Abstract
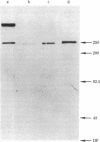
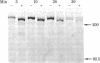
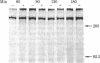
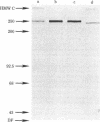
A monoclonal antibody, 9.2.27, with a high specificity for human melanoma cell surfaces has been utilized for biosynthetic studies in M21 human melanoma cells to define a unique antigenic complex consisting of a 250-kilodalton N-linked glycoprotein and a high molecular weight proteoglycan component larger than 400 kilodaltons. The 250-kilodalton glycoprotein has endoglycosidase H-sensitive precursors and shows a lower apparent molecular weight after treatment with neuraminidase. The biosynthesis of the proteoglycan component is inhibited by exposure of M21 cells to the monovalent ionophore monensin, this component can be labeled biosynthetically with 35SO4, is sensitive to beta-elimination in dilute base, and is degraded by both chondroitinase AC and ABC lyases, suggesting that it is a chondroitin sulfate proteoglycan. These data demonstrate that the antigenic determinant recognized by monoclonal antibody 9.2.27 is located on a glycoprotein-proteoglycan complex which may have unique implications for the interaction of glycoconjugates at the human melanoma tumor cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Callahan G. N., Walker L. E., Martin W. J. Biochemical comparison of H-2K antigen isolated from C3HfB/HeN and C3H/HeN mice. Immunogenetics. 1981 Mar 1;12(5-6):561–568. doi: 10.1007/BF01561696. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Vertel B. M., Schwartz N. B. Immunological methods in the study of chondroitin sulfate proteoglycans. Curr Top Dev Biol. 1980;14(Pt 2):169–198. doi: 10.1016/s0070-2153(08)60194-5. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Fries E., Rothman J. E. Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3870–3874. doi: 10.1073/pnas.77.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R., McCabe R. P., Pellegrino M. A., Ferrone S., Reisfeld R. A. Tumor-associated antigens in spent medium of human melanoma cells: immunochemical characterization with xenoantisera. J Immunol. 1981 Jan;126(1):62–66. [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Glimelius B., Norling B., Westermark B., Wasteson A. Composition and distribution of glycosaminoglycans in cultures of human normal and malignant glial cells. Biochem J. 1978 Jun 15;172(3):443–456. doi: 10.1042/bj1720443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney-Kieras J., Kieras F. J. Glycosaminoglycans synthesized by tumorigenic and nontumorigenic mouse melanoma cells in culture. J Natl Cancer Inst. 1980 Dec;65(6):1345–1350. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Kawakami H., Terayama H. Cell surface proteoglycans as a negative modulator in concanavalin A-mediated agglutination of hepatoma cells. Biochim Biophys Acta. 1980 Jun 20;599(1):301–314. doi: 10.1016/0005-2736(80)90076-0. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Thonar E. J., Hascall V. C., Reiner A., Poole A. R. Identification of core protein, an intermediate in proteoglycan biosynthesis in cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Aug 10;256(15):7890–7897. [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981 Apr 3;212(4490):55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Lalley K., Kiang W. L., Crockett C., Margolis R. K. Isolation and properties of a soluble chondroitin sulfate proteoglycan from brain. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1018–1024. doi: 10.1016/0006-291x(76)90224-2. [DOI] [PubMed] [Google Scholar]
- Mitchell K. F., Fuhrer J. P., Steplewski Z., Koprowski H. Biochemical characterization of human melanoma cell surfaces: dissection with monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7287–7291. doi: 10.1073/pnas.77.12.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. C., Jr, Galloway D. R., Reisfeld R. A. Production and characterization of monoclonal antibody to a melanoma specific glycoprotein. Hybridoma. 1981;1(1):27–36. doi: 10.1089/hyb.1.1981.1.27. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tajiri K., Uchida N., Tanzer M. L. Undersulfated proteoglycans are secreted by cultured chondrocytes in the presence of the ionophore monensin. J Biol Chem. 1980 Jul 10;255(13):6036–6039. [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]