Abstract
Background
Prolonged storage of platelets could improve availability and logistical management and decrease wastage. Immunobiochemical methods can be used to guarantee the quality of platelets after prolonged storage.
Objective
The aim of this study was to compare storage-related changes in buffy coat-derived platelet concentrations versus platelet-rich plasmal.
Methods
Units of whole blood were drawn using a quadruple-bag blood container system. Platelet-rich plasma and buffy coat prepared from whole blood following standard methods were stored for 9 days. During this period test samples were aseptically collected for analysis on Days 1, 2, 3, 5, 7 and 9.
Results
The highest CD42b expression was greater than 95%. The percentage of CD62p was significantly lower than the CD42b expression. The pH remained fairly stable during storage. Measurement of pO2 and pCO2 showed that oxygen levels were significantly higher than carbon dioxide levels. There were no significant differences in bicarbonate levels, glucose consumption and lactate production between the groups. The swirling effect with platelet-rich plasma samples decreased after 5 days of storage and after 7 days of storage for buffy coat samples. There was a significant twenty-fold increase in the mean IL-1β after 5 days of storage for both groups. Slight increases in IL-6 and IL-8 levels were seen at 5 days.
Conclusion
The quality of platelet concentrates remained acceptable during 7 days of storage in respect to the swirling effect, pH and platelet activation. There were no significant differences between buffy coat-derived platelets and platelet-rich plasma in this study.
Keywords: Platelet-rich plasman, Blood buffy coat, Flow cytometry, Cytokines, Blood preservation
Introduction
Despite continuous efforts to improve the process and in spite of major advances in establishing optimized storage conditions, platelet storage lesions still occur. These are characterized by loss of function and changes in morphophysiological characteristics associated with platelet activation, vacuolization, microvesiculation and fragmentation. A poor quality of platelets may lead to a decrease in recovery after transfusions and shorter survival times of circulating platelets.(1-4)
Image analysis shows that changes of platelets during storage are correlated to the CD62p expression.(5,6)
Cytokine accumulation in blood components during storage contribute to non-hemolytic febrile reactions and allergic reactions associated with transfusions.(6,7) Some studies have shown that the presence of RANTES, TGF-β, IFN-γ, IL-1, IL-6 and IL-8 in platelet concentrates (PCs) contribute to febrile non-hemolytic transfusion reactions and that the residual leukocyte content and storage time are the determining factors for the occurrence of this type of reaction.(8) Adverse reactions occur even after the use of leukocyte filters due to the presence of cytokines in platelet concentrates. Some studies have even shown that cytokine levels released by platelets are higher in filtered products.(9) Thus, it seems that the use of filters may stimulate cytokine release; this occurs when platelets are exposed to the different types of artificial surfaces of filters.(10) These reactions can range from simple urticaria, febrile non-hemolytic reactions, hypotension, chills and nausea to more severe reactions such as anaphylaxis and acute noncardiogenic pulmonary edema. The severity of reactions depends on the blood components and the patient's condition. The occurrence of febrile non-hemolytic reactions is more frequent after platelet transfusions (5-31%) than after red blood cell transfusions (1-6.8%).(11) This difference is a reflection of the platelet concentrate storage conditions at 22ºC which favors a greater accumulation of cytokines in these products over the 5-day storage period; red blood cells are stored at 4ºC for no more than 42 days.(12)
The amount of residual leukocytes in platelet concentrates depends on the method used to prepare the product. In the Fundação Hemominas, platelet concentrates are obtained from whole blood donations or aphaeresis. In preparation from whole blood, the platelet concentrate is obtained by soft-spin and fractionation of the platelet-rich plasma (PRP). A second centrifugation concentrates the platelets and the plasma supernatant is then extracted leaving approximately 60 mL of plasma in platelet concentrates.(13)
Another method to prepare platelet concentrates from whole blood is by Top-and-Bottom bags using high-spin centrifugation of fresh platelet-poor plasma; the red blood cell concentrates and the buffy coat (BC) containing the platelets is extracted from whole blood.
This study aimed to evaluate some parameters related to the viability of platelets during storage using different types of platelet concentrates.
Methods
Preparation of buffy coat-derived platelets
Units of whole blood (450 ± 45 mL) were drawn from healthy donors, using a quadruple blood bag system with an additive solution to preserve red blood cells (top and bottom with SAG-mannitol - Leucotrap RC T&B RCB438CN, NY, USA). The bags were centrifuged (Cryofuge 6000i Heraeus, Hanau, Germany) at 4236 x g for 13 minutes at 22ºC within 3 hours of collection and immediately separated into red blood cells, plasma and BC using a blood component separator (Optipress II, Baxter, Nivelles, Belgium). The BC of approximately 60 mL was stored at 22ºC overnight without agitation.
Preparation of platelet-rich plasma
PRP was prepared from whole blood following standard methods. A volume of 450 mL of blood was collected in standard blood bags with CPDA1 anticoagulant (JP Pharmaceuticals, Ribeirão Preto, Brazil). PRP was prepared by centrifugation (350 x g for 10 min) and the concentrates were stored in a 'Thrombomixer' (Travenol, Oslo, Norway) at 22ºC according to the method described by Hervig et al.(14)
Study design
Twenty samples of PRP and twenty samples of BC were stored for nine days. During this period samples for analysis were aseptically collected on the day of preparation (D1), the second day (D2), third day (D3), fifth day (D5), seventh day (D7) and the ninth day (D9). The platelet count, residual leukocytes, biochemical analysis, morphological analysis, cytokine levels and expression of CD41/CD62P were analyzed at each time point. Metabolic parameters (pH, pO2, pCO2, lactate, and glucose) were measured with a blood gas analyzer (ABL 705, Radiometer Medical A/S, Denmark) at 37ºC.
Expression of CD42b and CD62p
Measurements of CD42b and CD62p were performed according to the recommendations of the Biomedical Excellence for Safer Transfusion (BEST) collaborative group.(3) Immediately after collection of the PC sample (500 x 109 platelets/L), 10 mL of the sample was stained with 10 mL of fluorochrome-labeled monoclonal antibodies (FITC labeled anti-CD42b and phycoerythrin labeled anti-CD62p; Becton Dickinson, San Jose, CA, USA). The samples were incubated for 15 minutes at 37ºC in the dark. Nonspecific binding was controlled using mouse IgG1 (Becton Dickinson, San Jose, CA, USA). After incubation, samples were diluted to 1 mL of FACSFlow and analyzed by flow cytometry. A total of 500,000 events were collected and analyzed using FITC/PE dot plot and quadrant analysis (Cell Quest program, Becton Dickinson, San Jose, CA, USA). The lower left quadrant was set to include 99% of events from the control. At least 95% of events of the activated control sample were in the upper right quadrant. The percentage of platelets observed in the upper right quadrant was defined as the percentage of cells expressing CD62P compared to controls.
Recording of the swirling effect
Swirling of platelets was scored by the naked eye against a strong light. Swirling was graded as 3 (excellent), 2 (good), 1 (poor) and 0 (no swirling).(14,15) For the normal quality of PCs, swirling was categorized either by the presence of 'hairs' formed by moving discoid platelets or by the loss of them. Swirling negative platelets were prepared by storing platelets at 4ºC for 24 hours; this was used to compare with swirling positive platelets.
Plasma processing for cytokine analysis
Supernatant plasma samples harvested from platelet concentrates were stored in aliquots at -70ºC and assayed in the same experiment for the presence of various cytokines. Prior to the assay, the aliquots were thawed at room temperature and centrifuged for 10 min at 9000 x g in a refrigerated microcentrifuge.
Quantification of cytokines
The levels of IL-1β, IL-6, IL-8 and TNF-α were measured in the stored platelet supernatants using commercially available assays (BioSource International Inc., Camarillo, CA, USA) following the manufacturer's instructions. The minimum detection limits were 1.5 pg/mL for IL-1β, 1 pg/mL for IL-6, 3 pg/mL for IL-8 and 10 pg/mL for TNF-α.
Statistical analysis
Comparisons between groups were analyzed using the Student t-test for independent samples. Results were expressed as mean values and 95% confidence intervals. P-values below 0.05 were considered statistically significant. We used the standard statistics Instat Software (GraphPad Software, San Diego, CA, USA).
Results
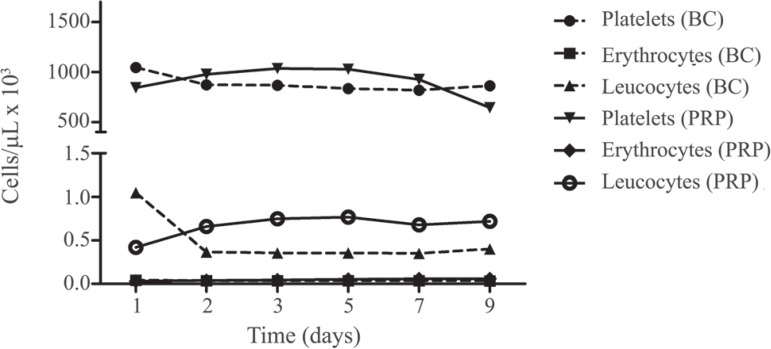
The mean platelet and white blood cell (WBC) counts in BC were 883.1 ± 82.24 x 109/L and 0.4796 ± 0.280 x109/L, respectively, while the mean platelet and WBC counts in PRP were 909.1 ± 148.5 x 109/L and 0.667 ± 0.127 x 109/L, respectively on the day of preparation. The residual mean red blood cell count in BC was 0.0370 ± 0.0025 and in PRP it was 0.0472 ± 0.013. These counts did not differ statistically between groups at any time point (Figure 1).
Figure 1.
Cell concentrations in buffy coat-derived platelet concentrations (BC) and platelet-rich plasma (PRP) over nine days of storage
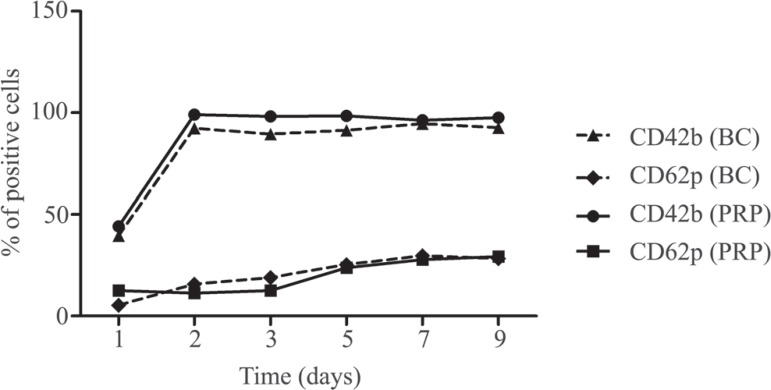
The highest CD42b expression was greater than 95%. Its expression increased significantly between D1 and D2. Thereafter, the CD42b levels remained uniformly high until D9. The percentage CD62p expression was significantly lower than the CD42b expression. Its highest rates of expression did not exceed 20% of that observed at D9 (Figure 2).
Figure 2.
Platelet surface expression of the CD42b and CD62p activation markers during nine days of storage of buffy coat-derived platelet concentrations (BC) and platelet-rich plasma (PRP)
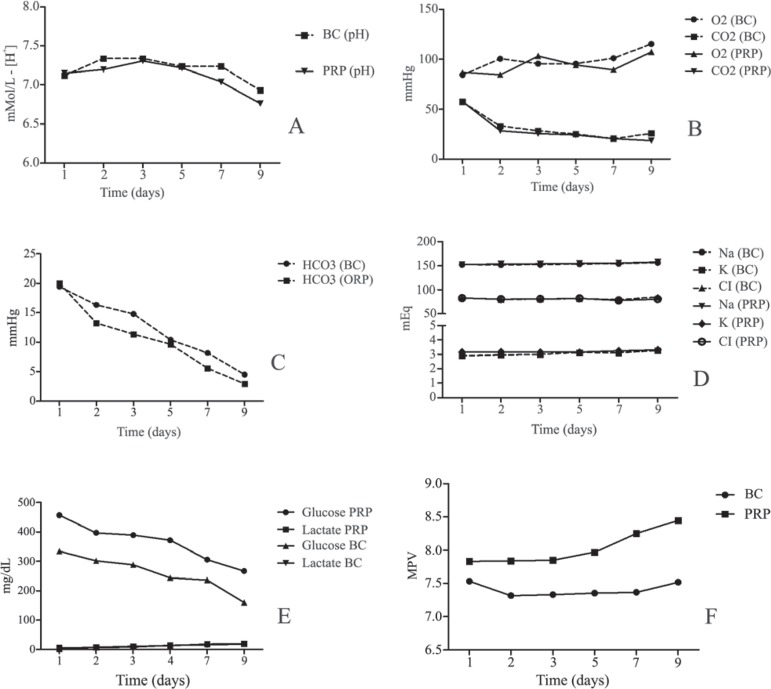
During storage, the pH values remained fairly stable in the range of 6.76 - 7.31 for the PRP platelets and 6.93 - 7.34 for BC-derived platelets (Figure 3A). The BC maintained a mean pH level of 7.02 ± 0.15 during the storage period and the PRP a mean of 7.11 ± 0.19. Plasma pH was constant during storage without any significant differences between the groups. Thus the pH at D3 of storage was similar to that at D7. At all times the pH remained above 6, which is critical for platelet function.
Figure 3.
Biochemical characteristics of the buffy coat-derived platelet concentrations (BC) and platelet-rich plasma (PRP) aliquots during storage over nine days
(A) – Extracellular pH
(B) – Oxygen consumption and CO2 production
(C) – Production of bicarbonate
(D) – Levels of sodium, potassium and chlorine ions
(E) – Glucose consumption and lactate production
(F) – Mean platelet volume
Each point represents the average of ten blood bags
The platelet respiratory activity was evaluated by measuring the pO2 and pCO2; oxygen levels were significantly higher than levels of carbon dioxide (p-value < 0.05) but no significant differences were seen on comparing the BC and PRP groups ( Figure 3B).
There were no significant differences in bicarbonate levels, glucose consumption and lactate production between the groups during storage (Figure 3C & 3E).
The effect of ions on the quality of platelets stored for up to nine days were analyzed. The levels of sodium and chlorine were significantly higher than the levels of potassium (p-value < 0.05). For the different ion levels, there were no significant differences between groups over the nine days of storage (Figure 3D).
All PCs showed similar storage-promoted glycolytic production of lactate, leading to total depletion of bicarbonate and a progressive decline in the pH. It is important to mention that the glucose consumption was statistically different between the groups (p-value < 0.05) at each time point (Figure 3E).
Mean platelet volumes of the PRP and BC were 8.032 ± 0.259 and 7.404 ± 0.096, respectively giving a significant difference between the two groups (p-value = 0.0022 - Figure 3F); significant differences were seen at all time points.
It is known that potassium and magnesium can reduce the rate of glycolysis and better maintain the pH and induce a response to hypotonic shock.
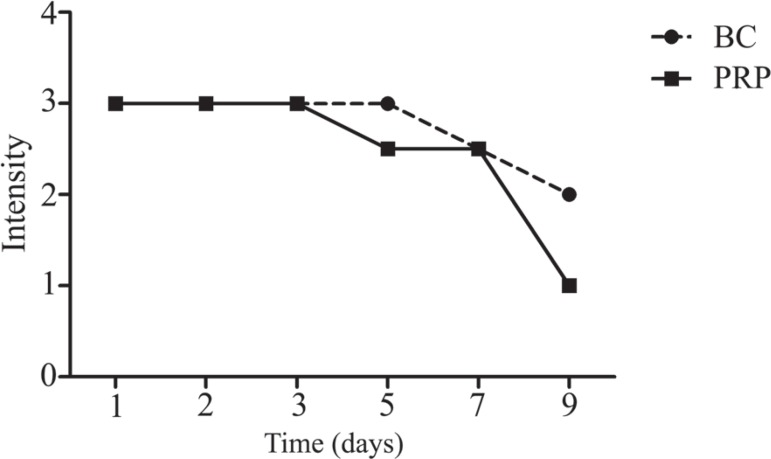
Discoid platelets may reflect the time of storage. The best way to measure the shape of platelets is by swirling analysis. Generally, the swirling effect was excellent throughout the first 5 days of storage: all PCs had the highest score.
The swirling effect started to decrease after five and seven days of storage for PRP and BC, respectively; the decrease was significant after seven days of storage for both BC-derived platelet concentrates and PRP concentrates (Figure 4).
Figure 4.
Effect of storage of platelet concentrates on the swirling effect from 0 (no swirling) to 3 (excellent swirling) in buffy coat-derived platelet concentrations (BC) and platelet-rich plasma (PRP)
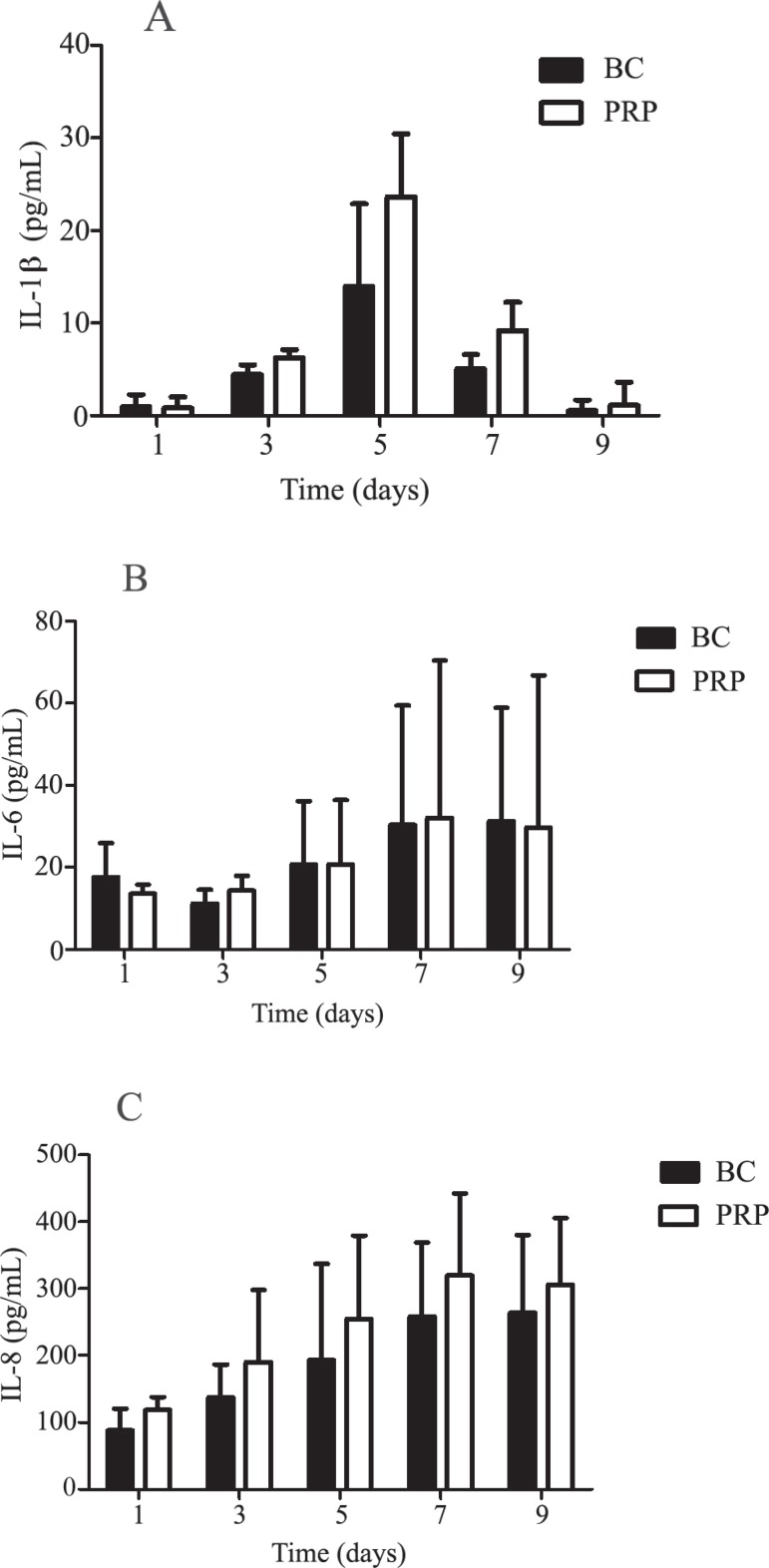
The concentrations of TNF-α were undetectable (below the sensitivity of tests) in BC and PRP during the whole storage period (nine days). There was a significant twenty-fold increase in mean IL-1β at D5 compared to D1 for BC and PRP (Figure 5A). Slight increases in IL-6 (15%) and IL-8 (28%) levels were seen at D5 but without any significant difference between concentrates. There were no significant differences in the generation of cytokines between BC and PRP ( Figure 5B & 5C).
Figure 5.
Level of IL-1β (A), IL-6 (B) and IL-8 (C) cytokines in buffy coat-derived platelet concentrations (BC) and platelet-rich plasma (PRP)
Discussion
The use of enzyme linked immunosorbent assay (ELISA) has become very popular for the determination of cytokine levels. These assays are sensitive, rapid and monospecific due to the use of monoclonal antibodies.(16,17)
Many cytokines, especially those associated with inflammatory response, can be produced by the WBCs that were activated during storage. Previous studies have suggested that cytokines, such as IL-1β, IL-6, IL-8 and TNF-α, which are released by leucocytes and other cell types during the storage of platelets, may play a role in adverse reactions.(16,18)
A number of factors can activate WBCs to generate cytokines during PC storage including activated complement components and thrombin or cytokines released from damaged white cells.(19)
There was no evidence of any detectable TNF-α and only a small amount of IL-1β was detected in the plasma of PCs. This may be attributed to the short serum half-life of TNF-α (approximately 6 min.) Similar results were found by Dietrich et al.(19)
From D1 to D5 the PCs produced increasing levels of IL-1β. Therefore, the increased plasma levels result from the active synthesis and release of cytokines by activated cells most probably monocytes as was proposed by Gottschall et al.(20) However, it is not clear how the synthesis of cytokines is triggered. Monocytes may be activated not only by activated complement components or by thrombin(21,22) but also by cytokines released from leukocytes that were activated or damaged during the collection or preparation processes of platelet concentrates or lysed during storage. It is known that some T-cells respond to subpicomolar concentrations of IL-1β.(23) Once the process has started, the released IL-1β is probably responsible for the induction of other cytokines.(24)
IL-6 is a pyrogenic cytokine; significantly increased serum levels have been found in patients experiencing febrile reactions after transfusion.(25) It is not yet clear whether this effect is due to endogenously produced cytokines responding to the binding of leukocyte antibodies to donor leukocytes or whether this is a direct effect of the transfusion of exogenously produced cytokines.(24) We detected very low IL-6 levels (on average < 20 pg/mL) for BC and PRP at all time points. Thus, these low IL-6 levels could be associated with a low incidence of transfusion reactions recorded with the use of PCs produced by this institution.
IL-8 is a chemoattractant for neutrophils, T-lymphocytes and basophils. It increases neutrophil degranulation. Intravenous injections of IL-8 in primates result in a rapid but transient granulocytopenia followed by granulocytosis.(24) Unfortunately, very limited data are available on the in vivo effects of transfused cytokines, and their clinical significance remains unknown. Thus, the effect of IL-8 administration in humans remains to be investigated. Transfusion-related adverse reactions have been attributed to the presence of substantially high levels of IL-8.(7) Our results show increased amounts of IL-8 in the plasma of PCs from D1 onwards. This may be related to WBC activation or its lysis.(7) However, only amounts of IL-8 < 100 pg/mL were present at D1, and these levels remained greater than 100 pg/mL during the rest of storage, that is, at levels similar to other studies.(9,19,26)
Conversely, an accumulation of IL-8 during storage can serve as an indicator of the quality of the platelet product.(27) As stated before, our IL-8 levels are in agreement with the levels found by other researchers, so the quality of the PCs in this study are similar to other groups in respect to IL-8.(7)
We did not investigate febrile non-hemolytic transfusion reactions during the transfusion of PCs as this was not the aim of the study, but we suggest that further studies should investigate the relationship between these cytokines and this type of reaction.
However, from the general in vitro results of the current study it seems that storage is acceptable for seven days with no significant differences between the product types, with regard to the swirling effect, pH and extent of platelet activation. According to Gottschall et al.,(20) platelet transfusion in thrombocytopenic patients with 7-day-old PCs stored in plasma have proved to be adequate. There were only slight differences between BC-derived platelets and PRP in this study, especially regarding the content of residual leukocytes, which was greater in platelet concentrates obtained by PRP. We doubt that these differences are of major clinical importance.
Acknowledgments
This study was supported by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais)
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Gulliksson H.Defining the optimal storage of platelets. Transfus Med Rev. 2003; 17(3): 209-15 [DOI] [PubMed] [Google Scholar]
- 2.VandenBroeke T, Dumont LJ, Hunter S, Nixon J, Murphy S, Roger J, Herschel L, AuBuchon JP, Gulliksson H, Dengler T, Hornsey V, Prowse C, Biomedical Excellence for Safer Transfusion Working Party of the International Society of Blood Transfusion Platelet storage solution effects on the accuracy of laboratory tests for platelet function: a multi-laboratory study. Vox Sang. 2004; 86 (3): 183-8 [DOI] [PubMed] [Google Scholar]
- 3.Bayraktarogglu Z, Yilmaz N, Çicek HK, Karafak A, Gul E.Platelet storage time and cytokine (IL2-r, Il-8, TNFa) levels. East Mediterr Health J. 2007; 13(1): 79-84 [PubMed] [Google Scholar]
- 4.Murphy S, Rebulla P, Bertolini F, Holme S, Moroff G, Snyder E, et al. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion Transfus Med Rev; 1994; 8(1): 29-36 [DOI] [PubMed] [Google Scholar]
- 5.Wagner T, Vetter A, Dimovik N, Guber SE, Helmberg W, Kröll W, et al. Ultra structural changes and activation differences in platelet concentrates stored in plasma and additive solution. Transfusion. 2002; 42(6): 719-27 [DOI] [PubMed] [Google Scholar]
- 6.Devine DV, Bradley AJ, Maurer E, Levin E, Chahal S, Serrano K, et al. Effects of prestorage white blood cell reduction on platelet aggregate formation and the activation state of platelets and plasma enzyme systems. Transfusion. 1999; 39(7): 724-34 Comment in: Transfusion. 2000;40(5):615-6 [DOI] [PubMed] [Google Scholar]
- 7.Wadhwa M, Krailadsiri P, Dilger P, Gaines Das R, Seghatchian MJ, Thorpe R.Cytokine levels as performance indicators for white blood cell reduction of platelet concentrates. Vox Sang. 2002; 83(2): 125-36 [DOI] [PubMed] [Google Scholar]
- 8.Cardigan R, Williamson LM.The quality of platelets after storage for 7 days. Transfus Med. 2003; 13(4): 173-87 [DOI] [PubMed] [Google Scholar]
- 9.Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, et al. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006; 46(7): 1184-89 [DOI] [PubMed] [Google Scholar]
- 10.Seghatchian MJ.An agarose gel method for evaluating F VIII procoagulant electrophoretic distribution. Ann N Y Acad Sci. 1981; 370: 236-40 [DOI] [PubMed] [Google Scholar]
- 11.Shanwell A, Falker C, Gulliksson H.Storage of platelets in additive solutions: the effects of magnesium and potassium on the release of RANTES, b thhromboglobulin, platelet factor 4 and interleukin 7, during storage. Vox Sang. 2003; 85(3): 206-12 [DOI] [PubMed] [Google Scholar]
- 12.Agência Nacional de Vigilância Sanitária Resolução RDC nº 153, de 14 de junho de 2004. Determina o regulamento técnico para os procedimentos hemoterápico, incluindo a coleta, o processamento, a testagem, o armazenamento, o transporte, o controle de qualidade e o uso humano de sangue, e seus componentes, obtidos do sangue venoso, do cordão umbilical, da placenta e da medula óssea [Internet]. Brasília: Ministério da Saúde; 2004[cited 2011 Nov 21] Available from: http://portal.saude.gov.br/portal/arquivos/pdf/resolucao_153_2004.pdf [Google Scholar]
- 13.Seghatchian J.Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006; 34(1): 125-30 [DOI] [PubMed] [Google Scholar]
- 14.Hervig T, Vølundardottir T, Bakken AM, Farstad M.Thrombin-induced serotonin release as an in vitro indicator of the functional integrity in stored platelets. Clin Chem. 1990; 36(1): 28-31 [PubMed] [Google Scholar]
- 15.Wagner SJ, Robinette D.Evaluation of swirling, pH, and glucose tests for the detection of bacterial contamination in platelet concentrates Transfusion 1996; 36(11-12): 989-93 [DOI] [PubMed] [Google Scholar]
- 16.Wadhwa M, Seghatchian MJ, Dilger P, Sands D, Krailadisiri P, Contreras M, et al. Cytokines in WBC-reduced apheresis PCs during storage: a comparison of two WBC-reduction methods. Transfusion. 2000; 40(9): 1118-26 [DOI] [PubMed] [Google Scholar]
- 17.House RV.Cytokine technology in basic and applied research on the hematopoietic system. Toxicol Pathol. 1993; 21(2): 251-7 [DOI] [PubMed] [Google Scholar]
- 18.Stack G, Snyder EL.Cytokine generation in stored platelet concentrates. Transfusion. 1994; 34(1): 20-25 [DOI] [PubMed] [Google Scholar]
- 19.Diedrich B, Sandgren P, Jansson B, Gulliksson H, Svensson L, Shanwell A.In vitro and in vivo effects of potassium and magnesium on storage up to 7 days of apheresis platelet concentrates in platelet additive solution. Vox Sang. 2008; 94(2): 96-102 [DOI] [PubMed] [Google Scholar]
- 20.Gottschall JL, Johnston VL, Rzad L, Anderson AJ, Aster RH.Importance of white blood cells in platelet storage. Vox Sang. 1984; 47(2): 101-7 [DOI] [PubMed] [Google Scholar]
- 21.Bode AP, Miller DT, Newman S.Generation of complement activation peptides during storage of platelet concentrates (PLC). Thromb Haemost. 1987; 58: 512 Proceedings of the 11th International Congress on Thrombosis and Haemostasis; 1987 July 6-10; Brussels, Belgium [Google Scholar]
- 22.Bode AP, Miller DT.Generation and degradation of fibrinopeptide A in stored platelet concentrate. Vox Sang. 1986; 51(3): 192-6 [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA.Interleukin-1 and interleukin-1 antagonism. Blood. 1991; 77(8): 1627-52 [PubMed] [Google Scholar]
- 24.Ferrer F, Rivera J, Corral J, González-Conejero R, Vicente V.Evaluation of leukocyte-depleted platelet concentrates obtained by in-line filtration. Vox Sang. 2000; 78(4): 235-41 [DOI] [PubMed] [Google Scholar]
- 25.Sacher RA, Boyle L, Freter CE.High circulating interleukin 6 levels associated with acute transfusion reaction: cause or effect? Transfusion. 1993; 33(11): 962-3 Comment on: Transfusion. 1993;33(3):195-9 [DOI] [PubMed] [Google Scholar]
- 26.Seghatchian MJ., Wadhwa, M, Thorpe R.Cytokines in platelet concentrates: a comparison of aphaeresis platelet (haemonetics) and filtered and unfiltered pooled buffy-coat derived platelet concentrates. Transfus Sci. 1997; 18(1): 103-7 [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary R, Aggarwal A, Khetan D, Dayal R.Cytokine generation in stored platelet concentrate: comparison of two methods of preparation. Indian J Med Res. 2006; 124(4): 427-30 [PubMed] [Google Scholar]