Abstract
The starch was isolated from jackfruit seeds and evaluated for its preformulation properties, like tapped density, bulk density, and particle size. The fourier transform infrared (FTIR) analysis was done and compared with that of the commercially available starch which confirmed the properties. Using the various concentrations of jackfruit seed starch, the microspheres were prepared, combining with gelatin by ionotropic gelation technique. The developed microspheres were subjected to analysis of particle size, drug content, entrapment efficiency, and percentage yield. The spectral analysis confirmed the presence of drug and absence of interactions. Scanning electron microscope image showed that the particles were in spherical shape with a rough surface. The in vitro drug release in water for 12 hours proved to be in the range of 89 to 100%. The various kinetic models were applied using release data to confirm the mechanism of drug. It was concluded that the jackfruit starch-gelatin microspheres gave satisfactory results and met pharmacopieal limits.
Keywords: FTIR, ionotropic gelation technique, jackfruit seed starch, microsphere
INTRODUCTION
Jackfruit (Artocarpus heterophyllus Lam) is a popular fruit crop that is widely grown in Thailand and Southern parts of India. The ripe fruit contains well flavored yellow sweet bulbs and seeds. Seeds have high carbohydrates and protein content. Starch is widely used as a food additive and has very good laxative activity. Alginate is a complex polysaccharide whose composition varies on the basis of the proportion of its monomeric units namely, mannuronic acid and glucuronic acid. It is widely used for drug dilution system.[1–4]
Guaifenesin is an expectorant which enhances the output of respiratory tract fluid by reducing the adhesiveness and surface tension. It also enhances the removal of viscous mucus, which helps in making the non-productive coughs more productive and less frequent. Moreover, this is also used to reduce chest congestion, caused by common cold, infections, or allergies.
Microspheres are micron-sized particulate systems, made up of polymeric and other protective materials such as biodegradable synthetic polymers and modified starches, gums, proteins, fats, and waxes. The drug materials can be incorporated within the microspheres in liquid or solid state. Microspheres possess large surface to volume ratio due to the fact that they are of micron size. Each particle is basically a matrix of drug dispersed in a polymer or protein from which release occurs by first order process and drug release is controlled by dissolution or degradation of matrix. The drug molecules are dispersed in solution or crystalline form. Biodegradable materials have the potential for controlled release of drug.[5–7] The aim of this study is develop the sustained release microbeads of highly metabolized and short half-life drug (1 hour) guaifenesin which helps to increase the more bioavailability and stability of drug in biological fluids.
MATERIALS AND METHODS
Materials
Guaifenesin was obtained as a gift sample from Granules India, Hyderabad. Jackfruit starch was isolated from jackfruit seeds, which were procured from local market, Thanjavur, Tamilnadu, India (Kamaraj vegetable market). Sodium alginate and calcium chloride were obtained from Loba Chemie Pvt. Ltd, Chennai, India, and all other reagents were of analytical grade.
Isolation of Starch from Jackfruit Seeds
The main objectives of the isolation of starch from seeds of jackfruit were to asses and identify the properties and activity of starch. Starch isolation from flour was carried out following the basic procedure; the seeds collected from the market were shade dried and the brown skin layer was removed then made into flour using electrical mixer. The ground flour was mixed with 3 parts of distilled water and made into slurry. The slurry was filtered through 70 mesh sieve to eliminate seed fibers and debris. The starch suspension was allowed to settle and liquid was decanted at less than 10°C using ice cooled condition. This step was repeated several times until the supernatant was clear. The starch was dried in a conventional oven at 40°C to 60°C until the moisture content was less than 13%, then ground with a mortar and pestle and passed through 70-mesh sieve to get uniform and free-flowing powder. The isolated starch was stored in an air-tight container at room temperature until use.[8]
Formulation of Starch-Gelatin Complex Microspheres
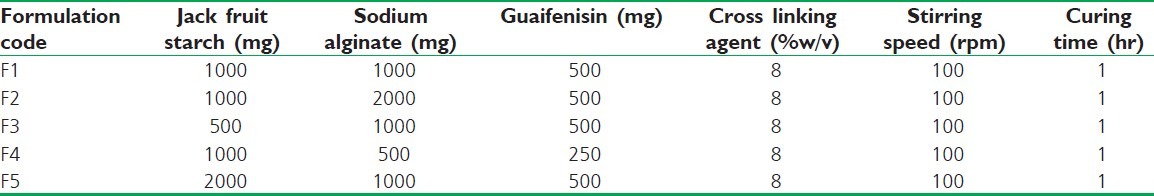
The starch-gelatin complex microspheres were formulated by using well-established ionotropic gelation technique[9,10] to entrap Guaifenesin due to its simplicity, low cost, high yield, and its success with poorly soluble drugs and the production of microspheres. The microspheres were prepared using different concentrations of starch (500 mg, 1 000 mg, and 2 000 mg), drug (250 mg and 500 mg), and sodium alginate (500 mg, 1 000 mg, and 2 000 mg). Various concentrations of starch-sodium alginate complex solutions were prepared by mixing sodium alginate in water to get proper mixing and distribution. Guaifenesin was added to the above mixture and dispersed thoroughly with initial stirring and sonicated for 5 minutes in bath sonicator. The drug polymer dispersion was added manually drop wise with a syringe (gauge size 28) into 100 ml of calcium chloride (8%) solution by stirring with a magnetic stirrer, maintaining the approximately same height and pressure. Gelation time of 1 hour was allowed to complete the curing reaction to produce spherical and rigid microspheres. The spheres so obtained were collected by decantation, washed with distilled water, and dried for 24 hours at room temperature, as shown in Table 1.
Table 1.
Composition variables and processing parameters of microspheres of Guaifenesin
EVALUATION TECHNIQUES
Particle Size Analysis
Uniformity of particle size is important for uniform distribution of drug and maintaining the drug release in plasma. The particle size was measured using optical microscope (Khera, New Delhi, India). It is the most important direct technique for the measurement of size and size distribution. 100 mg of microspheres were mounted on the glass slide and measured using calibrated eye piece micrometer and the size and distribution were calculated and tabulated.
Hydration Studies
Swelling of the particles was studied by observing the water or moisture from the environment. The mass of the spheres were found to be directly proportional to the rate of water uptake. 100 mg of microspheres were accurately weighed and placed in 100-ml beakers, containing water at 37°C. At regular time intervals, the diameter (size) and weight of the microspheres were measured using vernier caliper and digital electronic balance (BL-220H, Shimadzu Corporation, Japan). The mean and standard deviation was calculated and tabulated.[11,12]
Drug Content
100 mg of dried microspheres were accurately weighed and crushed using a mortar and pestle and transferred to 100-ml volumetric flask containing water. From the above solution, suitable dilutions were made and the absorbance was measured spectrophotometrically at λmax 273 nm using UV-VIS spectrophotometer (Perkin-Elmer Lifesciences, USA) and the drug content was calculated from standard calibration curve.
Entrapment Efficiency
About 100 mg of accurately weighed drug-loaded microspheres were weighed and were crushed in a glass mortar and pestle and added to 100 ml water. The resulting mixture was stirred well. From the stock solution, suitable dilutions were made and analyzed spectrophotometrically at 273 nm using Perkin-Elmer Life Sciences, USA, UV-VIS spectrophotometer. The drug entrapment efficiency was calculated using the formula:[13]
Entrapment Efficiency (% EE) = [(Practical drug content) / (Theoretical drug content)] ×100
In vitro Release Study
The drug release behaviors of the microspheres were evaluated in water. The basket method was used to conduct the dissolution tests. The basket position was set at 2.5 cm from the bottom of the flask and the speed was adjusted to 100 rpm. The in vitro dissolution studies were carried out in 900 ml of water maintained at 37 ± 0.5°C and microspheres containing 100 mg of drug were placed in each case.[14,15] Aliquots of 10 ml sample were withdrawn at predetermined time intervals up to 12 hours and immediately replaced with 10 ml of fresh and warm dissolution medium, to maintain a constant volume of 900 ml, for achieving sink condition. The samples were filtered using Whatman No 1 filter paper and the absorbance was determined at 273 nm using a Perkin-Elmer Life Sciences (USA) UV-VIS spectrophotometer against water as blank.[15]
Scanning Electron Microscopy
The surface topography of microparticles was examined using scanning electron microscope [Jeol, JSM-6360, (Japan, 15 kV)]. Microspheres were freeze dried and mounted on a screw-shaped tube using double-sided carbon adhesive tape. Samples were coated with platinum in an argon atmosphere under vacuum condition by using ion sputter chamber for making it conductive in nature and were examined at 15 000 V accelerating voltage.[16]
Fourier Transform Infra-Red Spectroscopy
A KBr pellet is prepared by grinding the solid sample, which is previously dried and moisture free, with solid Potassium bromide (KBr), and pellets were prepared on KBr-press under hydraulic pressure of 150 kg/cm2, applying high pressure to the dry mixture. KBr is chosen because of the fact that it is transparent to infrared radiation. Spectra were scanned over the wave number range of 3600 to 400 cm at ambient temperature.[11]
RESULTS AND DISCUSSION
Guaifenesin microspheres with varying proportions of Guaifenesin, polymer, and cross-linked starch were prepared by ionotropic gelation method. While dispersing the mixture of sodium alginate, drug, and starch into the calcium chloride solution, the microspheres are formed by the mechanism of gelation which occurs due to the electrostatic interaction between the positively charged calcium ion and negatively charged sodium alginate ions.[17,18] Hence, as the concentration of the sodium alginate increase, there will be increase in the rigidity of the microspheres.
The effect of various formulation parameters are shown in Table 1. Guaifenesin microspheres were found to be in the size range of 45 to 90 μm. Guaifenesin loading amount, stirring speed, curing time, polymer concentration, and cross linking agent seem to influence the particle size.
Description of the Microspheres
Stage microscopy showed that the microspheres obtained were spherical and free flowing [Figure 1a]. The yield obtained in all the batches was good which was above 75% and none of the variables affected the yield. It indicated that Guaifenesin microspheres had a discrete spherical structure without aggregation.
Figure 1.

Microscopic images of starch-sodium alginate Microspheres of optimized formulation (F2). a) Optical microscopy, b) Scanning Electron microscopy
Particle Size Analysis
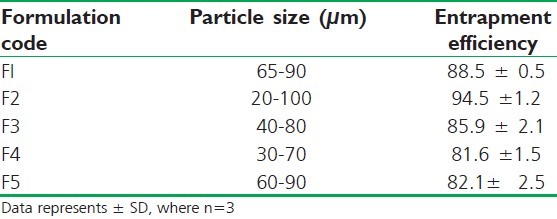
All particles were found to be in micrometer range, i.e., from 20 to 100 μm. There is no much difference in particle size by changing the composition in the formulations and the size distribution was found to be even and uniform; it helps in the content uniformity of drug [Table 2].
Table 2.
Particle size and entrapment efficiency of starch-sodium alginate microspheres
Percentage Entrapment Efficiency (% EE)
The EE was found to be in the range of 81.6 ± 1.5 to 94.5 ± 1.2. These results show that this was good entrapment efficiency. F2 formulation containing 1 part of starch and 2 parts of sodium alginate shows the maximum EE and formulations F4 and F5 show least EE. It reveals that by increasing the concentration of starch, the entrapment of the drug get reduces [Table 2].
Hydration Studies
The uptake studies were carried out to assess the maximum uptake of water by starch-sodium alginate microspheres using swelling by size and weight method. Particle size was found to be increased after swelling. After 3 days, there was no significant change in the size of the particles. Swelling might be due to starch which absorbs water. After 3days because of saturation of starch, there was no significant change in the particle size [Figures 2 and 3].
Figure 2.
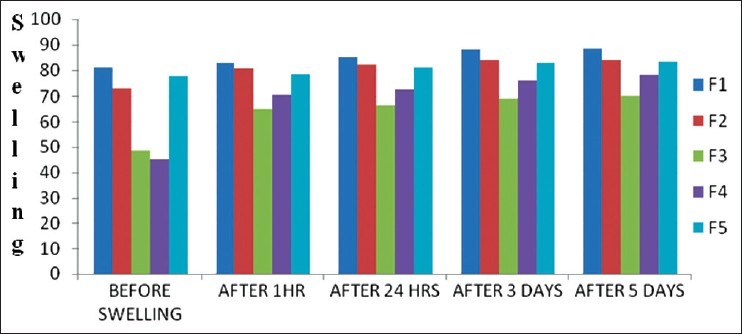
Determinations of water uptake of microspheres by particle size mode (μm)
Figure 3.
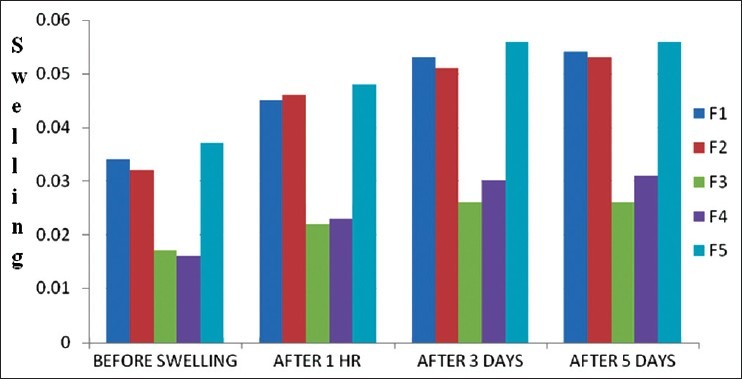
Determinations of water uptake of microspheres by particle Weight (mg)
In vitro Drug Release
The in vitro drug release of starch-alginate microspheres was conducted in 900 ml of water as dissolution media for 12 hours [Figure 4]. The drug release was found to be in the range of 63 to 97% at the end of study. Formulation F1 shows the less drug release and F5 shows maximum drug release. This may be due to the influences of starch in the formulations. Formulation F1 contains the equal amount of starch and F5 contains lesser than sodium alginate, it may be due to the reason that starch swells and dissolves faster than the alginate. It was supported by the swelling studies.
Figure 4.
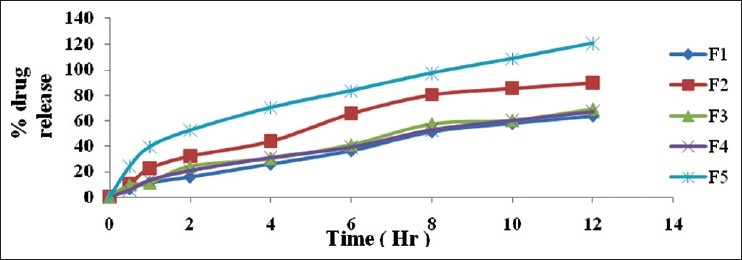
Comparative in vitro drug release studies of Starch-sodium alginate complex microspheres in water
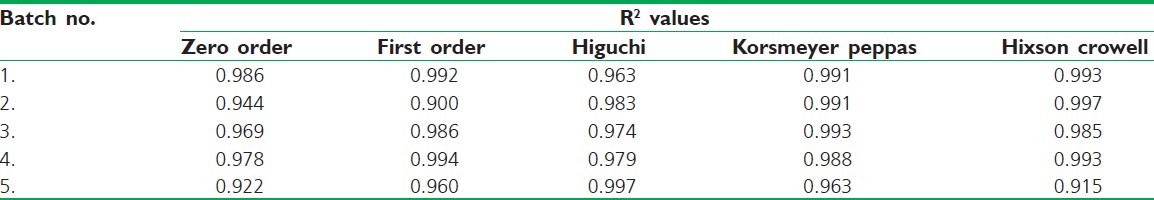
Release Kinetics
The release kinetics of the above data reveals that all the formulations obey Hixson Crowell kinetic model. It shows that the drug release is based on non-Fickian diffusion mechanism. This release mechanism proves the influence of both starch and sodium alginate for sustained drug release Table 3.
Table 3.
Release kinetics of in-vitro drug release data of different batches starch-sodium alginate microspheres
Microscopic Evaluations
The microspheres were examined under Scanning Electron Microscopy (SEM) to observe surface morphology of optimized formulation [Figure 1b]. Microspheres were found to be spherical with rough texture. Small fibers were visible on the surface, which might be due to the presence of starch. Optimized formulation was observed through optical microscope [Figure 1a]. The microspheres were found to be round and in the size range of 10 to 120 μm.
Fourier Transform Infra-Red Spectroscopy
Peaks of Fourier Transform Infra-Red Spectroscopy of formulation (F2) were observed compared with pure drug and starch IR peaks [Figure 5a–c]. It reveals that there is bond formation between the drug and starch-sodium alginate complexes; it confirms the entrapment of drug into the mixture. There is mild shift in the wave number from 3241.87 to 3432.65, 2941.00 to 2921.39, and 1594.37 to 1635.01; this may be due to the bond formation. The finger print region looks shorter in the peaks heights and disappearance of some of the peaks compared with pure drug may be due to the masking of drug by the mixture of starch and sodium-alginate complex. The peaks shown in pure drug at wave number 2928.29/cm, 1455.5/cm, 1256.49 cm, and 1021.77/cm shows –CH–OH stretching vibration, -CH scissoring and bending (of alkane compound), aryl-O stretching, and -C-O stretching, respectively.[16] The peaks of starch at 2 941/cm due to –CH–OH stretching vibration, 1 645.69/cm shows -C=C- stretching, 1 242.12/cm, 1 155.59/cm, and 1 018.92 cm shows -C-O stretching for three peaks, and 710.76/cm shows –C-H bend, respectively. These peaks were also seen in the formulation with slight shifts which shows that the formulation had significant characteristics of starch and the drug.
Figure 5.
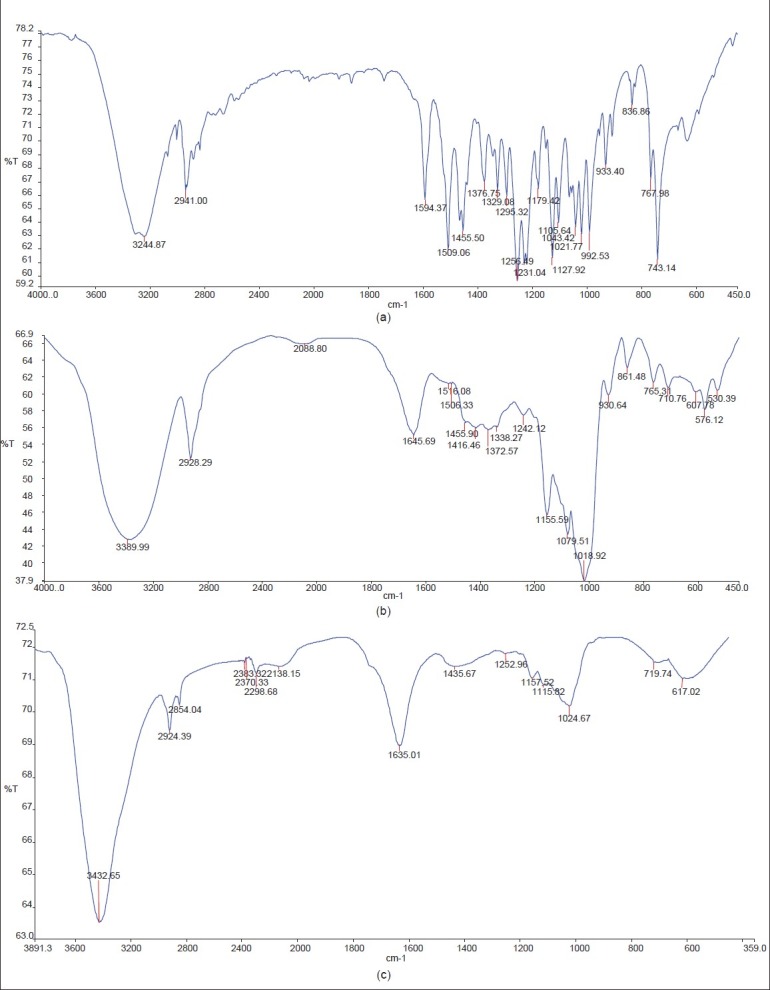
FT-IR Spectroscopy images of a) Pure drug, b) starch, c) Formulation F2
CONCLUSION
The microspheres were successfully prepared by using ionotropic gelation technique using starch and sodium alginate. From this study, it can be concluded that the drug polymer ratio and stirring speed are important for obtaining desired spherical particles. The yield was found to be high in all the formulations. The release rates depend upon the amount of drug and polymer used. Therefore, from these studies, it reveals that the prepared polymer system could be used in the drug delivery studies in future.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Jha AK, Bhattacharya A. Preparation and evaluation of sweet potato starch-blended sodium alginate microbeads. Asian J Pharma. 2009;4:299–302. [Google Scholar]
- 2.Thombre NA, Chaudhari MR, Kadam SS. Preparation and characterization of rofecoxib microspheres using cross-linked starch as novel drug delivery system. Int J PharmTech Rese. 2009;1:1394–402. [Google Scholar]
- 3.Babitha S, Soccol CR, Pandey A. Jackfruit seed for production of monascus pigments. Food Technol Biotechnol. 2006;44:465–71. doi: 10.1016/j.biortech.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Mukprasirt A. Kamontip Sajjaanantakul. Int J Food Sci Technol. 2004;39:271–6. [Google Scholar]
- 5.Rajkumar M, bhise SB. Carbamazepine loaded porous microspheres for short term sustained drug delivery. J Young Pharmacist. 2010;18:7–14. doi: 10.4103/0975-1483.62206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdi G, Pnchel G, Dunchene D. An original method for studying in-vitro the enzymatic degradation of cross-linked starch microsphere. J Control Release. 1998;55:193–201. doi: 10.1016/s0168-3659(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 7.Mani N, Park MO, Jun HW. Effects of formulation variables and characterization of guaifenesin wax microspheres for controlled release. Pharm Dev Technol. 2005;10:71–83. doi: 10.1081/pdt-49658. [DOI] [PubMed] [Google Scholar]
- 8.Tulyathan V, Tananuwong K, Songjinda P, Jaiboon N. Some Physicochemical Properties of Jackfruit (Artocarpus heterophyllus Lam) Seed Flour and Starch. Sci Asia. 2002;28:37–41. [Google Scholar]
- 9.Vedha HB, Brahma RA, Samyuktha RB. Floating drug delivery of Nevirapine as a gastro retentive system. J Young Pharm. 2010;2:350–5. doi: 10.4103/0975-1483.71622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S, Budhiraja RD. Chitosan-alginate microcapsules of amoxicillin for gastric stability and mucoadhesion. J Adv Pharm Tech Res. 2012;3:68–74. doi: 10.4103/2231-4040.93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathiyaraj S, Devi RD, Hari VB. Lornoxicam gastro retentive floating matrix tablets: Design and in vitro evaluation. J Adv Pharm Tech Res. 2011;2:156–62. doi: 10.4103/2231-4040.85531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangeetha S, Sakthisaravanan V, Komala M, Harish G, Sivakumar V. Design and evaluation of gastroretentive beads of theophylline By Ionotropic Gelation. Int J Pharm Pharm Sci. 2010;2:99–101. [Google Scholar]
- 13.Yadav A, Jain DK. In-vitro characterization of gastroretentive microballoons prepared by the emulsion solvent diffusion method. J Adv Pharm Tech Res. 2010;1:56–67. [PMC free article] [PubMed] [Google Scholar]
- 14.Sathiya Sundar R, Murugesan A, Venkatesan P, Manavalan R. Formulation development and evaluation of carprofen Microspheres. Int J PharmTech Res. 2010;2:1674–6. [Google Scholar]
- 15. U.S. Pharmacopoeia, pharmacopeial forum, United States Pharmacopeial Co, Vol 30. P. 107, USP29-NF24, p 1030.
- 16.Samyuktha R, Vedha HB. Niosomal Formulation of orlistat: formulation and in-vitro evaluation. Int J drug dev res. 2011;3:300–11. [Google Scholar]
- 17.Nayak AK, Pal D. development of pH-sensitive tamarind seed polysaccharide–alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int J Biol macromol. 2011;49:784–93. doi: 10.1016/j.ijbiomac.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Veerareddy PR, Tedla S, Banda SR, Bandari S, Jukanti R. Preparation and evaluation of mucoadhesive cefdinir microcapsules. J Adv Pharm Tech Res. 2011;2:115–20. doi: 10.4103/2231-4040.82955. [DOI] [PMC free article] [PubMed] [Google Scholar]


