, Rhodes LE. Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group. Br J Dermatol. 2002;146:552–567. doi: 10.1046/j.1365-2133.2002.04719.x. [DOI] [PubMed] [Google Scholar]
- [41].Jeremic G, Brandt MG, Jordan K, Doyle PC, Yu E, Moore CC. Using photodynamic therapy as a neoadjuvant treatment in the surgical excision of nonmelanotic skin cancers: prospective study. J Otolaryngol Head Neck Surg. 2011;40(Suppl 1):S82–89. [PubMed] [Google Scholar]
- [42].Warren CB, Karai LJ, Vidimos A, Maytin EV. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol. 2009;61:1033–1043. doi: 10.1016/j.jaad.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Owens DM, Wei S, Smart RC. A multihit, multistage model of chemical carcinogenesis. Carcinogenesis. 1999;20:1837–1844. doi: 10.1093/carcin/20.9.1837. [DOI] [PubMed] [Google Scholar]
- [44].Agarwal R, Korman NJ, Mohan RR, Feyes DK, Jawed S, Zaim MT, Mukhtar H. Apoptosis is an early event during phthalocyanine photodynamic therapy-induced ablation of chemically induced squamous papillomas in mouse skin. Photochem Photobiol. 1996;63:547–552. doi: 10.1111/j.1751-1097.1996.tb03082.x. [DOI] [PubMed] [Google Scholar]
- [45].Kalka K, Ahmad N, Criswell T, Boothman D, Mukhtar H. Up-regulation of clusterin during phthalocyanine 4 photodynamic therapy-mediated apoptosis of tumor cells and ablation of mouse skin tumors. Cancer Res. 2000;60:5984–5987. [PubMed] [Google Scholar]
- [46].Mukhtar H, Agarwal R, Athar M, Lewen RL, Elmets CA, Bickers DR. Photodynamic therapy of murine skin tumors using Photofrin-II. Photodermatol Photoimmunol Photomed. 1991;8:169–175. [PubMed] [Google Scholar]
- [47].Kyriazi M, Alexandratou E, Yova D, Rallis M, Trebst T. Topical photodynamic therapy of murine non-melanoma skin carcinomas with aluminum phthalocyanine chloride and a diode laser: pharmacokinetics, tumor response and cosmetic outcomes. Photodermatol Photoimmunol Photomed. 2008;24:87–94. doi: 10.1111/j.1600-0781.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- [48].Boiy A, Roelandts R, de Witte PA. Photodynamic therapy using topically applied hypericin: comparative effect with methyl-aminolevulinic acid on UV induced skin tumours. J Photochem Photobiol B. 2011;102:123–131. doi: 10.1016/j.jphotobiol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- [49].Togsverd-Bo K, Lerche CM, Poulsen T, Wulf HC, Haedersdal M. Photodynamic therapy with topical methyl- and hexylaminolevulinate for prophylaxis and treatment of UV-induced SCC in hairless mice. Exp Dermatol. 2010;19:e166–172. doi: 10.1111/j.1600-0625.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- [50].Anderson CY, Freye K, Tubesing KA, Li YS, Kenney ME, Mukhtar H, Elmets CA. A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem Photobiol. 1998;67:332–336. [PubMed] [Google Scholar]
- [51].Ferrario A, Von Tiehl K, Wong S, Luna M, Gomer CJ. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer Res. 2002;62:3956–3961. [PubMed] [Google Scholar]
- [52].Bai L, Guo J, Bontempo FA, 3rd, Eiseman JL. The relationship of phthalocyanine 4 (pc 4) concentrations measured noninvasively to outcome of pc 4 photodynamic therapy in mice. Photochem Photobiol. 2009;85:1011–1019. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- [53].Baran TM, Giesselman BR, Hu R, Biel MA, Foster TH. Factors influencing tumor response to photodynamic therapy sensitized by intratumor administration of methylene blue. Lasers Surg Med. 2010;42:728–735. doi: 10.1002/lsm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Donnelly RF, McCarron PA, Woolfson AD. Drug delivery of aminolevulinic acid from topical formulations intended for photodynamic therapy. Photochem Photobiol. 2005;81:750–767. doi: 10.1562/2004-08-23-IR-283. [DOI] [PubMed] [Google Scholar]
- [55].Gerritsen MJ, Smits T, Kleinpenning MM, van de Kerkhof PC, van Erp PE. Pretreatment to enhance protoporphyrin IX accumulation in photodynamic therapy. Dermatology. 2009;218:193–202. doi: 10.1159/000183753. [DOI] [PubMed] [Google Scholar]
- [56].Amo T, Kawanishi N, Uchida M, Fujita H, Oyanagi E, Utsumi T, Ogino T, Inoue K, Shuin T, Utsumi K, Sasaki J. Mechanism of cell death by 5-aminolevulinic acid-based photodynamic action and its enhancement by ferrochelatase inhibitors in human histiocytic lymphoma cell line U937. Cell Biochem Funct. 2009;27:503–515. doi: 10.1002/cbf.1603. [DOI] [PubMed] [Google Scholar]
- [57].Feuerstein T, Schauder A, Malik Z. Silencing of ALA dehydratase affects ALA-photodynamic therapy efficacy in K562 erythroleukemic cells. Photochem Photobiol Sci. 2009;8:1461–1466. doi: 10.1039/b9pp00007k. [DOI] [PubMed] [Google Scholar]
- [58].Greenbaum L, Katcoff DJ, Dou H, Gozlan Y, Malik Z. A porphobilinogen deaminase (PBGD) Ran-binding protein interaction is implicated in nuclear trafficking of PBGD in differentiating glioma cells. Oncogene. 2003;22:5221–5228. doi: 10.1038/sj.onc.1206723. [DOI] [PubMed] [Google Scholar]
- [59].Soergel P, Hillemanns P. Photodynamic therapy for intraepithelial neoplasia of the lower genital tract. Photodiagnosis Photodyn Ther. 2010;7:10–14. doi: 10.1016/j.pdpdt.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [60].Istomin YP, Lapzevich TP, Chalau VN, Shliakhtsin SV, Trukhachova TV. Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with Photolon. Photodiagnosis Photodyn Ther. 2010;7:144–151. doi: 10.1016/j.pdpdt.2010.06.005. [DOI] [PubMed] [Google Scholar]
- [61].Soergel P, Loehr-Schulz R, Hillemanns M, Landwehr S, Makowski L, Hillemanns P. Effects of photodynamic therapy using topical applied hexylaminolevulinate and methylaminolevulinate upon the integrity of cervical epithelium. Lasers Surg Med. 2010;42:624–630. doi: 10.1002/lsm.20979. [DOI] [PubMed] [Google Scholar]
- [62].Campbell SM, Gould DJ, Salter L, Clifford T, Curnow A. Photodynamic therapy using meta-tetrahydroxyphenylchlorin (Foscan) for the treatment of vulval intraepithelial neoplasia. Br J Dermatol. 2004;151:1076–1080. doi: 10.1111/j.1365-2133.2004.06197.x. [DOI] [PubMed] [Google Scholar]
- [63].Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100:271–275. doi: 10.1016/j.ygyno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- [64].Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, Kitchener HC. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008;14:5292–5299. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- [65].Axcrona K, Brennhovd B, Alfsen GC, Giercksky KE, Warloe T. Photodynamic therapy with methyl aminolevulinate for atypial carcinoma in situ of the penis. Scand J Urol Nephrol. 2007;41:507–510. doi: 10.1080/00365590701428590. [DOI] [PubMed] [Google Scholar]
- [66].Webber J, Fromm D. Photodynamic therapy for carcinoma in situ of the anus. Arch Surg. 2004;139:259–261. doi: 10.1001/archsurg.139.3.259. [DOI] [PubMed] [Google Scholar]
- [67].Nardelli AA, Stafinski T, Menon D. Effectiveness of photodynamic therapy for mammary and extra-mammary Paget's disease: a state of the science review. BMC Dermatol. 2011;11:13. doi: 10.1186/1471-5945-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Housel JP, Izikson L, Zeitouni NC. Noninvasive extramammary Paget's disease treated with photodynamic therapy: case series from the Roswell Park Cancer Institute. Dermatol Surg. 2010;36:1718–1724. doi: 10.1111/j.1524-4725.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- [69].Menon D, Stafinski T, Wu H, Lau D, Wong C. Endoscopic treatments for Barrett's esophagus: a systematic review of safety and effectiveness compared to esophagectomy. BMC Gastroenterol. 2010;10:111. doi: 10.1186/1471-230X-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, Sivak MV, Jr., Nishioka N, Barr H, Marcon N, Pedrosa M, Bronner MP, Grace M, Depot M. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- [71].Dunn JM, Banks MR, Oukrif D, Mackenzie GD, Thorpe S, Rodriguez-Justo M, Winstanley A, Bown SG, Novelli MR, Lovat LB. Radiofrequency ablation is effective for te treatment of high-grade dysplasia in Barrett's esophagus after failed photodynamic therapy. Endoscopy. 2011;43:627–630. doi: 10.1055/s-0030-1256443. [DOI] [PubMed] [Google Scholar]
- [72].Prasad GA, Wang KK, Halling KC, Buttar NS, Wongkeesong LM, Zinsmeister AR, Brankley SM, Fritcher EG, Westra WM, Krishnadath KK, Lutzke LS, Borkenhagen LS. Utility of biomarkers in prediction of response to ablative therapy in Barrett's esophagus. Gastroenterology. 2008;135:370–379. doi: 10.1053/j.gastro.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jerjes W, Upile T, Alexander Mosse C, Hamdoon Z, Morcos M, Morley S, Hopper C. Prospective evaluation of 110 patients following ultrasound-guided photodynamic therapy for deep seated pathologies. Photodiagnosis Photodyn Ther. 2011;8:297–306. doi: 10.1016/j.pdpdt.2011.08.002. [DOI] [PubMed] [Google Scholar]
- [74].Biel MA. Photodynamic therapy of head and neck cancers. Methods Mol Biol. 2010;635:281–293. doi: 10.1007/978-1-60761-697-9_18. [DOI] [PubMed] [Google Scholar]
- [75].Hopper C, Kubler A, Lewis H, Tan IB, Putnam G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer. 2004;111:138–146. doi: 10.1002/ijc.20209. [DOI] [PubMed] [Google Scholar]
- [76].Karakullukcu B, van Oudenaarde K, Copper MP, Klop WM, van Veen R, Wildeman M, Bing Tan I. Photodynamic therapy of early stage oral cavity and oropharynx neoplasms: an outcome analysis of 170 patients. Eur Arch Otorhinolaryngol. 2011;268:281–288. doi: 10.1007/s00405-010-1361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol. 2007;83:1063–1068. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- [78].Separovic D, Bielawski J, Pierce JS, Merchant S, Tarca AL, Bhatti G, Ogretmen B, Korbelik M. Enhanced tumor cures after Foscan photodynamic therapy combined with the ceramide analog LCL29. Evidence from mouse squamous cell carcinomas for sphingolipids as biomarkers of treatment response. Int J Oncol. 2011;38:521–527. doi: 10.3892/ijo.2010.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Simone CB, 2nd, Friedberg JS, Glatstein E, Stevenson JP, Sterman DH, Hahn SM, Cengel KA. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis. 2012;4:63–75. doi: 10.3978/j.issn.2072-1439.2011.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Friedberg JS. Photodynamic therapy for malignant pleural mesothelioma: the future of treatment? Expert Rev Respir Med. 2011;5:49–63. doi: 10.1586/ers.11.1. [DOI] [PubMed] [Google Scholar]
- [81].Kosharskyy B, Solban N, Chang SK, Rizvi I, Chang Y, Hasan T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Res. 2006;66:10953–10958. doi: 10.1158/0008-5472.CAN-06-1793. [DOI] [PubMed] [Google Scholar]
- [82].Zhou X, Chen B, Hoopes PJ, Hasan T, Pogue BW. Tumor vascular area correlates with photosensitizer uptake: analysis of verteporfin microvascular delivery in the Dunning rat prostate tumor. Photochem Photobiol. 2006;82:1348–1357. doi: 10.1562/2006-03-25-ra-858. [DOI] [PubMed] [Google Scholar]
- [83].Ortel B, Sharlin D, O'Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87:1321–1327. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Leggett CL, Gorospe EC, Montori VM, Baron TH, Murad MH, Wang KK. Photodynamic Therapy for Unresectable Cholangiocarcinoma: A Comparative Effectiveness Systematic Review and Meta-analyses. Photodiag Photodynam Ther. doi: 10.1016/j.pdpdt.2012.03.002. doi:10.1016/j.pdpdt.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [85].Pereira SP, Hughes SK, Roughton M, O'Donoghue P, Wasan HS, Valle J, Bridgewater J. Photostent-02; Porfimer Sodium Photodynamic Therapy Plus Stenting Versus Stenting Alone in Patients with Advanced Or Metastatic Cholangiocarcinomas and Other Biliary Tract Tumours: A Multicentre, Randomised Phase III Trial. Annals Oncol. 2010;21:250. [Google Scholar]
- [86].Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, Mossner J, Hauss J, Witzigmann H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer. 2003;97:2783–2790. doi: 10.1002/cncr.11401. [DOI] [PubMed] [Google Scholar]
- [87].Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huggett MT, Baddeley RNB, Sandanayake MS, Webster GJM, Bown SG, Lovat LB, Gillams A, Pogue BW, Hasan T, Pereira SP. Kessel D, Hasan T, editors. Photodynamic therapy of pancreatic cancer and elastic scattering spectroscopy of the duodenal mucosa for the detection of pancreaticobiioliary malignancy. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XX, Society of Photo-Optical Instrumentation Engineers. 2011:718860J718861–718869. [Google Scholar]
- [89].Yusuf TE, Matthes K, Brugge WR. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video) Gastrointest Endosc. 2008;67:957–961. doi: 10.1016/j.gie.2007.08.020. [DOI] [PubMed] [Google Scholar]
- [90].Xie Q, Jia L, Liu YH, Wei CG. Synergetic anticancer effect of combined gemcitabine and photodynamic therapy on pancreatic cancer in vivo. World J Gastroenterol. 2009;15:737–741. doi: 10.3748/wjg.15.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg Med. 2011;43:565–574. doi: 10.1002/lsm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Eljamel S. Photodynamic applications in brain tumors: a comprehensive review of the literature. Photodiagnosis Photodyn Ther. 2010;7:76–85. doi: 10.1016/j.pdpdt.2010.02.002. [DOI] [PubMed] [Google Scholar]