Abstract
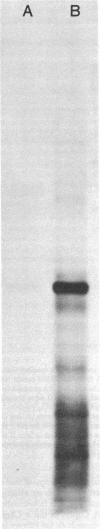
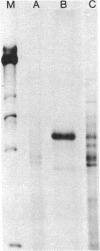
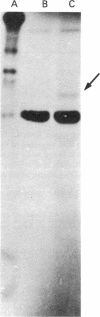
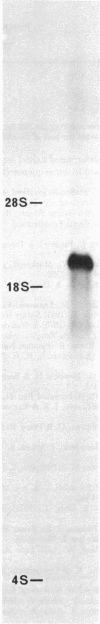
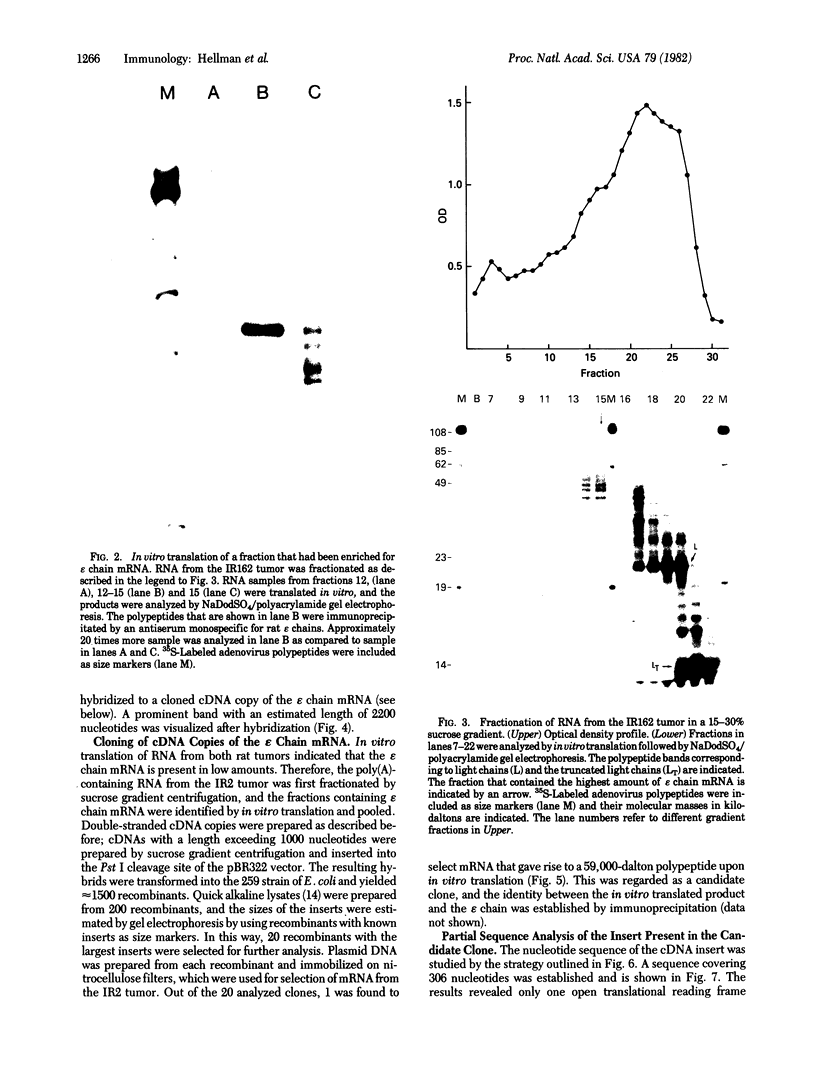
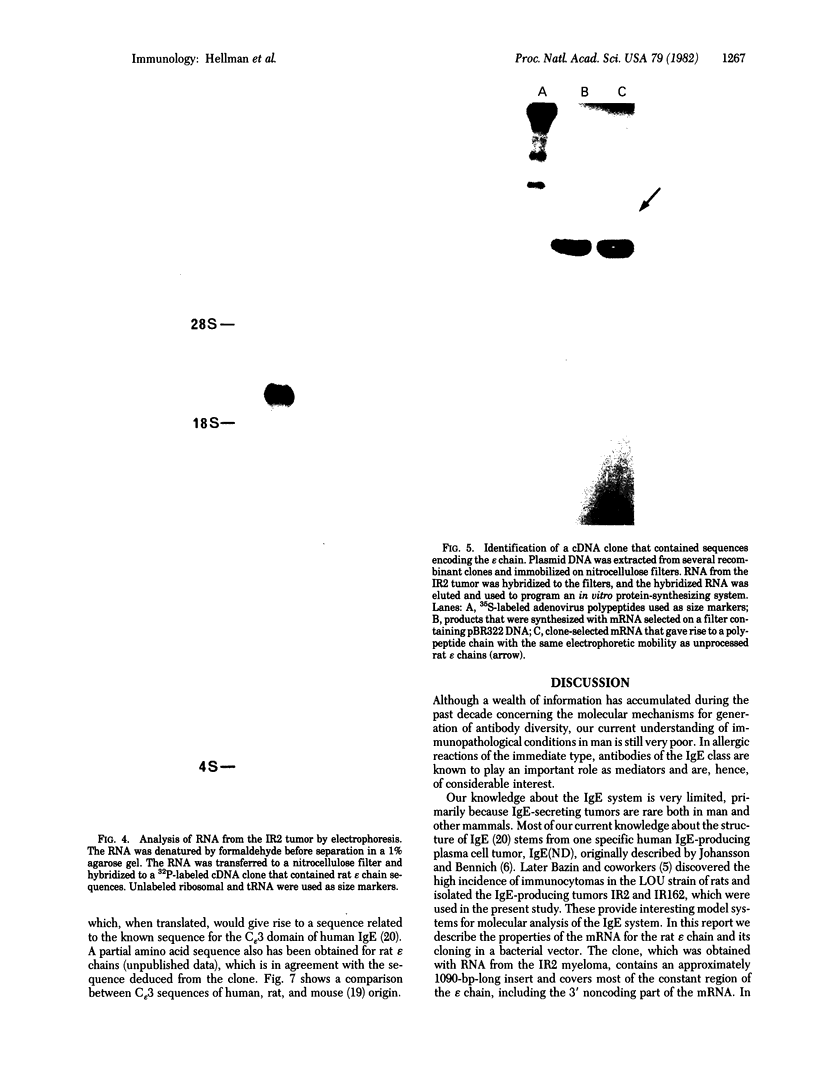
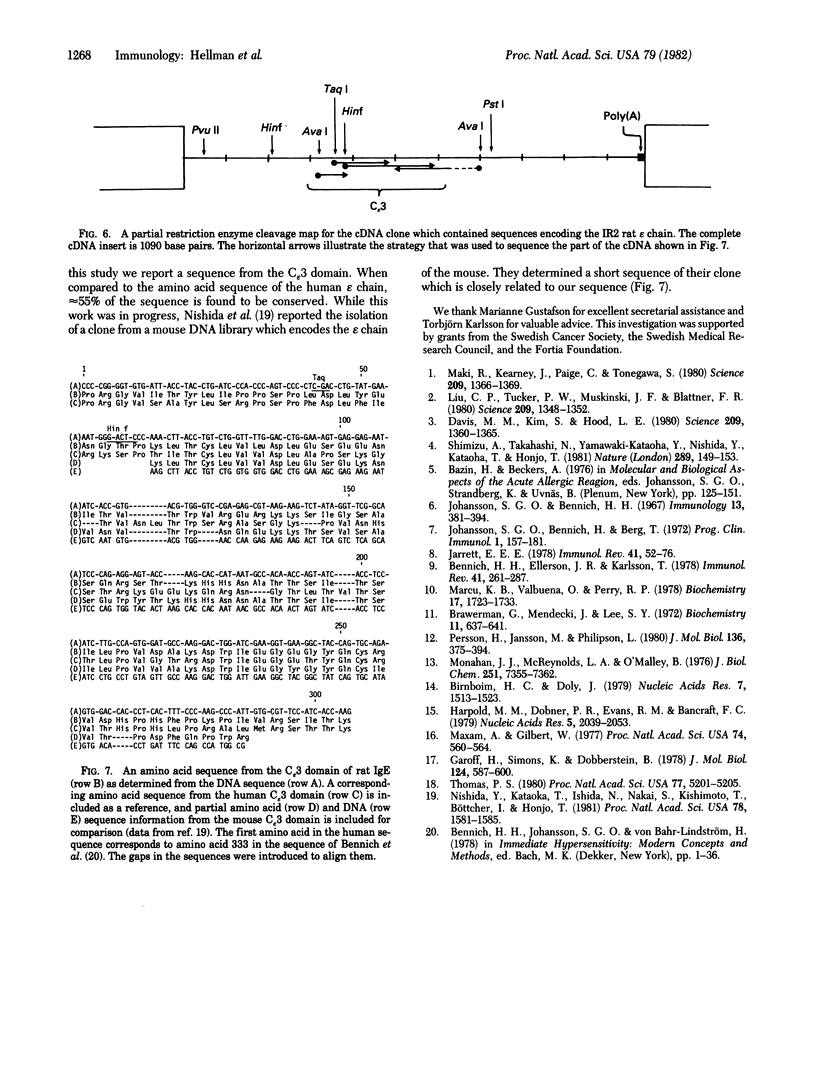
We report a study of the mRNA for the heavy (epsilon) chain of rat IgE. Cytoplasmic RNA was prepared from the two rat immunocytomas IR2 and IR162 and fractionated by sucrose gradient centrifugation. An enriched fraction containing approximately 5% mRNA for the epsilon chain was obtained in this way. When translated in vitro, it produced a 59,000-dalton polypeptide, which in the presence of a membrane fraction yielded a 90,000-dalton polypeptide, presumably through posttranslational modification. Both polypeptides were precipitated by rabbit antisera that were monospecific for rat epsilon chains. The epsilon chain mRNA was estimated to be approximately 2200 nucleotides long and constitutes a minute fraction in the total mRNA both in the IR2 and the IR162 tumors, unlike the mRNA for light chains. Double-stranded cDNA copies prepared frm the RNA fraction, which was enriched for epsilon chain mRNA, were inserted into the Pst I cleavage site of the pBR322 vector. Twenty clones with inserts exceeding 1000 base pairs were used for selection of mRNA from the IR2 tumor. By in vitro translation of the selected mRNA, one clone was identified that yielded a polypeptide with the same size as the unprocessed epsilon chain. The nucleotide sequence was determined for part of the inserted cDNA in this candidate clone and was found to be homologous to a sequence in the constant region (C) of the human epsilon chain. In this communication we report a sequence from the C epsilon 3 domain of the rat IgE. When compared to the corresponding sequence of human IgE, 55% of the amino acids in the rat sequence were found to be conserved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennich H. H., Ellerson J. R., Karlsson T. Evaluation of basic serum IgE levels and the IgE antibody response in the rat by radioimmunoassays. Immunol Rev. 1978;41:261–287. doi: 10.1111/j.1600-065x.1978.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E. Stimuli for the production and control of IgE in rats. Immunol Rev. 1978;41:52–76. doi: 10.1111/j.1600-065x.1978.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Johansson S. G., Bennich H. H., Berg T. The clinical significance of IgE. Prog Clin Immunol. 1972;1:157–181. [PubMed] [Google Scholar]
- Johansson S. G., Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967 Oct;13(4):381–394. [PMC free article] [PubMed] [Google Scholar]
- Liu C. P., Tucker P. W., Mushinski J. F., Blattner F. R. Mapping of heavy chain genes for mouse immunoglobulins M and D. Science. 1980 Sep 19;209(4463):1348–1353. doi: 10.1126/science.6774414. [DOI] [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Valbuena O., Perry R. P. Isolation, purification, and properties of mouse heavy-chain immunoglobulin mRNAs. Biochemistry. 1978 May 2;17(9):1723–1733. doi: 10.1021/bi00602a022. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. J., McReynolds L. A., O'Malley B. W. The ovalbumin gene. In vitro enzymatic synthesis and characterization. J Biol Chem. 1976 Dec 10;251(23):7355–7362. [PubMed] [Google Scholar]
- Nishida Y., Kataoka T., Ishida N., Nakai S., Kishimoto T., Böttcher I., Honjo T. Cloning of mouse immunoglobulin epsilon gene and its location within the heavy chain gene cluster. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1581–1585. doi: 10.1073/pnas.78.3.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Jansson M., Philipson L. Synthesis and genomic site for an adenovirus type 2 early glycoprotein. J Mol Biol. 1980 Feb 5;136(4):375–394. doi: 10.1016/0022-2836(80)90396-4. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yamawaki-Kataoka Y., Nishida Y., Kataoka T., Honjo T. Ordering of mouse immunoglobulin heavy chain genes by molecular cloning. Nature. 1981 Jan 15;289(5794):149–153. doi: 10.1038/289149a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]