Abstract
The Rab3 small G protein family consists of four members, Rab3A, -3B, -3C, and -3D. Of these members, Rab3A regulates Ca2+-dependent neurotransmitter release. These small G proteins are activated by Rab3 GDP/GTP exchange protein (Rab3 GEP). To determine the function of Rab3 GEP during neurotransmitter release, we have knocked out Rab3 GEP in mice. Rab3 GEP−/− mice developed normally but died immediately after birth. Embryos at E18.5 showed no evoked action potentials of the diaphragm and gastrocnemius muscles in response to electrical stimulation of the phrenic and sciatic nerves, respectively. In contrast, axonal conduction of the spinal cord and the phrenic nerve was not impaired. Total numbers of synaptic vesicles, especially those docked at the presynaptic plasma membrane, were reduced at the neuromuscular junction ∼10-fold compared with controls, whereas postsynaptic structures and functions appeared normal. Thus, Rab3 GEP is essential for neurotransmitter release and probably for formation and trafficking of the synaptic vesicles.
INTRODUCTION
Rab small G proteins represent the largest branch of the small G protein superfamily and are recognized as key molecules in vesicle trafficking and organelle dynamics in eukaryotic cells (Nuoffer and Balch, 1994; Novick and Zerial, 1997; Martinez and Goud, 1998; Schimmoller et al., 1998; Takai et al., 2000). Recycling between the GDP/GTP-bound forms of Rab small G proteins is coupled with its translocation between the cytosol and vesicular membranes, which plays mechanistic roles for exocytosis (Südhof, 1997; Gonzalez et al., 1999; Takai et al., 2000). The Rab3 family consists of four members: Rab3A, -3B, -3C, and -3D. Of these members, the function and mode of action of Rab3A have most extensively been studied. Rab3A plays a key regulatory role in Ca2+-dependent exocytosis of neurotransmitter release (Takai et al., 1996, 2000; Südhof, 1997; Geppert and Südhof, 1998; Gonzalez et al., 1999). Knockout studies on Rab3A have revealed an important insight into Rab3A function: Rab3A is not essential for basal neurotransmission but modulates synaptic plasticity. In Rab3A−/− mice, synaptic depression is increased after short trains of repetitive stimuli in the CA1 region of the hippocampus (Geppert et al., 1994), and mossy fiber LTP in the CA3 region is abolished (Castillo et al., 1997). Rab3A is suggested to play roles in either recruitment of synaptic vesicles or, more likely, Ca2+-triggered membrane fusion, because a more-than-usual number of exocytic events occur within a brief time after arrival of the nerve impulse in Rab3A−/− mice (Geppert et al., 1997).
The Rab3 family members are regulated by three regulators: Rab GDP dissociation inhibitor (Rab GDI), Rab3 GDP/GTP exchange protein (Rab3 GEP), and Rab3 GTPase-activating protein (Rab3 GAP) (Takai et al., 1996, 2000). Of these regulators, Rab GDI is active on all the Rab family members, whereas the other two are specific for the Rab3 family members. It remains unknown, however, how these regulators are involved in exocytotic events in animals. We have recently knocked out Rab GDIα, the neuron-specific isoform of Rab GDI (Ishizaki et al., 2000). In the CA1 region of the hippocampus of Rab GDIα−/− mice, synaptic potentials display larger enhancement during repetitive stimulation, which is apparently opposite to the phenotype of Rab3A−/− mice (Geppert et al., 1994). Rab GDIα plays a specialized role in Rab3A recycling to suppress hyperexcitability via modulation of presynaptic forms of plasticity, which may explain the pathogenesis of X-linked mental retardation (D'Adamo et al., 1998).
To understand the physiological role of Rab3 GEP in neurotransmitter release, we knocked out here this regulator. We report the phenotype of Rab3 GEP-deficient mice, providing genetic evidence that Rab3 GEP is essential for vesicle trafficking at the neuromuscular junction and is implicated in synaptic vesicle formation.
MATERIALS AND METHODS
DNA Library Screening
A Mouse Rab3 GEP cDNA was isolated from a brain cDNA library λ TriplEx (Clontech, Palo Alto, CA) using a rat Rab3 GEP cDNA and sequenced using ABI DNA sequencer. A cDNA fragment encoding the N-terminal half region of Rab3 GEP was subcloned into appropriate plasmid vectors and used as a probe for homology screening of 129SVJ mouse genomic library λ FIXII (Stratagene, La Jolla, CA).
Generation of Rab3 GEP−/− Mice
A targeting construct was made to replace 3′ half of the coding exon 1 and 5′ half of the following exon 2 with a neo-resistance gene cassette. Gene targeting of Rab3 GEP in 129/Sv-derived RW4 embryonic stem (ES) cells was carried out using positive–negative selection as previously described (Koera et al., 1997). Homologous recombinants were verified by Southern hybridization using 5′- and 3′-external probes and the neo-resistance gene probe. ES cells were microinjected into E3.5 C57BL/6J blastocysts and transferred to MCH pseudopregnant foster mothers to generate chimeras that were mated with BDF1 mice for germline transmission. Mice were genotyped using primers for PCR in the neo gene (5′-GGGCGCCCGGTTCTTTTTGTC-3′ and 5′-GCCATGATGGATACTTTCTCG-3′) and in the replaced Rab3 GEP gene (5′-ACTCCCAGACCTTATTTCCAT-3′ and 5′-CAAGATGATCAGCACCTTAGC-3′). The PCR mixtures were denatured at 95°C for 2 min and annealed at 55°C for 1 min. PCR was performed 25 cycles as follows: extend at 72°C for 2 min, denature for at 95°C for 30 s, and anneal at 55°C for 1 min. Samples were extended at 72°C for additional 5 min. PCR products were visualized on 4% 3:1 NuSieve agarose (Takara, Kusatsu)/TAE gels.
Western Blot Analysis and Assay for Rab3 GEP Activity
An anti-Rab3 GEP antibody was raised against the hydrophilic region of Rab3 GEP, 365–447 amino acid residues, fused to the GST protein. Anti-rabphilin-3 and Rab GDIα antibodies were raised as described (Shirataki et al., 1994; Ishizaki et al., 2000). Anti-Rab3A and -3C antibodies were gifts from Ahmed Zahraoui (Institut Curie, Paris, France). Antibodies against synapsin Ia/b, synaptotagmin, and actin (C-11) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Embryonic mouse brains were homogenized in a lysis buffer of 320 mM sucrose, 20 mM Tris-Cl, pH 7.5, 2 mM EDTA, and 10 mM phenylmethylsulfonyl fluoride. Fifty micrograms of proteins were separated by SDS-PAGE, transferred to Immobilon membrane (Millipore, Bedford, MA), and blocked for 1 h in Tris-buffered saline containing 5% skimmed milk. After incubation with each antibody for 1 h and then with the peroxidase-conjugated secondary antibody for 1 h, the blots were developed with ECL (Amersham Pharmacia Biotech, Piscataway, NJ).
Embryonic mouse brains were homogenized in a buffer containing 20 mM Tris-Cl, pH 7.5, 1 mM DTT, and 10 μg/ml leupeptin, followed by ultracentrifugation at 100,000 × g for 60 min. Aliquots of the homogenates and the supernatant (cytosol) and pellet (membrane) fractions were subjected to the assay for Rab3 GEP activity as described (Wada et al., 1997).
Electrophysiology
Whole embryos at embryonic day (E)18.5 were surgically dissected and used for electrophysiological examination. To record electromyograms, electrical stimulation was delivered through bipolar electrodes at the spinal cord and the phrenic and sciatic nerves, and then action potentials were recorded at the diaphragm, the quadriceps, and gastrocnemius muscles, respectively, with the Nicolet Viking QuestTM system (Nicolet Biomedical, Madison, WI). To test nerve conductivity, action potentials were recorded at the spinal cord and the phrenic nerve that were surgically removed from the embryos and properly incubated during experiments in Ringer's solution of 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 25 mM dextrose, saturated with O2. Acetylcholine-induced action potentials of the diaphragm were recorded with surging 1.4 μmol acetylcholine chloride.
Electron Microscopy
E18.5 embryos were fixed with 2.5% glutaraldehyde and 2% paraformaldehyde in phosphate-buffered saline, followed by fixation with 1% OsO4 in 0.1 M cacodylate-HCl, pH 7.4, for 1 h. The samples were dehydrated, embedded in Epon812, and examined using an electron microscope. Acetylcholine esterase was used as an indicator to localize synaptic clefts at the neuromuscular junction.
Whole Mounts
To visualize peripheral nerves, whole embryos were fixed overnight in 4% paraformaldehyde. The embryos were made permeable with 10% Triton X-100 for 30 min, washed in Tris-buffered saline containing Triton X-100 (TBST) of 10 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.15% Triton X-100, incubated in 1% periodic acid solution at room temperature for 10 min, and then blocked in TBST containing 5% skimmed milk and 0.1% sodium azide. The antibody 2H3 against neurofilaments (Dodd et al., 1988) was incubated overnight at room temperature in TBST containing 5% skimmed milk and 0.1% sodium azide. After washing, the embryos were incubated with horseradish peroxidase–conjugated anti-mouse IgG (Amersham Pharmacia Biotech) in TBST containing 5% skimmed milk and soaked in 0.025% 3,3′-diaminobenzidene substrate solution as described (Dodd et al., 1988). The diaphragm extracted from E18.5 embryos was fixed in 4% paraformaldehyde in phosphate-buffered saline for 1 h. After washing three times, samples were incubated with rhodamine-labeled α-bungarotoxin (Molecular Probes, Eugene, OR) diluted 1:1000 in phosphate-buffered saline containing 0.3% bovine serum albumin at 37°C for 1 h and extended on a slide glass.
RESULTS
Generation of Rab3 GEP−/− Mice
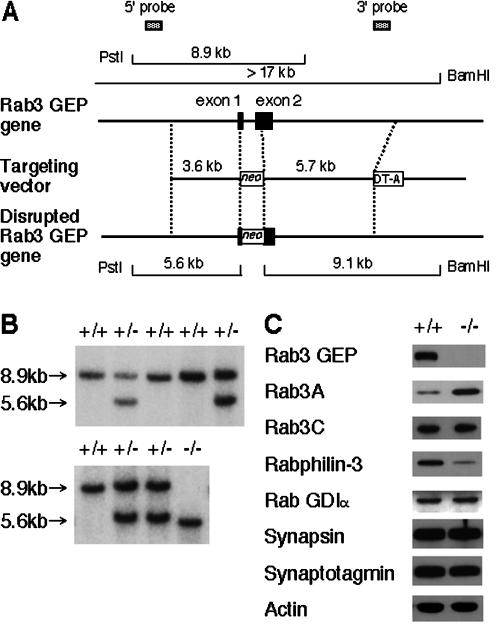
The whole structure of the mouse Rab3 GEP gene is currently unknown because it covers many exons that encode 1602 amino acids in total. To disrupt Rab3 GEP in ES cells, gene targeting was used to replace the 3′ half of the first coding exon and the 5′ half of the following exon 2 with an MC1-neomycin resistance cassette (Figure 1A). Because the targeted allele is disrupted by frame-shift mutation, it would not produce the intact Rab3 GEP protein except for the short peptide truncated at the N-terminal region. Because we have previously shown that the Rab3 GEP mutant lacking a C-terminal domain totally abolished its activity in Ca2+-dependent exocytosis of PC12 cells (Oishi et al., 1998), we conclude that this disruption results in mice that are functionally null for Rab3 GEP. The targeting vector was electroporated into RW4 ES cells, and six G418-resistant colonies heterozygous for the Rab3 GEP gene were selected. Cells from two independent ES clones were used to generate chimeric mice and successfully contributed to germline transmission. Rab3 GEP heterozygotes were intercrossed to produce homozygous mutant offspring (Figure 1B). Mice homozygous for the disrupted allele expressed neither the intact Rab3 GEP protein as analyzed by immunoblots (Figure 1C) nor the reverse transcriptase PCR product derived from the Rab3 GEP mRNA. To examine loss of GEP activity in the Rab3 GEP−/− mouse, we analyzed biochemical abilities of the homogenates and the cytosol and membrane fractions from the brains of the wild-type and Rab3 GEP−/− mice to stimulate GDP/GTP exchange on purified Rab3A as described (Wada et al., 1997). However, the GEP activity was at barely detectable levels in all the samples tested. Therefore, we conclude that GEP activity at least dependent on Rab3 GEP is absent in the Rab3 GEP−/− mice, but it remains unknown whether there are other factors than Rab3 GEP that enhance the GDP/GTP exchange activity of Rab3A.
Figure 1.
Targeted disruption of the Rab3 GEP gene. (A) Top: partial structure of the mouse Rab3 GEP gene with the first and second coding exons. A targeting vector was designed to remove the genomic DNA segment encompassing 3′ half of the exon 1 and 5′ half of the exon 2. The construct contained 3.6-kb 5′-flanking DNA sequences and 5.7-kb 3′ flanking DNA sequences. The diphtheria toxin DT-A cassette was inserted at the 3′ end. In the targeted allele, the MC1-neo cassette replaces 1.1 kb of genomic DNA region. Homologous recombination was verified by using informative restriction fragments and diagnostic probes as indicated. (B) Southern hybridization using PstI-digested DNA extracted from ES cells (top panel) and mouse tails (bottom panel) and the 5′ external probe shown in A. Genotypes of ES cells and mice, identified by the 8.9-kb wild-type and the 5.6-kb mutant fragments, are indicated above each lane. (C) Western blot analysis of synaptic proteins extracted from the brains of the wild-type and Rab3 GEP−/− embryos at E18.5. The 200-kDa band of the Rab3 GEP protein was detected in the brain of the wild-type embryo but not in that of the Rab3 GEP−/− embryo.
To examine how Rab3 GEP deficiency affects the levels of Rab and relevant proteins, we analyzed the homogenate of E18.5 embryo brains by Western blotting (Figure 1C). Rab3A levels in Rab3 GEP−/− mice were increased ∼2.5-fold, but Rab3C levels were not changed. Rabphilin-3 levels were decreased to 25% of those of the wild-type mice, which coincides with the findings in Rab3A−/− mice (Geppert et al., 1994). These results probably indicate that the GTP-bound form of Rab3A is essential for the interaction with rabphilin-3 and that free rabphilin-3 is a rather unstable protein. Other synaptic proteins, Rab GDIα, synaptotagmin I, and synapsin I a/b, were at similar levels between the wild-type and Rab3 GEP−/− mice. Thus, Rab3A and rabphilin-3 levels were selectively altered among the synaptic proteins tested, reflecting a close relationship between Rab3 GEP and Rab3A recycling in mice.
Requirement of Rab3 GEP for Neonatal Survival
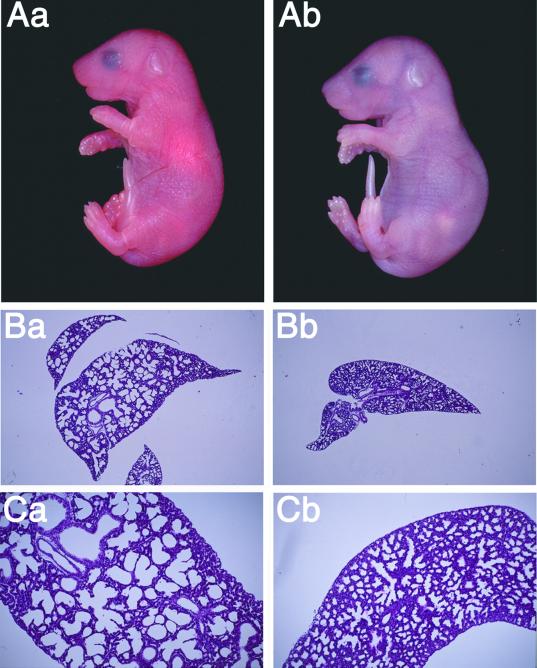
No homozygous null mice were observed in a total of 256 live mice at 1 to 21 days after birth (Table 1). These mice were derived from Rab3 GEP heterozygous intercrosses using either of the two founder lines. The wild-type and heterozygous mice were born at the expected frequencies and appeared normal and healthy. To determine when Rab3 GEP−/− mice die, we analyzed embryos from heterozygous intercrosses at various points of gestation. However, genotyping showed no evidence of embryonic lethality, suggesting that Rab3 GEP is not essential for mouse development but is implicated in neonatal survival. We then dissected E18.5 embryos from the uterus and determined the survival percent of Rab3 GEP−/− mice. Nearly 25% of mice died within 30 min after birth, which were identified as Rab3 GEP−/− (Figure 2A). These mice were refractory to resuscitation and showed few responses to tactile stimulation. These Rab3 GEP−/− mice were likely to die from acute respiratory failure because pulmonary tracts were not dilated histologically (Figure 2, B and C). Except for the closed lungs, however, no clear difference was seen in the gross morphologies between the wild-type and Rab3 GEP−/− E18.5 embryos. Development of the CNS was apparently normal in light microscopic analysis during embryonic stages of Rab3 GEP−/− mice.
Table 1.
Genotyping of live mice arising from Rab3 GEP heterozygous crosses
| Stage | Genotype of Rab3 GEP-deficient mice
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| E13.5 | 8 /28 (29%) | 14 /28 (50%) | 6 /28 (21%) |
| E15.5 | 5 /21 (24%) | 12 /21 (57%) | 4 /21 (19%) |
| E16.5 | 3 /24 (13%) | 14 /24 (58%) | 7 /24 (29%) |
| E18.5 | 14 /64 (22%) | 33 /64 (51%) | 17 /64 (27%) |
| P0 | 9 /24 (38%) | 15 /24 (63%) | 0 /24 (0%) |
| P21 | 101 /256 (39%) | 155 /256 (61%) | 0 /256 (0%) |
Genotype of Rab3 GEP-deficient mice is indicated as +/+, +/−, and −/−. Genotyping was performed by Southern hybridization or PCR analysis at the age indicated as embryonic (E) and postnatal (P) days.
Figure 2.
Marked cyanotic appearance of Rab3 GEP−/− mice and histology of the closed lungs. (A) Appearance of the wild-type (Aa) and Rab3 GEP−/− newborn mice (Ab) at P0. Rab3 GEP−/− mice developed normally but showed extreme cyanosis due to hypoxia. (B) Histology of lung sections stained with hematoxylin and eosin. Images of the wild-type (Ba and Ca) and Rab3 GEP−/− mice (Bb and Cb) at low magnification (top panels) and at high magnification (bottom panels).
Defective Neuromuscular Transmission in Rab3 GEP−/− Mice
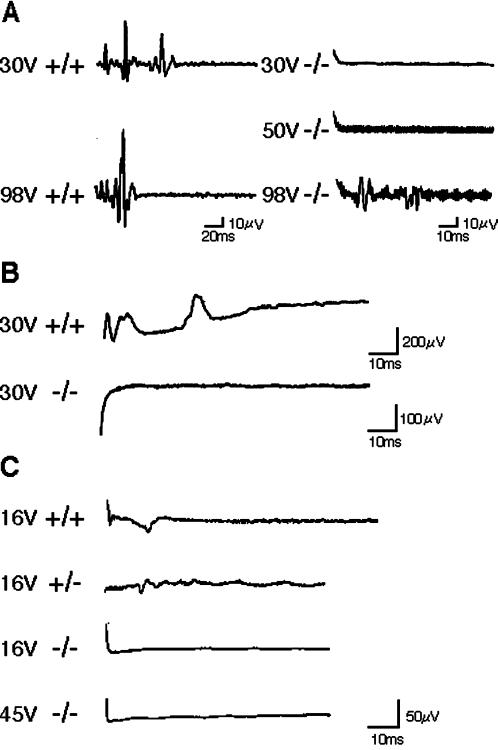
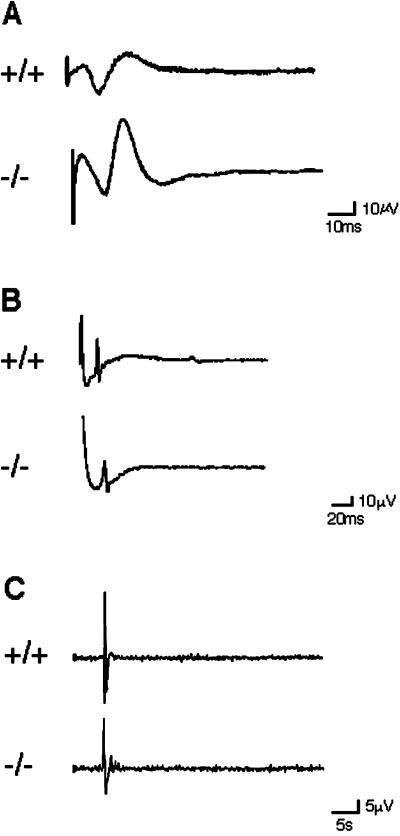
Rab3 GEP−/− mice showed infrequent voluntary motions of the limbs, few responses to tactile stimulation, and impaired respiratory movement. All these findings could be explained by any defects in pathways from motor neurons to muscles. To investigate the cause of death in Rab3 GEP−/− newborn mice, we examined electrophysiological characteristics in neuromuscular transmission. First, electrical stimuli were given at the spinal cord with bipolar electrodes at the lumbosacral level, and action potentials were recorded at the quadriceps muscle (Figure 3A). Second, electrical stimuli were given at the sciatic nerve, and action potentials were recorded at the gastrocnemius muscle (Figure 3B). The wild-type and heterozygous embryos at E18.5 appeared normal, with spikes of motor evoked potentials to electrical stimuli at 10- to 30-ms point. In contrast, all Rab3 GEP−/− embryos showed no spikes in response to mild (30 V) and moderate (50 V) stimuli, even though low-grade spikes were detected at the maximal intensity (98 V) of stimuli (n = 6; Figure 3A). Similarly, Rab3 GEP−/− embryos showed no spikes at the diaphragm in response to stimuli at the phrenic nerve, compared with normal spikes in the wild-type and heterozygous embryos (n = 6; Figure 3C). On the other hand, nerve conduction of the spinal cord (Figure 4A) and the phrenic nerve (Figure 4B) showed no significant difference between the wild-type and Rab3 GEP−/− embryos (n = 6). To explore postsynaptic defects, we isolated the diaphragm from embryos and incubated it with acetylcholine to examine physiological responses of the acetylcholine receptor. Reaction levels of the acetylcholine receptor were almost the same between the wild-type and Rab3 GEP−/− mice (Figure 4C). We conclude from these data that neuromuscular transmission is primarily impaired in Rab3 GEP−/− mice.
Figure 3.
Impairment of neuromuscular transmission detected by electromyograms of the wild-type and Rab3 GEP−/− embryos at E18.5. (A) Action potentials of the quadriceps muscle evoked with electrical stimulation of the spinal cord. (B) Action potentials of the gastrocnemius muscle evoked with electrical stimulation of the sciatic nerve. (C) Action potentials of the diaphragm evoked with electrical stimulation of the phrenic nerve. The voltage of stimuli and the genotype of mice are indicated at the left side of panels.
Figure 4.
Normal axonal conductivity at the spinal cord and the phrenic nerve of the wild-type and Rab3 GEP−/− embryos at E18.5. Samples were surgically removed from embryos, incubated in Ringer's solution, and used for electrophysiological analysis. Bipolar electrodes placed at both ends of the spinal cord and the phrenic nerve; stimuli were given at the proximal end and action potentials were recorded at the distal end. (A) Spinal cord; (B) phrenic nerve; (C) action potentials of the diaphragm evoked with acetylcholine chloride that was added at 1.4 μmol per experiment.
Reduced Numbers of Synaptic Vesicles at the Neuromuscular Junction in Rab3 GEP−/− Mice
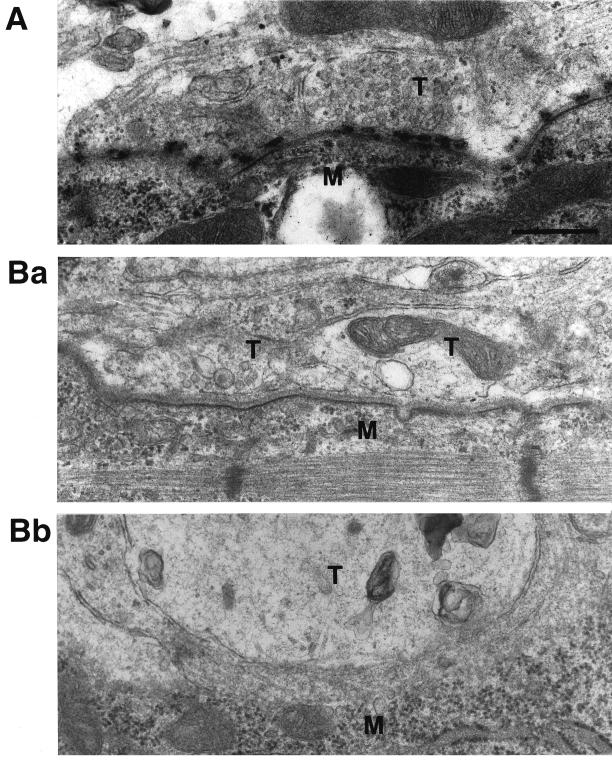
The observation that Rab3 GEP−/− embryos grow normally until E18.5 makes it possible to investigate the critical question of how Rab3 GEP is involved in vesicle trafficking. We examined extensively by electron microscopy the structure of synapses at the neuromuscular junction of the phrenic nerve and the diaphragm (Figure 5). In the wild-type embryos, synaptic components at the neuromuscular junction were fully developed at E18.5 stage, and there were >40 mature synaptic vesicles of equal size in the wild-type axon terminal section (Figure 5A). In contrast, there were only a few synaptic vesicles or almost none in the Rab3 GEP−/− axon terminal section (Figure 5Ba). Structural abnormalities such as enlargement of the axon terminal, degeneration of mitochondria, and large synaptic vesicles with no content were frequently detected in the Rab3 GEP−/− embryo (Figure 5Bb). The number of synaptic vesicles in Rab3 GEP−/− embryos was drastically decreased to 11.8 ± 3.6% of that in the wild-type embryos (n = 3). In addition, ultrastructural localization of synaptic vesicles was different between the wild-type and Rab3 GEP−/− embryos. Abundant synaptic vesicles located near the presynaptic plasma membrane in the wild-type embryos were supposed to be docked/fused to active zones and to immediately release neurotransmitters in response to stimuli. However, most of the synaptic vesicles in Rab3 GEP−/− embryos were located apart from the presynaptic plasma membrane, indicating that they did not readily undergo exocytosis. Therefore, it is conceivable that formation of synaptic vesicles as well as their docking/fusion at the presynaptic plasma membrane are impaired in Rab3 GEP−/− embryos. Active zones at the presynaptic plasma membrane were not well- developed, reflecting the presence of chronic defects in exocytosis during gestation.
Figure 5.
Reduced numbers of synaptic vesicles at the neuromuscular junction analyzed by electron micrographs of the Rab3 GEP−/− diaphragm. (A) The wild-type mouse neuromuscular junction. More than 40 synaptic vesicles of an equal size are found in the axon terminal. Clusters of synaptic vesicles are accumulated in the close proximity to the presynaptic plasma membrane. (Ba) The Rab3 GEP−/− mouse neuromuscular junction. There are only a few synaptic vesicles in the left axon terminal and none in the right axon terminal. Synaptic vesicles are located apart from the presynaptic plasma membrane. Unusual large vesicles are found in both axon terminals. (Bb) The Rab3 GEP−/− mouse neuromuscular junction. The axon terminal that is enlarged up to 3 μm in diameter contains no synaptic vesicles. Degenerated mitochondria and vesicles larger than normal synaptic vesicles are frequently visible. These morphological changes indicate the impairment of synaptic vesicle trafficking in axon terminals of Rab3 GEP−/− mice. T, axon terminal; M, muscle cell. Bar, 500 nm.
Absence of Developmental and Postsynaptic Defects in Rab3 GEP−/− Mice
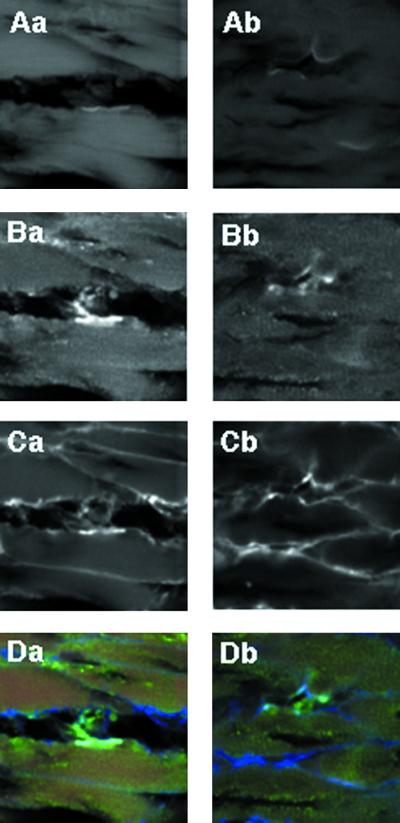
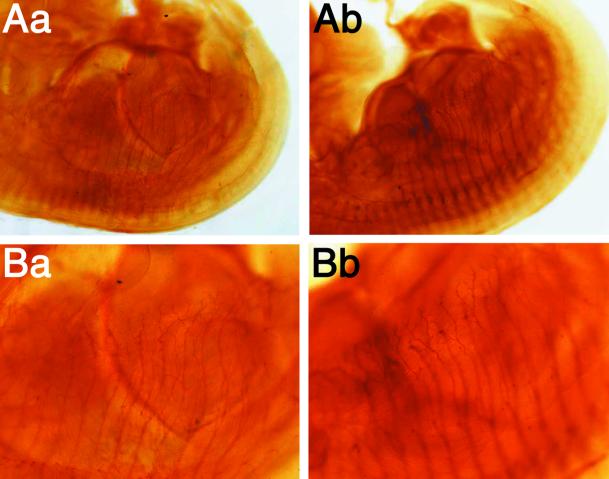
The reduced numbers of synaptic vesicles in Rab3 GEP−/− embryos appear to indicate presynaptic defects at the neuromuscular junction. However, it is important to exclude postsynaptic defects, developmental defects, or delay in maturity in the peripheral nervous system. First, we investigated clustering of the acetylcholine receptor of the diaphragm by staining with the labeled α-bungarotoxin (Figure 6A). We have observed no significant difference in the number and distribution between the acetylcholine receptor clustering of the wild-type and Rab3 GEP−/− embryos. Although the findings were not analyzed statistically, they were consistent with the normal responses of the acetylcholine receptor as observed in experiments using the Rab3 GEP−/− diaphragm (Figure 4C). Second, we investigated the distribution of Rab3A as well as synaptotagmin by immunostaining (Figure 6, B and C). No difference was observed in that of the two molecules between the wild-type and Rab3 GEP−/− embryos. These results were shown in the merged profiles of the three molecules (Figure 6D). Finally, we investigated the development of the intercostal nerves by immunohistochemistry using an anti-neurofilament antibody at various stages of gestation. We observed no difference in the size and distribution between the intercostal nerves of the wild-type and Rab3 GEP−/− embryos (Figure 7). Thus, we conclude that Rab3 GEP is primarily implicated in presynaptic vesicle trafficking at the neuromuscular junction.
Figure 6.
Normal numbers and distribution of the acetylcholine receptor, Rab3A, and synaptotagmin in the Rab3 GEP−/− nerve terminals analyzed by staining with α-bungarotoxin and specific antibodies. (A) Staining of the acetylcholine receptor with α-bungarotoxin. (B) Immunostaining with the anti-Rab3A antibody. (C) Immunostaining with the anti-synaptotagmin antibody. (D) Merged images of the acetylcholine receptor (red), Rab3A (green), and synaptotagmin (blue). (Aa, Ba, Ca, and Da) Wild-type embryos. (Ab, Bb, Cb, and Db) Rab3 GEP−/− embryos.
Figure 7.
Normal distribution of Rab3 GEP−/− intercostal nerves analyzed by whole-mount immunostaining with the anti-neurofilament antibody. Embryos were delivered by Caesarean section, fixed in 4% paraformaldehyde, permeabilized, and blocked. To stain neurofilaments, samples were incubated with the 2H3 antibody, treated with the second horseradish peroxidase–conjugated antibody, and observed by the peroxidase method with 3,3′-diaminobenzidene. (Aa and Ba) Wild-type embryos at E12.5. (Ab and Bb) Rab3 GEP−/− embryos at E12.5. Images at low magnification were shown in the top panels, and images at high magnification were shown at the bottom panel.
DISCUSSION
We have shown that Rab3 GEP−/− embryos at E18.5 have defects in synaptic vesicle trafficking at the neuromuscular junction but do not show postsynaptic abnormalities and that Rab3 GEP appears not essential for development of neuronal cells or morphogenesis of synapses. The striking observation is that numbers of synaptic vesicles, especially those docked at the presynaptic plasma membrane, are drastically decreased, which may cause reduced neurotransmission in Rab3 GEP−/− mice. It is notable that Caenorhabditis elegans with mutations of Aex-3, a Rab3 GEP homolog, shows reduced synaptic transmission that is consistent with our findings in Rab3 GEP−/− mice (Iwasaki et al., 1997). The mechanistic implication of Rab3 GEP, however, is not readily known because of lacking of the exact information about how Rab3 recycling is linked to vesicle formation, vesicle trafficking, and release probability. Because numbers of synaptic vesicles are not altered in mice lacking Rab3A or Rab GDIα, impaired Rab3A recycling during targeting/fusion alone appears unable to explain the entire phenotype of Rab3 GEP−/− mice. Thus, we hypothesize that Rab3 GEP is implicated in formation of synaptic vesicles as well as vesicle trafficking and that increased levels of the GDP-bound Rab proteins presumably inhibit synaptic vesicle biosynthesis in the absence of Rab3 GEP function.
In nerve terminals, the recycling of synaptic vesicles is well established (De Cammili and Takei et al., 1996). Synaptic vesicles are targeted and fused to the active zone in response to a rise in Ca2+ to release neurotransmitters and are immediately retrieved from the membrane to the cytosol by endocytosis via clathrin-dependent or -independent mechanisms. The retrieved vesicles then fuse to the endosomes where new synaptic vesicles are to bud-off. This rapid regeneration of synaptic vesicles after exocytosis is believed to be sufficient for the assembly of synaptic vesicles in nerve terminals. On the other hand, the components of synaptic vesicles are newly synthesized and transported to nerve terminals. Recycled and newly synthesized synaptic vesicle proteins are recognized to converge at the endosomes to produce new synaptic vesicles. Thus, defects in any step during the process of recycling and formation are supposed to result in decreased numbers of synaptic vesicles.
Then how and what Rab3 isoforms are likely to take part in each secretory process? Rab3 GEP shows GDP/GTP exchange activity on Rab3A, -3C, and -3D but not on Rab3B (Wada et al., 1997). Rab3A and -3C are primarily expressed in the neuronal tissues and localized on synaptic vesicles (Mizoguchi et al., 1990; Fischer von Mollard et al. 1990, 1994), whereas Rab3D is expressed in all tissues, predominantly in heart, lung, and liver (Adachi et al., 2000) and is found on the secretory granules of exocrine glands and mast cells (Tang et al., 1996; Valentijin et al., 1996; Tuvim et al., 1999). Consistent with the tissue and subcellular distributions, Rab3A is involved in the process of targeting/fusion (Fischer von Mollard et al., 1994; Geppert et al., 1994, 1997), although the role of Rab3C remains unknown. Because synaptic vesicles incorporated into the plasma membrane via exocytosis has recently been found to be a sufficient trigger of synaptic vesicle endocytosis (Gad et al., 1998), inhibition of Rab3-mediated exocytosis may affect or delay endocytosis in Rab3 GEP−/− mice. It is possible that Rab3D is involved in the synthesis or transport of synaptic vesicle precursors in the neuronal cell body. Recently, some Rab proteins have been found to link the vesicles and organelles to the microtubules and to stimulate their transport along the microtubules (Echard et al., 1998; Mammoto et al., 1999; Nielsen et al., 1999; White et al., 1999). Therefore, Rab3D may serve to link the synaptic vesicle precursors to the microtubules in the axon and to stimulate the axonal transport, resulting in synaptic vesicle formation in nerve terminals. Because Rab3D is a ubiquitous protein, Rab3 GEP deficiency is likely to cause more generalized phenotype than Rab3A or Rab3C deficiency alone. Taken together, the dysfunction involving all these Rab3 isoforms appears to induce the profound phenotype of Rab3 GEP−/− mice, even though we cannot exclude a possibility that part of the phenotype of Rab3 GEP−/− mice is caused by the defective function of Rab3 GEP that may not be related to the Rab3 family.
Biological issue requiring further investigation is whether synaptic plasticity of the CA1 and CA3 regions of the hippocampus is altered by the absence of Rab3 GEP. Unfortunately, it is technically difficult to keep Rab3 GEP mice alive up to 3 weeks of age, which prevents us from analyzing hippocampal synaptic plasticity. In addition, we cannot exclude possible abnormalities in the peripheral and CNS, such as carotid pressure receptor to oxygen or the medulla oblongata responsible for regulating respiration, which may also lead to the lethality of Rab3 GEP−/− mice. To address these questions, we have to develop conditional targeting to disrupt the Rab3 GEP gene, in which mice could survive at least for 3 weeks. Alternatively, we are currently looking for defects in synaptic transmission of embryonic neuronal cells in culture.
ACKNOWLEDGMENTS
We are grateful to Dr. Ahmed Zahraoui for providing anti-Rab3A and -3C antibodies. The 2H3 monoclonal antibody developed by Dodd et al. (1988) was obtained from the Developmental Studies Hybridoma Bank maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
Footnotes
† Takai Biotimer Project was closed in September 1999.
REFERENCES
- Adachi R, Nigam R, Tuvim MJ, DeMayo F, Dickey BF. Genomic organization, chromosomal localization, and expression of the murine RAB3D gene. Biochem Biophys Res Commun. 2000;273:877–883. doi: 10.1006/bbrc.2000.3032. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fiber long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–139. doi: 10.1038/487. [DOI] [PubMed] [Google Scholar]
- De Cammili P, Takei K. Molecular mechanisms in synaptic vesicle endocytosis and recycling. Neuron. 1996;16:481–486. doi: 10.1016/s0896-6273(00)80068-9. [DOI] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, Jahn R, Südhof TC. Rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA. 1990;87:1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stahl B, Khokhlatchev A, Südhof TC, Jahn R. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicles after stimulation of exocytosis. J Biol Chem. 1994;269:10971–10974. [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Südhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Südhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Geppert M, Südhof TC. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Scheller RH. Regulation of membrane trafficking: Structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/s0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Ishizaki H, Miyoshi J, Kamiya H, Togawa A, Tanaka M, Sasaki T, Endo K, Mizoguchi A, Ozawa S, Takai Y. Role of Rab GDP dissociation inhibitor α in regulating plasticity of hippocampal neurotransmission. Proc Natl Acad Sci USA. 2000;97:11587–11592. doi: 10.1073/pnas.97.21.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Koera K, Nakamura K, Nakao K, Miyoshi J, Toyoshima K, Hatta T, Otani H, Aiba A, Katsuki M. K-ras is essential for the development of the mouse embryo. Oncogene. 1997;15:1151–1159. doi: 10.1038/sj.onc.1201284. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ohtsuka T, Hotta I, Sasaki T, Takai Y. Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein, implicated in vesicle recycling. J Biol Chem. 1999;274:25517–25524. doi: 10.1074/jbc.274.36.25517. [DOI] [PubMed] [Google Scholar]
- Martinez O, Goud B. Rab proteins. Biochem Biophys Acta. 1998;1404:101–112. doi: 10.1016/s0167-4889(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Kim S, Ueda T, Kikuchi A, Yorifuji H, Hirokowa N, Takai Y. Localization and subcellular distribution of smg p25A, a ras p21-like GTP-binding protein, in rat brain. J Biol Chem. 1990;265:11872–11879. [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosome on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicle traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Oishi H, Sasaki T, Nagano F, Ikeda W, Ohya T, Wada M, Ide N, Nakanishi H, Takai Y. Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J Biol Chem. 1998;273:34580–34585. doi: 10.1074/jbc.273.51.34580. [DOI] [PubMed] [Google Scholar]
- Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, director of vesicle docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Shirataki H, Yamamoto T, Hagi S, Miura H, Oishi H, Jin-no Y, Senbonmatsu T, Takai Y. Rabphilin-3A is associated with synaptic vesicles through a vesicle protein in a manner independent of Rab3A. J Biol Chem. 1994;269:32717–32720. [PubMed] [Google Scholar]
- Südhof TC. Function of Rab3 GDP-GTP exchange. Neuron. 1997;18:519–522. doi: 10.1016/s0896-6273(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in Ca2+-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-Binding Proteins. Physiology Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tang LH, Gumkowski FD, Sengupta D, Modlin IM, Jamieson JD. Rab3D protein is a specific marker for zymogen granules in gastric chief cells of rats and rabbits. Gastroenterology. 1996;110:809–820. doi: 10.1053/gast.1996.v110.pm8608891. [DOI] [PubMed] [Google Scholar]
- Tuvim MJ, Adachi R, Chocano JF, Moore RH, Lampert RM, Zera E, Romero E, Knoll BJ, Dickey BF. Rab3D, a small GTPase, is localized on mast cell secretory granules and translocates to the plasma membrane upon exocytosis. Am J Respir Cell Mol Biol. 1999;20:79–89. doi: 10.1165/ajrcmb.20.1.3279. [DOI] [PubMed] [Google Scholar]
- Valentijin JA, Sengupta D, Gumkowski FD, Tang LH, Konieczko EM, Jamieson JD. Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur J Cell Biol. 1996;70:33–41. [PubMed] [Google Scholar]
- Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3A subfamily small G proteins. J Biol Chem. 1997;272:3875–3878. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reisch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EHK. Rab6 coordinate a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]