Abstract
Background:
Sexually transmitted infections (STIs) remain an important public health issue in sub-Saharan Africa. STIs in HIV-positive women are associated not only with gynecological complications but with increased risk of HIV transmission to HIV-negative partners and newborns.
Aims:
The aims of this study are to determine the prevalence of chlamydia (CT) and gonorrhea (GC) and examine the demographic characteristics and risk behaviors associated with these STIs in a group of HIV-positive women in Lusaka, Zambia.
Settings and Design:
Cross-sectional study of a sample of HIV-infected women enrolled in two large studies conducted in urban Lusaka, Zambia.
Materials and Methods:
HIV-seropositive women (n = 292) were assessed for demographic and behavioral risk factors and tested for CT and GC. Univariate analysis was used to determine the demographic characteristics and risk behaviors associated with having CT or GC.
Results:
The identified prevalence of CT was 1% and of GC was 1.4%. There was an association of CT/GC with the use of alcohol before sex (OR = 9.I, CI = 0.59-0.15, P = 0.03).
Conclusions:
Rates of CT and GC are described in this sample of HIV-positive women. While being in HIV care may serve to increase medical care and condom use, alcohol use should be addressed in this population.
Keywords: Africa, Chlamydia, Gonorrhea, Human immunodeficiency virus, Women
INTRODUCTION
Sexually transmitted infections (STIs) are a major cause of acute disease and obstetric and gynecological complications in women. Genital infections with chlamydia (CT) and gonorrhea (GC) are associated with an increased risk of genital acquisition and transmission of Human immunodeficiency virus (HIV) in both men and women.[1] In settings with a high prevalence of HIV, such as sub-Saharan Africa, STIs in HIV-infected women may increase HIV transmission to HIV-negative partners and newborns.
In Zambia, the HIV epidemic is so devastating that teenagers currently aged 15 have more than a 50% risk of dying of Acquired Immunodeficiency Syndrome (AIDS) before the age of 35.[2] In this country, it is estimated that 10% of all outpatient attendances to health institutions are related to STIs, but there are no data on the prevalence of these infections in women that are infected with HIV.[2]
This study examines the prevalence of CT and GC in a group of HIV-infected women in Lusaka, Zambia. It also describes the demographic characteristics and risk behaviors associated with CT and GC in the study population.
MATERIALS AND METHODS
This study is a cross-sectional analysis of a convenience sample of HIV-infected women drawn from two larger studies conducted at the University Teaching Hospital (UTH) and Community Health Centers (CHCs) in urban Lusaka. This report presents demographic and risk factor data drawn from participants recruited in these studies and rates of CT and GC at the time of enrollment. Institutional Review Board (University of Miami Miller School of Medicine) and Research Ethics Committee (University of Zambia) approvals and client informed consent were obtained before recruitment, assessment and any study-related interventions.
Study candidates were referred from the voluntary counseling and testing (VCT) at the UTH and CHCs. Eligible participants were women 18 years of age or older, sexually active, HIV-positive and living in the Lusaka Metropolitan Area. Demographic, medical and risk factors’ questionnaires were administered to all participants. History of prior STIs (CT, GC, syphilis, chancroid, genital herpes, genital warts or trichomoniasis) was provided by self-report and prior infections had been treated prior to enrollment.
Outcome measures
Demographic questionnaire
This questionnaire included data collection on age, educational level, employment status, date of HIV infection, mode of infection with HIV, history of drug use or alcohol use, marital status, current partner HIV status, living situation, number of children and children's HIV serostatus.
Sexual activities’ questionnaire
This 55-item scale was adapted from the widely used sexual risk behavior assessment schedule (SERBAS).[3] Responses indicated the frequency of heterosexual sexual intercourse (vaginal, oral, anal) with both primary partners (most frequent sexual relations) and non-primary partners (any other male partners). The questionnaire also assessed sexual barrier use, HIV status of the partner, known sexual practices of the partner, and alcohol or drug use. The SERBAS is a structured interview that identifies frequency as “all the time or 9 times out of 10, most times or 7 times out of 10, half of the time or 5 times out of 10, sometimes or 3 times out of ten, or never.” Responses were combined by adding the number of responders and converted to dichotomous variable (0 = never, 1 = combination of other frequencies or yes).
Laboratory assessments
Testing for CT and GC was performed using urine real-time Nucleic Acid Amplification testing (BD ProbeTec systemä). All samples were analyzed at the center for infectious diseases’ research of Zambia (CIDRZ). Since CT and GC share the same transmission patterns, cases of CT and GC were added and used to calculate associations between CT, GC and other variables of interest. Outcomes were analyzed using a dichotomous scale (0 = no, 1 = yes).
Predictive Analytics Software 18 (PASWÒ) statistics was used for analysis (SPSS Microsoft Corp, Chicago). Univariate analysis using odds ratios was used to determine factors associated with CT/GC; a P value of less than 0.05 was considered to be significant.
RESULTS
HIV-positive women (n = 292) were enrolled and included in the analysis. The median age was 34.6 years. Baseline characteristics of the women tested are presented in Table 1. The sample was composed of very low income, middle-aged women, married and living with stable HIV-positive partners. The primary identified risk factor for HIV transmission was heterosexual contact. Over 60% of women reported condom use most of the time or always (48% most of the times and, 12% all the times). Five percent used condom half of the times, 12% sometimes and 21% never. Less than 5% reported additional non-primary sexual partners. Almost 75% of the women reported chronic HIV infection (between one and five years) and over two-thirds were receiving antiretroviral therapy. The most common self-reported prior STI was syphilis (14%). Ten percent of women reported use of alcohol prior to sexual activity and 8% had engaged in anal sex in the prior month.
Table 1.
Baseline Sociodemographic characteristics and risk behaviors in the study population (n = 292)
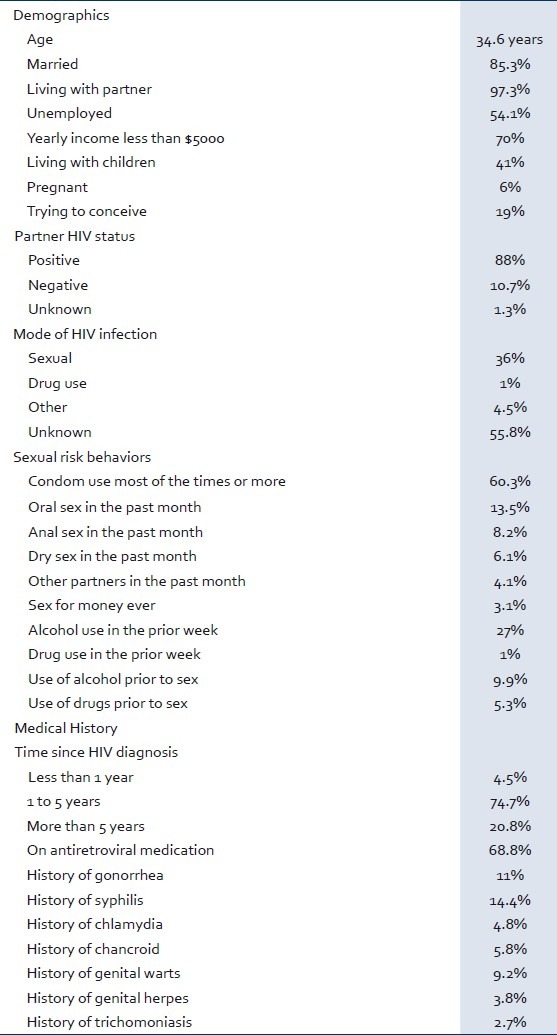
Prevalence of CT was 1% and GC 1.4%. No participants had dual infection with CT/GC.
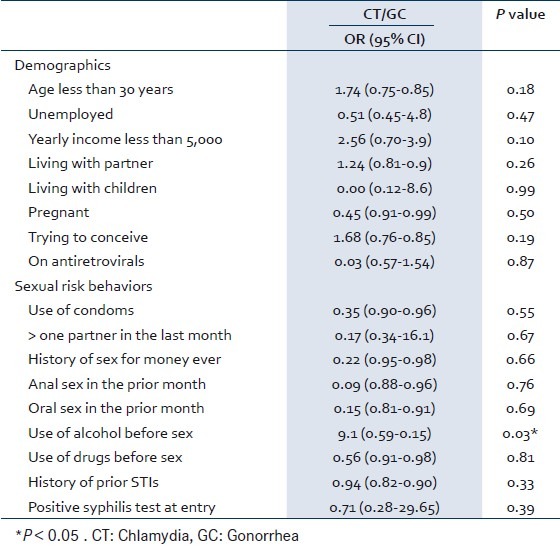
Univariate analysis of potential factors associated with having a lower genital STI infection (CT or GC) is presented in Table 2. There was no association between demographic characteristics, including relationship status, previous history of STIs, plans to conceive, use of antiretrovirals, condom use, transactional sex and sex with non-primary sexual partners, and the presence of CT/GC. In terms of sexual risk behaviors, the use of alcohol before sex was associated with the presence of CT/GC.
Table 2.
Odds’ Ratio for having CT/GC
DISCUSSION
This study examined the prevalence of chlamydia and gonorrhea in a group of HIV-infected women in Lusaka, Zambia. It also reports the relationships between the presence of CT or GC, demographic characteristics and sexual risk behaviors in the study population. The prevalence of CT and GC was 1% and 1.4%. There was no association between demographic characteristics and the presence of STIs, but the use of alcohol before sex was significantly associated with having CT or GC.
Other studies from sub-Saharan Africa have reported variable rates of STIs. In a sample of commercial sex workers in Benin, Ahoyo identified rates of CT and GC as being approximately 5%, and Braunstein identified rates of CT and GC of 5% and 11.6% respectively in a similar population in Rwanda.[4,5] In Tanzania, in a sample of HIV-infected pregnant women, Msuya found a high prevalence of chlamydia (30%) and 1.6% prevalence of gonorrhea.[6] Other large studies such as the Partners in Prevention HSV/HIV transmission study, however, have found lower rates of these infections.[7]
In Zambia, Kapina et al. found rates of CT of 2.7% and of GC 3.4% in 2010.[8] The prevalence of 1% of CT and 1.4% of GC among our sample of HIV-seropositive women is slightly lower. The lower rates obtained are likely due to low levels of risk behaviors reported. Most participants were in stable relationships and less than 5% reported other sexual partners or history of exchanging sex for money. In addition, due to their HIV status, participants were more likely to be receiving ongoing medical care and syndromic management for STI symptoms. These suggest that engaging in HIV care may play a role for controlling rates of STI in this population. However, although the STI rates were lower in this sample, and most of the women had an HIV-positive partner, they remain important to identify as they can increase the risk of HIV transmission to HIV-negative partners and newborns. Studies addressing rates and risk factors of CT/GC in HIV-positive and -negative women will help to determine if engaging in HIV care could have a protective effect against these infections.
In this study, participants were recruited from VCT centers that routinely provide information about HIV prevention and sexual barrier methods. Sixty percent of women reported condom use in more than 70% of sexual encounters. Rates of condom use in other studies have been found to be very low. Van der Straten found only 30% condom use in a large HIV prevention trial in sub-Saharan Africa.[9] This finding suggests that VCT may be useful in improving condom use but additional interventions may be of value to achieve higher rates.
Risk factors associated with CT/GC were also examined in this study. Different studies have evaluated the factors associated with STI in sub-Saharan Africa. Younger age, presence of other STIs, lower level of education and nulliparous status were found to be associated with CT/GC in pool data from four microbicide trials.[10] In Southern African, Venkatesh et al. in the “Methods for Improving Reproductive Health in Africa” trial found that younger age, not living with primary partner, new sex partners, HSV-2 infection, and recent HIV infection were associated with CT/GC.[11] Concurrent STIs have also been associated with CT/GC in other studies.[7,12]
In our study, significant associations were not found in most of the examined variables and this could be due to the low numbers of women with STIs. We did not find any association with demographic characteristics, concurrent or prior history of STIs. We found an association of alcohol use before sex with CT/GC. Previous studies have suggested that alcohol use in sub-Saharan Africa is associated with HIV infection as well as the behaviors that lead to STIs such as unprotected sex, multiple partnering and commercial sex.[13,14] In our sample, we did not find high rates of recent alcohol use but alcohol prior to sexual intercourse was associated with STI. Our study appears to be the first study to report rates of alcohol and drug use before intercourse in African HIV-positive women and the association with STIs. A case control study in HIV-infected women will further clarify the relationship between sexual risk factors and CT and GC in this population.
This study has several limitations, including having a convenience sample and participant self-report of sexual risk behaviors and STI history.
CONCLUSION
This study reports the rates of STIs among HIV-positive women living in urban Lusaka, Zambia. The rates of CT and GC found in this sample are lower than what has been reported in other studies in females in sub-Saharan Africa and may be as a result of engaging in routine HIV care. The use of alcohol as a factor that may contribute to STIs needs to be further investigated. Further studies addressing the rates of and risk factors for STIs in HIV-positive women in this region need to be conducted. Controlling STIs and identifying factors associated with such diseases continues to be an important element in the design of interventions targeting STIs and as a result, HIV prevention in sub-Saharan Africa.
ACKNOWLEDGMENTS
We thank all those in our research team at the University Teaching Hospital in Lusaka, community sites providing referrals, and the women participating in this study.
The opinions reflected in this report are those of the authors and do not necessarily reflect those of the funding agencies and participating institutions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 2.Zambia Country Report. Multi-sectorial AIDS response monitoring and evaluation biennial report. Zambia Ministry of Health. 2008 [Google Scholar]
- 3.Meyer-Bahlberg HF, Ehrhardt AA, Exner TM, Gruen RS. Sexual risk behavior assessment schedule: Adult. New York: Psychiatric Institute and Columbia University; 1991. (SERBAS-A-DF-4) [Google Scholar]
- 4.Ahoyo AB, Alary M, Ndour M, Labee AC, Ahoussinou C. HIV and sexually transmitted diseases among female sex workers in Benin. Med Trop (Mars) 2009;69:457–62. [PubMed] [Google Scholar]
- 5.Braunstein SL, Ingabire CM, Kestelyn E, Uwizera AU, Mwamarangwe L, Ntirushwa J, et al. High human immunodeficiency virus incidence in a cohort of Rwandan female sex workers. Sex Transm Dis. 2011;38:385–94. doi: 10.1097/olq.0b013e31820b8eba. [DOI] [PubMed] [Google Scholar]
- 6.Msuya SE, Uriyo J, Hussain A, Mbizo E, Jeansson S, Sam N, et al. Prevalence of sexually transmitted infections among pregnant women with known HIV status in northern Tanzania. Reprod Health. 2009;6:4. doi: 10.1186/1742-4755-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthrie BL, Kiarie JN, Morrison S, John-Stewart GC, Kinutthia J, Whittington WL, et al. Sexually transmitted infections among HIV-1 discordant couples. PLoS One. 2009;4:e8276. doi: 10.1371/journal.pone.0008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapina M, Reid C, Roman K, Cyrus-Cameron E, John-Stewart GV, Kwicien A, et al. HIV incidence rates and risk factors for urban women in Zambia: Preparing for a microbicide clinical trial. Sex Transm Dis. 2009;36:129–33. doi: 10.1097/OLQ.0b013e318190191d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Straten A, Cheng H, Chidanyika A, De Bruyn G, Padian N MIRA Team. Vaginal practices and associations with barrier methods and gel use among sub-saharan African women enrolled in an HIV prevention trial. AIDS Behav. 2010;14:590–9. doi: 10.1007/s10461-010-9690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldblum P, Lie C, Weaver M, Van Damme L, Harpern V, Adeiga A, et al. Baseline factors associated with incident HIV and STI in four microbicide trials. Sex Transm Dis. 2010;37:594–601. [PubMed] [Google Scholar]
- 11.Venkatesh T, Vander Straten A, Mayer K, Blanchard K, Ramjee G, Lurie M, et al. African women recently infected with HIV-1 and HSV-2 have increased risk of acquiring Neisseria gonorrhoeae and Chlamydia trachomatis in the methods for improving reproductive health in Africa trial. Sex Transm Dis. 2011;38:562–70. doi: 10.1097/OLQ.0b013e31820a8c2c. [DOI] [PubMed] [Google Scholar]
- 12.Fritz K, Woelk G, Bassett M, McFarland W, Routh J, Tobaiwa O, et al. The association between alcohol use, sexual risk behavior and HIV infection among men attending beer halls in Harare, Zimbabwe. AIDS Behav. 2002;6:221–8. [Google Scholar]
- 13.Simbayi LC, Kalichman SC, Jooste S, Mathiti V, Cain D, Cherry C. Alcohol use and sexual risks for HIV infection among men and women receiving sexually transmitted infection clinic services in Cape Town, South Africa. J Stud Alcohol. 2004;65:434–42. doi: 10.15288/jsa.2004.65.434. [DOI] [PubMed] [Google Scholar]
- 14.Ghebremichael M, Paintsil E, Larsen U. Alcohol abuse, sexual risk behaviors, and sexually transmitted infections in women in Moshi urban district, northern Tanzania. Sex Transm Dis. 2009;36:102–7. doi: 10.1097/OLQ.0b013e31818b20e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


