Abstract
In the present work, Caesalpinia bonduc seed coat extract (CBSCE) has been evaluated for anti-inflammatory and analgesic activity C. bonduc seeds have been attributed with anti-inflammatory and analgesic properties in the folklore medicine. Here in our study, we have tried to carry out the systematic evaluation of the seed coat extract of C. bonduc to substantiate these claims. C. bonduc seed coat was extracted with 95% ethanol and concentrated; further, the extract was screened for anti-inflammatory and analgesic activity. The studies were carried using Carrageenan-induced Paw Edema, Egg albumin-induced paw edema, Eddy's Hot Plate Test, Tail Immersion Method so as to prove acclaimed properties. The data was analyzed statistically by Students’ ‘t’ test. The results indicate that seed coat extract has the ability to decrease the induced inflammation at varied doses in Carrageenan model as well as in the Egg albumin model in rats. The antinociceptive results indicate that the extract has the ability to increase the pain threshold of the animals and reduce the pain factor, thereby inducing analgesia. Thus, it can be concluded that CBSCE posses analgesic and anti-inflammatory activity.
Keywords: Analgesic, anti-inflammatory, Caesalpinia bonduc, tail flick
INTRODUCTION
Caesalpinia bonduc seeds have been used in the folklore medicine since long time. The use of this plant has been quoted in the Ayurvedic and traditional scriptures.[1] Plant is reported to have multiple restorative properties like anthelmintic, antibacterial, antidiuretic and recently it has received considerable attention to treat Diabetes.
C. bonduc F. (family: Caesalpiniaceae) is a large, scandent, prickly shrub found throughout the hotter and southern parts of India. It is a large straggling, thorny shrub, the branches are armed with hooks and straight, hard yellow prickles. The leaves are compound. The flowers are pale yellow in color, in supra-axillary racemes at the top. The fruits are inflated pods, covered with prickles, 6 cm long, and 1-2 seeds per pod. The seeds are globular, hard, bluish grey in color with a smooth shiny surface.
C. bonduc has numerous synonyms like Duhsparsa – difficult to touch, Kantaki karanja – having prickles; Vajra bijaka - having hard seeds, Kanta phala – has fruits covered with prickles, etc. It is attributed to be an aphrodisiac and general tonic helping in the rejuvenation of the body.[2] The roasted seed powder is used as an antileprotic. The seeds are useful as anti-inflammatory,[3] antidiabetic, antiperiodic, antipyretic, etc.[4,5]
The seeds contain various chemical constituents such as furanoditerpene's: a-caesalpin, β-caesalpin, γ-caesalpin, δ-caesalpin, ε-caesalpin, and caesalpin –F; fatty acids: palmitic, stearic, octadeca-4-enoic, octadeca-2, 4-dienoic, lignoceric, oleic and linoleic acids, phytosterinin, b-sitosterol, homoisoflavone bonducellin; amino acids: aspartic acid, arginine, and citrulline; carbohydrates: starch and sucrose; β-carotene, glycoside-bonducin, gums, and resins.[6]
The seeds of C. bonduc have been attributed with anti-inflammatory properties in the folklore medicine. The seed oil and the kernel extracts have been screened for anti-inflammatory and antipyretic activity. The terpenoidal and steroidal moieties have been reported from the seed kernels, which may be responsible for the claimed attributes. Till date, there has been no systematic study carried on the seed coat of C. bonduc to substantiate these claims.
EXPERIMENTAL
Plant Material
Seeds of C. bonduc were collected from vicinity of Kannur village, Dist. Bijapur, Karnataka, India. Herbarium was preserved and authenticated by Dr. P. S. N. Rao, Deputy Director, Botanical Survey of India, Pune, India (Specimen Voucher No: DMK-281002).
Preparation of the Extract
The plant material was dried and seeds were broken so as to separate the kernel and the seed coat. The seed coat was coarsely powdered and extracted with 95% ethanol in a Soxhlet extractor; further, the extract was filtered and concentrated on Rota-evaporator,[7] to get a sticky reddish brown extract (C. bonduc seed coat extract [CBSCE]) (yield 12.3% w/w).
Animals
The animal use protocol was approved by the SCES’ Indira College of Pharmacy, Pune, India (Animal Eths Comm /1265/ac/09/CPCSEA), and was in accordance with international standard on the care and use of experimental animals. Healthy adult Swiss albino mice weighing between 30 and 40 g were used for antinociceptive studies and Wistar albino rats weighing between 120 and 140 g of either sex were used for the anti-inflammatory studies. Animals were housed under standard conditions and were fed ad-libitum with commercial pellet diet and had free access to water.
Extract and Standard Drugs
For each model, the animals were divided into four groups of six animals each, the Ist group served as Control (Distilled water and 1% CMC), IInd and IIIrd group animals were treated with CBSCE (200 and 400 mg/kg p.o.), respectively, the IVth group served as standard where Indomethacin (10 mg/kg b.w)[8] and Pentazocine (10 mg/kg b.w)[9,10] were administered by the intraperitoneal route and were used as a standard drugs in the anti-inflammatory activity and analgesic activity, respectively.
Screening of Anti-Inflammatory Activity
Carrageenan-induced paw edema in rats
In the Carrageenan-induced Paw Edema,[11–13] all the animals received their respective doses at fixed time once daily for 5 days except for the IVth group where the standard drug was injected one hour prior to the administration of carrageenan. On the fifth day, one hour after the drug administration, 0.1 ml Carrageenan (1%) suspended in normal saline was injected into the sub plantar region of the right hind paw.
The paw was immediately immersed in the Plethysmometer[14] up to the tibiotarsal articulation and the paw volume was measured,[15] which served as reading for 0 hr. The readings were taken similarly and paw volumes were measured after every 60-minute interval for next four hours. The average paw swelling in the groups of the CBSCE-treated animals were compared with control and that of the Standard group. Mean increase in paw volume was determined and expressed as mean paw displacement volume in ml.
The % inhibition was calculated at the end of fourth hour by the formula[16]
I = 100 [1-(a-x)/ (b-y)]
Where, I is the % inhibition, “a” is the mean volume at time = t of the test group, “x” is the mean volume at time = 0 of the test group, and “b” is the mean volume at time = t of the control group, “y” is the mean volume at time = 0 of the control group.
Egg albumin-induced paw edema
All the animals received their respective doses at a fixed time once daily for 5 days except for the IVth group where the standard drug was injected one hour prior to the administration of albumin. One hour after treatment, edema was induced by injecting fresh egg albumin (0.1 ml, 1% w/v)[17,18] solution in distilled water subcutaneously in the plantar surface of the right hind paw of all animals. The paw edema was measured at 30-minute interval at start and later on every hour from 1st to 4th hour by Plethysmometer.
The % inhibition was calculated at the end of fourth hour same as above.
Screening of Analgesic Activity
Eddy's hot plate test
Analgesic activity of the extract and Pentazocine was determined by Eddy's Hot Plate Test. In all the groups, the basal reaction time was recorded by observing hind paw licking or jump response in animals, when placed on a hot-plate[18] maintained at constant temperature (55°C). Normally, animals show such response in 6-8 seconds. A cut-off period of 15 seconds was observed to avoid damage to paws. The reaction time of animals on hot plate was recorded at 0, 30, 60, 90, and 120 minutes after the drug administration. Percentage increase in basal reaction time (index of analgesia) at each time interval was calculated.
Tail immersion method
The basal reaction time was recorded by observing tail flick response[19] in animals when tail is immersed in water maintained at constant temperature (55°C). Normally, animals show such response in 3 to 5 seconds. A cut-off period of 10 seconds was observed to avoid tissue damage. In all the four groups, the reaction time of animals in hot water was recorded at 0, 30, 60, 90, and 120 minutes after the drug administration. Percentage increase in basal reaction time – flicking/removal of tail (index of analgesia) at each time interval was calculated.
Statistical Analysis
Comparison between control and drug-treated groups was made by Students’ ‘t’ test.
RESULTS
Anti-Inflammatory Activity
Carrageenan-induced paw edema in rats
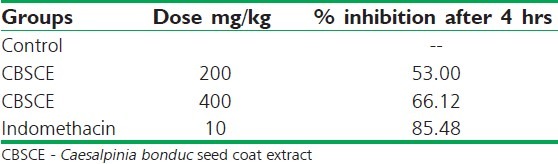
In the control group, all the animals exhibited severe edema formation which lasted for more than 4 hours. It was observed that CBSCE extract at both the doses, i.e., 200 mg/kg as well as 400 mg/kg reduced the inflammation in all the animals [Figure 1]. The results clearly indicate that CBSCE extract at a dose of 400 mg/kg was more effective and significant in controlling the inflammation which is clearly reflected in the % reduction in inflammation table [Table 1]. The CBSCE 400 animals exhibited reduction in inflammation after the 180-minute interval. In comparison to negative control, Standard drug Indomethacin exhibited highly significant action in controlling the edema and severely restricting the inflammation since the onset of inflammation. The % reduction of inflammation in both the groups at 240 minutes was highly significant.
Figure 1.
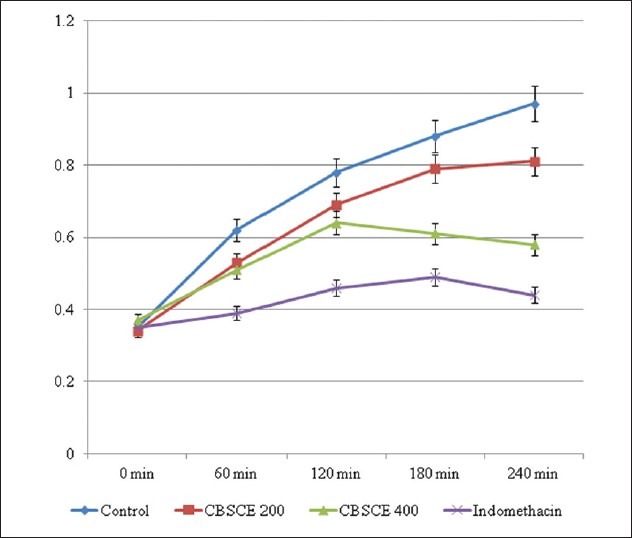
Effect of CBSCE extract on Carrageenan-induced Paw Edema in Rats Mean paw displacement volume in ml
Table 1.
Percentage inhibition of inflammation after 4 hours in carrageenan-induced inflammation
Egg albumin-induced acute inflammation
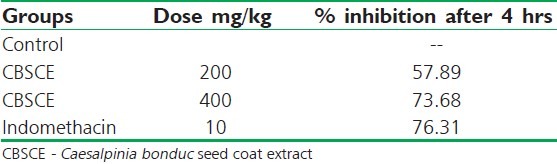
In the case of egg albumin-induced paw edema, the untreated control group exhibited severe edema in all the animals. The CBSCE extract possesses the ability to control acute inflammation [Figure 2] at both the doses. After a span of 120 minutes, it shows reversal and the edema decreases thereafter. CBSCE 400 extract is highly efficient in controlling the initial inflammatory response as compared to CBSCE 200 extract which is clearly reflected in the % reduction in inflammation table [Table 2]. The standard drug (Indomethacin) is highly significant in controlling the inflammation too. The onset of action in the case of the standard drug was within the first 30 minutes, whereas the extract exhibits delayed onset of action after 60 minutes.
Figure 2.
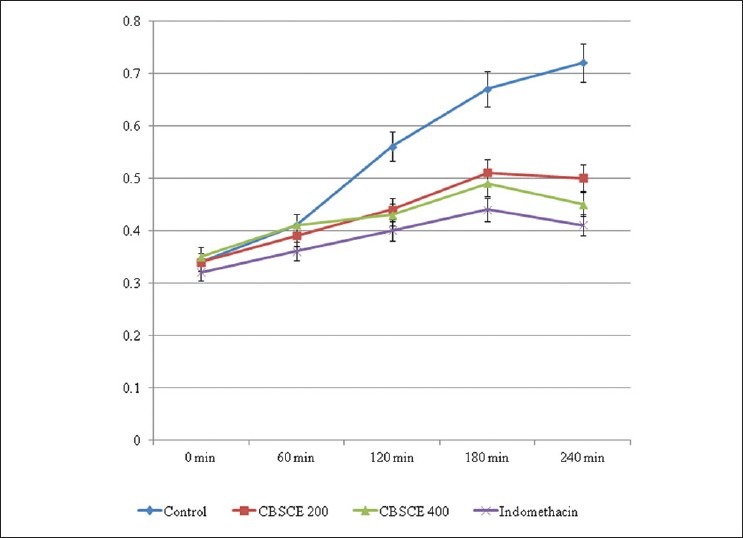
Egg Albumin-induced Acute Inflammation Mean Paw displacement volume in ml
Table 2.
Percentage inhibition of inflammation after 4 hours in egg albumin-induced inflammation
Analgesic Activity
Eddy's hot plate method
Antinociceptive activity of the extract at both the doses and Pentazocine was determined by Eddy's Hot Plate Test [Figure 3]. The basal reaction time was recorded by observing hind paw licking or jump response. The extract at both the doses exhibited significant analgesic action at 400 mg/kg; the reflex time was enhanced in comparison to 200 mg/ kg. Here too, the extract-treated groups showed delayed onset of action in comparison to the standard drug. The standard drug Pentazocine exhibited the most significant pain-controlling action. The increase in the basal reaction time duration was also high in the standard treated groups in comparison to the extract treated and the control animals.
Figure 3.
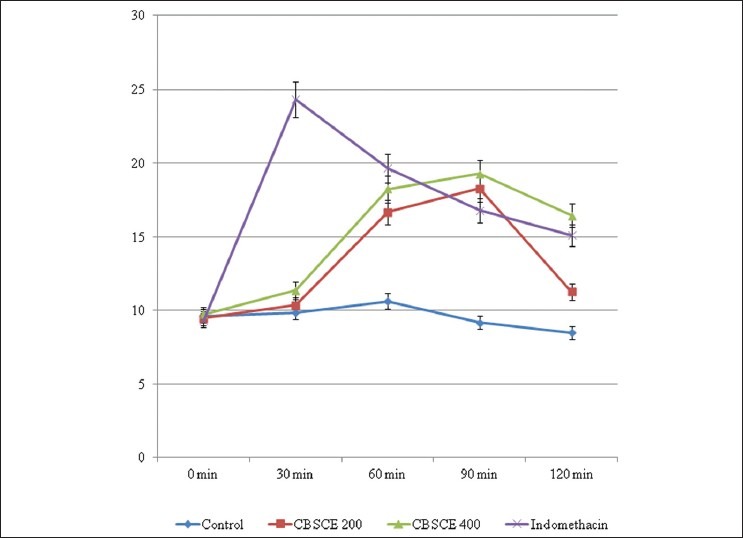
Antinociceptive activity by Eddy's Hot Plate Method Hind paw licking time in seconds at different time intervals
Tail immersion method
The basal reaction time was recorded by observing tail flick response in animals when tail is immersed in water maintained at constant temperature (55°C). CBSCE 200 and CBSCE 400 extracts exhibited a comparatively better analgesic action in comparison to untreated animals [Figure 4]; there was a gradual increase in the basal reaction time, Pentazocine showed the onset of action at 30 minutes, whereas the onset of action in the extract was observed after 60 minutes. In extract-treated animals, it was noted that the analgesia duration was prolonged in comparison to the standard drug. This could be due to delayed absorption and greater retention time.
Figure 4.
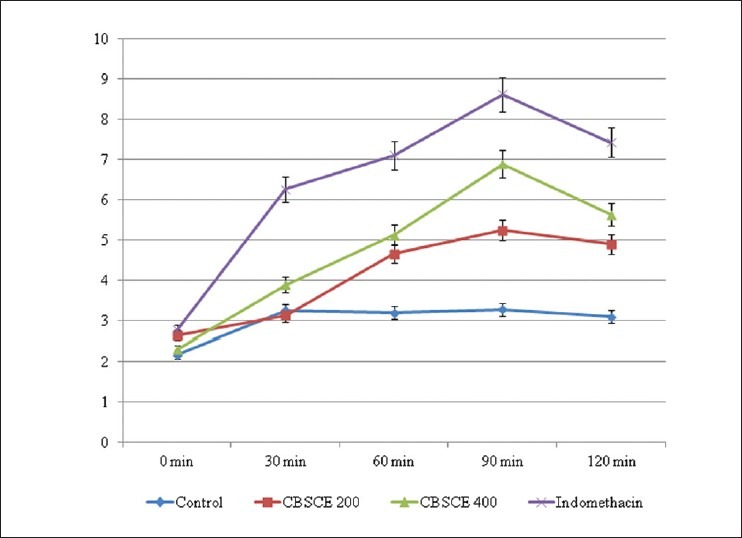
Basal Reaction Time in Tail Flick Method Tail Flick time in seconds at different time intervals
DISCUSSION
The C. bonduc seeds have been used in the household remedies for the analgesic and anti-inflammatory action. Even the seeds have been administered to infants in a pediatric traditional formulation known as “GUTTI.” The earlier studies have proven the significance of C. bonduc seed extracts as strong herbal agent possessing various pharmacological actions. It has been proven that the seed kernels posses anti-inflammatory action. The free radical-scavenging action too has been carried out.[20] The present study demonstrates that oral administration of CBSCE at varied doses produces consistent antinociceptive and anti-inflammatory effects in different models of pain and inflammation. The above results clearly indicate that C. bonduc seed coat posses anti-inflammatory action as claimed in folklore medicine. Though anti-inflammatory activity of C. bonduc seed kernels has been proven, this work is novel in proving the anti-inflammatory efficacy of the seed coat. The above results substantiate the claims that CBSCE posses significant anti-inflammatory action. Here in our study, it is observed that the inflammation reaches its maximum approximately at 3-hour interval post-treatment, after which it begins to decline. The late phase, which is a complement-dependent reaction, could be due to overproduction of prostaglandin in tissues.[21]
The central antinociceptive activity of the extract was evaluated by the hot-plate test as it is very effective and particularly is highly sensitive to strong analgesics and causes limited tissue damage. Our study clearly indicates that CBSCE enhances the tolerance to heat and increases the animal's reaction time, which reflects good analgesic action against the hot plate. In the case of the Tail flick method, the basal reaction time is increased with CBSCE-treated animals. The extract of the C. bonduc seed coat produces analgesia at both the doses and acts as an analgesic agent too. The activity exhibited by the extract of the seed coat can be attributed to the presence of triterpenoidal moieties in the seed coat extract. From the above results in both the models, we suggest that CBSCE has strong peripheral antinociceptive activity.
CONCLUSION
The above results confirm that C. bonduc seed coat possesses significant anti-inflammatory and analgesic activity. Our studies hereby confirm the traditional claims that seeds of C. bonduc possess anti-inflammatory as well as analgesic activity.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Kannur DM. Nuts & Seeds in Health and Disease Prevention. London: Academic Press Elsevier; 2011. Antidiabetic and antihyperlipidemic activity of bonducella (Caesalpinia bonducella) seeds; pp. 237–44. [Google Scholar]
- 2.Paranjpe P. Indian medicinal plants - forgotten healers. Delhi: Chaukhamba Sanskrit Pratishthan; 2005. p. 157. [Google Scholar]
- 3.Datte JY, Traore A, Offoumou AM, Ziegler A. Effects of leaf extract of Caesalpinia bonduc (Caesalpiniaceae) on the contractile activity of uterine smooth muscle of pregnant rats. J Ethnopharmacol. 1998;60:149–55. doi: 10.1016/s0378-8741(97)00144-x. [DOI] [PubMed] [Google Scholar]
- 4.Kannur DM, Hukkeri VI, Akki KS. Antidiabetic activity of Caesalpinia bonducella seed extracts in rats. Fitoterapia. 2006;77:546–9. doi: 10.1016/j.fitote.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Chunekar KC, Pandey GS. Bhavprakash Nighantu (Indian Materia Medica) of Sri Bhavmisra. Varanasi: Chaukhamba Bharati Academy; 2002. p. 417. [Google Scholar]
- 6.Williamson EM. Major Herbs of Ayurveda. India: The Dabur Research foundation & Dabur Ayurved limited; 2002. p. 83. [Google Scholar]
- 7.Kannur DM, Hukkeri VI, Akki KS. Adaptogenic activity of Caesalpinia bonduc seed extracts in rats. J Ethnopharmacol. 2006;108:327–31. doi: 10.1016/j.jep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Sagar MK, Upadhyaya K. Anti-inflammatory activity of root of Dalbergia sissoo (Roxb.) in carrageenan-induced paw edema in rats. Phcog J. 2010;2:427–30. [Google Scholar]
- 9.Maria EM, Luciane FB, Eniana CV, F’abio SM, Patricia DF. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J Ethnopharmacol. 2005;102:377–81. doi: 10.1016/j.jep.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Nonato FR, Barros TA, Lucchese AM, Oliveira CE, dos Santos RR, Soares MB, et al. Antiinflammatory and antinociceptive activities of Blechnum occidentale L. extract. J Ethnopharmacol. 2009;125:102–7. doi: 10.1016/j.jep.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Young J, Pil Y, Hee DK, Hee EH, Chul CY, Hwan RS, et al. Inhibitory effect of the Saponins derived from roots of Platycodon grandiflorum on Carrageenan-induced inflammation. Biosci Biotechnol Biochem. 2006;70:858–64. doi: 10.1271/bbb.70.858. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian A, Ramalingam K, Krishnan S, Christina AJ. Antiinflammatory activity of Morus indica Linn. IJPT. 2005;4:13–5. [Google Scholar]
- 13.Ratheesh M, Helen A. Anti inflammatory activity of Ruta graveolens Linn on carrageenan induced paw edema in Wistar male rats. Afr J Biotechnol. 2007;10:1209–11. [Google Scholar]
- 14.Gupta GD, Gaud RS. Anti-inflammatory activity of Tenoxicam gel on carrageenan-induced paw oedema in rats. Indian J Pharm Sci. 2006;68:356–9. [Google Scholar]
- 15.Borgi W, Ghedira K, Chouchane N. Antiinflammatory activity and analgesic activities of Zizyphus lotus root barks. Fitoterapia. 2007;78:16–9. doi: 10.1016/j.fitote.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Turner RA. Screening methods in Pharmacology. New York: Academic Press; 1965. p. 163. [Google Scholar]
- 17.Karthikeyan M, Deepa MK. Anti-inflammatory activity of Premna corymbosa (Burm.f.) Rottl. & Willd. leaves extracts in Wistar albino rats. Asian Pac J Trop Med. 2011;4:510–3. doi: 10.1016/S1995-7645(11)60136-3. [DOI] [PubMed] [Google Scholar]
- 18.Harer SL, Harer PS. Evaluation of analgesic and anti-inflammatory activity of Ficus racemosa Linn. Stem Bark Extract in Rats and Mice. Phcog J. 2010;2:65–70. [Google Scholar]
- 19.Harisha CR, Ashok BK, Rabinarayan A, Shukla VJ, Ravishankar B. Anti-inflammatory and Analgesic activities of root and stem of Cissus rependa vahl. Phcog J. 2011;2:49–53. [Google Scholar]
- 20.Jana K, Chatterjee K, Kazi MA, Ghosh A, Bera TK, Ghosh D. Antioxidant potential of hydro methanolic extract of seed of Caesalpinia bonduc: An In-vitro study. J Adv Pharm Technol Res. 2011;2:260–5. doi: 10.4103/2231-4040.90884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang X, Jinhui W, Maoxing L, Xiaolou M, Hu P, Yang Y, et al. Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia. 2011;82:716–20. doi: 10.1016/j.fitote.2011.03.001. [DOI] [PubMed] [Google Scholar]


