Abstract
Concanavalin A-stimulated murine spleen cells and antigen-stimulated B lymphocytes of normal mice express an antigen that reacts with goat antiserum against glycoprotein (gp) 70. Structural analysis of this antigen characterizes it as endogenous viral gp70 that is most likely of xenotropic origin. Activated nonspecific T suppressor cells and cytotoxic T lymphocytes express endogenous viral gp70, whereas nonactivated mouse T or B lymphocytes do not. The presence of endogenous retroviral gp70 is thus a novel marker for activated mouse lymphocytes in general.
Full text
PDF

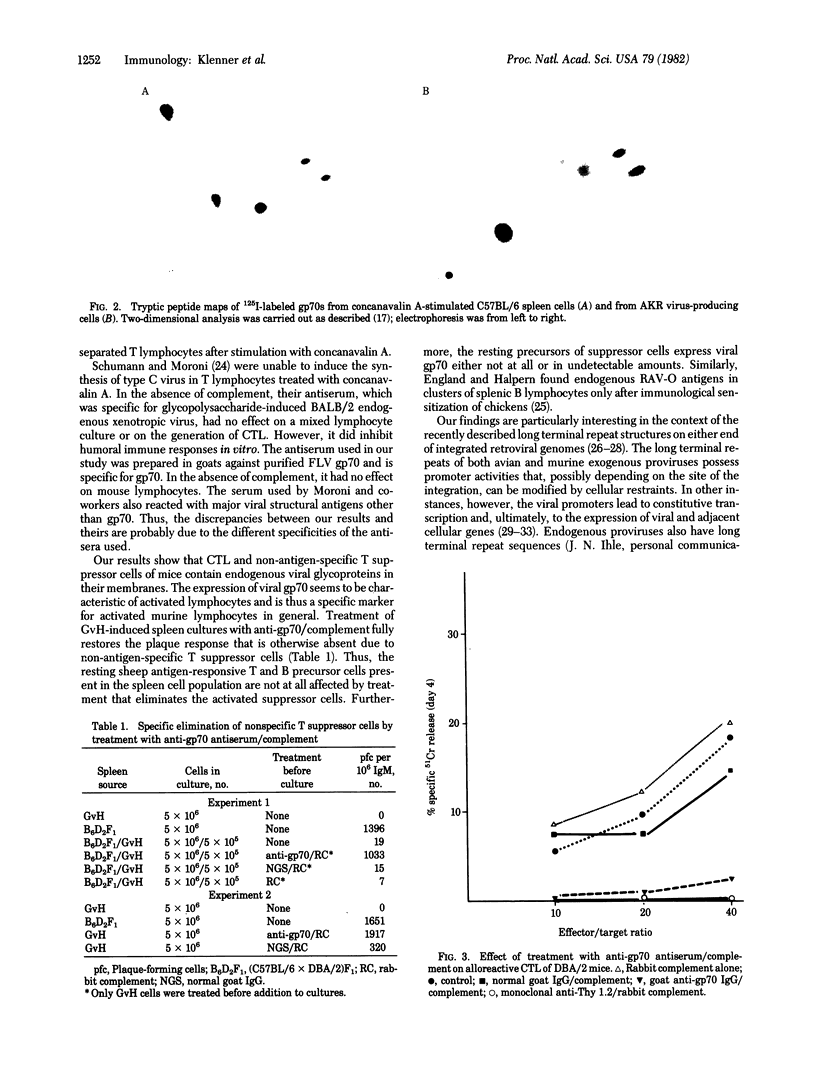
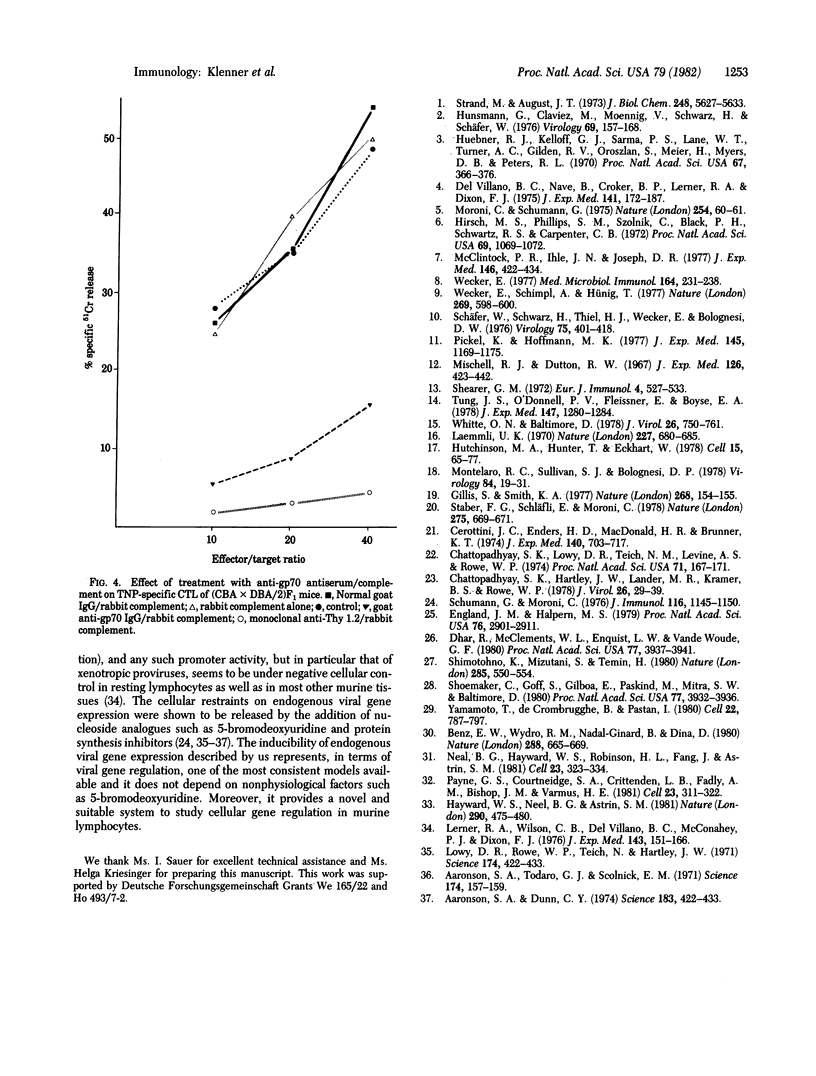
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Dunn C. Y. High-frequency C-type virus induction by inhibitors of protein synthesis. Science. 1974 Feb 1;183(4123):422–424. doi: 10.1126/science.183.4123.422. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Barbezat G. O., Grossman M. I. Intestinal secretion: stimulation by peptides. Science. 1971 Oct 22;174(4007):422–424. doi: 10.1126/science.174.4007.422. [DOI] [PubMed] [Google Scholar]
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Hartley J. W., Lander M. R., Kramer B. S., Rowe W. P. Biochemical characterization of the amphotropic group of murine leukemia viruses. J Virol. 1978 Apr;26(1):29–39. doi: 10.1128/jvi.26.1.29-39.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J. M., Halpern M. S. Endogenous oncornaviral antigen in the bursa of Fabricius of 15B X 7(2) chickens. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2908–2911. doi: 10.1073/pnas.76.6.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Phillips S. M., Solnik C., Black P. H., Schwartz R. S., Carpenter C. B. Activation of leukemia viruses by graft-versus-host and mixed lymphocyte reactions in vitro. Proc Natl Acad Sci U S A. 1972 May;69(5):1069–1072. doi: 10.1073/pnas.69.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Claviez M., Moennig V., Schwarz H., Schäfer W. Properties of mouse leukemia viruses. X. Occurrence of viral structural antigens on the cell surface as revealed by a cytotoxicity test. Virology. 1976 Jan;69(1):157–168. doi: 10.1016/0042-6822(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock P. R., Ihle J. N., Joseph D. R. Expression of AKR murine leukemia virus gp71-like and BALB(X) gp-71-like antigens in normal mouse tissues in the absence of overt virus expression. J Exp Med. 1977 Aug 1;146(2):422–434. doi: 10.1084/jem.146.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Sullivan S. J., Bolognesi D. P. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virology. 1978 Jan;84(1):19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Lipopolysaccharide induces C-type virus in short term cultures of BALB/c spleen cells. Nature. 1975 Mar 6;254(5495):60–61. doi: 10.1038/254060a0. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Pickel K., Hoffmann M. K. The Ly phenotype of suppressor T cells arising in mice subjected to a graft-versus-host reaction. J Exp Med. 1977 May 1;145(5):1169–1175. doi: 10.1084/jem.145.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Mitogen induction of murine C-type viruses. I. Analysis of lymphoid cell subpopulations. J Immunol. 1976 Apr;116(4):1145–1150. [PubMed] [Google Scholar]
- Schäfer W., Schwarz H., Thiel H. J., Wecker E., Bolognesi D. P. Properties of mouse leukemia viruses. XIII. Serum therapy of virus-induced murine leukemias. Virology. 1976 Dec;75(2):401–418. doi: 10.1016/0042-6822(76)90039-8. [DOI] [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber F. G., Schläfli E., Moroni C. Expression of endogenous C-type viral antigen on normal mouse haemopoietic stem cells. Nature. 1978 Oct 19;275(5681):669–671. doi: 10.1038/275669a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Tung J. S., O'Donnell P. V., Fleissner E., Boyse E. A. Relationships of gp70 of MuLV envelopes to gp70 components of mouse lymphocyte plasma membranes. J Exp Med. 1978 Apr 1;147(4):1280–1284. doi: 10.1084/jem.147.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker E. Expression of endogenous viral antigens during immune response. Med Microbiol Immunol. 1977;164(1-3):231–238. doi: 10.1007/BF02121317. [DOI] [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T. Expression of MuLV GP71-like antigen in normal mouse spleen cells induced by antigenic stimulation. Nature. 1977 Oct 13;269(5629):598–600. doi: 10.1038/269598a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]