Abstract
From the centuries, herbal medicines are used to treat various diseases and now they had become an item of global importance, with both medicinal and economic implications. The demand of herbal medicine is being increasing day by day due to their safety and efficacy. Now herbals had taken over the allopathic system due to their less side effect and efficient working mechanism. Herbals are playing and pivotal role in increasing the economy of the country and had taken the nation on to the new path to achieve the goal of development. Lygodium flexuosum (Linn) Sw. is a fern found nearly throughout India up to an elevation of 1500 meter. It belongs to the family Lygodiaceae and widely used in treating various ailments like jaundice, dysmenorrhea, wound healing and eczema. It is the rich source of alkaloids, flavonoids, saponins and cumarin. The main constitute of the plant is lygodinolide which is mainly used in wound healing. In the present review an attempt had been made to explore different aspects of L. flexuosum.
Keywords: Alkaloids, Lygodium flexuosum, lygodinolide, lygodiaceae
INTRODUCTION
Disease, decay and death have always coexisted with life, the study of disease and their treatment must also have been contemporaneous with the dawn of human intellect. The primitive man must have used plants as therapeutically active agents for curing various diseases. There is no authentic record of medicines used by the primitive man but the Rig Veda which is the oldest book in the library supplies curious information on this subject.[1] Nature has provided a complete remedy to cure all ailments of mankind. The history of herbal medicines is as old as human civilization.[2] Herbal medicines have often retained popularity for historical and cultural ingredients and are used primarily for treating mild and chronic ailments. They are considered to be medicinal if they possess pharmacological activities of possible therapeutic use. India has an ancient heritage of traditional medicines; Materia Medica of India provides lots of information on the folklore practices and traditional aspects of therapeutically important natural products. An Indian traditional medicine is based on various system including Ayurveda, Siddha and Unani. Natural products and especially those derived from higher plants have historically played a pivotal role in the discovery of new pharmaceuticals.[3] Modern allopathic system has developed many sophisticated and costly diagnostic methodologies which at the times have made it quite exorbitant and beyond the reach of common man. Many modern synthetic drugs may harm more than they help in curing diseases by its serious effects. On contrary, traditional medicines which make use of plants are much more esteemed being more safe without harmful effects and comparatively less expensive than many allopathic medicines.[4] Undoubtedly, the plant kingdom still holds many species of plants containing substances of medicinal value which have yet to be discovered.[5]
BOTANICAL DESCRIPTION
Large terrestrial climbing ferns are common everywhere on low lands in fairly open places. The dwarfed branches are up to 3-mm long.[6] It is slightly pubsent, substerete, flattened on one side, narrowly winged or tetragonous.[7] Rhizomes are short, creeping, underground, dichotomously branched, young regions clothed by stiff brown hairs and producing a single row of climbing leaves.[6] Rhizomatous perennial fern, with climbing rachis has up to 2.5-mm thickness. Primary rachis branches are not elongated. Secondary rachis branches are bearing alternate pinnately arranged leaflets, usually 3 or 4 (occasionally 5 or 6) on each side and a simple or forked terminal leaflet, the whole being 15-30 cm long. Basal leaflets often with large basal lobes, sometimes with two or three separate leaflets at the base. Largest leaflets 4-12 × 1-2.5 cm on hairy winged stalks 3-8-mm long, broadly rounded to cordate at the base and more or less distinctly jointed to the end of the stalk, narrowed gradually to the apex, edges of sterile leaflets finely toothed, texture thin but firm.[8–11] Rachis and petiole 0.1 inch or rarely more thick and several feet long, the rachis bearing short primary branches ending in a tuft of hairs, each primary branch bearing a pair of secondary branches which in their turn bear 3-4 alternately arranged pinnules and a simple or forked terminal pinnule. Pinnules 1-4-inch long and 0.25-1.0-inch broad, distinctly stalked, with the stalk articulated to the lamina, the base of the lamina rounded and gradually tapering to apex and with dichotomising free veins. The basal pinnules are with large basal lobes or even palmate. Fertile pinnules narrower than sterile ones and fringed by short narrow lobes about 0.32-inch long. Each lobe bears two lateral rows of sporangia attached individually to a short vein and covered by a marginal scale like indusium.[6] It is parallelogram and exine bearing compact leasurae (plate).[12] Lygodium fexuosum is extremely variable in the size and lobing of the sterile and sporogenous pinnules as well as the length of the sporogenous lobes. Occasionally the lower parts of the secondary pinnae are bipinnate.[13] Each lobe of the sporophore is interpreted as a reduced pinnule and contains a single sporangium enclosed by a flange [Figures 1 and 2].[14]
Figure 1.

Leaf of Lygodium flexuosum
Figure 2.

Single leaf of Lygodium flexuosum
Shoot morphology
The leaves of climbing fern Lygodium are born on dorsal surface of a subterranean rhizome and twining growth to form the aerial portion of the shoot. These leaves have a number of structural and functional analogies to the entire shoot of some twini. The determinate primary leaves and the inderterminate climbing leaves initiate from a single cell on the flank of the apical meristem and are strictly foliar in the nature, structurally homologues with each other and with leaves of the fern. Shoot of Lygodium is an example of the phenomenon of leaf elaboration common to many ferns. The complex nature of the leaves and simple morphology of the stem indicate specialization of function within the shoot rather than retention of primitive, poorly differentiated organographies.[15]
The root apex organization in L. flexuosum
It was fixed in formalin-acetic-alcohol and acetic-alcohol washed with water and preserved in 70% alcohol for study. Customary method of dehydration and embedding were followed. The initiation and further development of the root primordium has been studied in L. flexuosum. A cell in the growing region of the rhizome apex enlarges showing pyramidal appearance and produces cells laterally on all its faces. It appears that the surrounding cells in the expanding regions of the rhizome apex arrange themselves around the pyramidal cell and its derivatives and divide to form the developing root, primordium is almost next to the rhizome apex. A central pyramidal cell with four well-defined faces and number of surrounding cells usually in two tiers are seen. The procambial tissue differentiates between this group and the differentiating vascular tissue of the rhizome. A mature root apex is some what obtuse and is protected by a conical cap. Endodermis is double layered. A conspicuous tetrahedral cell is observed at the central region of the apex. In lygodium, the apical cells divide during early development of the root but no divisions were seen subsequently.[16] In Lygodium spores, a proximal cell formed by an initial division of the spore cut off a protonemal cell, a rhizoid and a wedge shaped cell by walls parallel to the polar axis.[17]
Effect of Gibberellic acid on the ultrastructural morphology of the cells of L. flexuosum
The effect of Gibberellic acid (GA) on the ultrastructure of chloroplast and the content of chlorophyll was seen in the cells of dwarf maize. Spores of Lygodium flexuosum were sown in two separate petridishes, each having approximately 500 spores in 1% knop's solution. After 5-6 days when germination takes place 2 mg/ml of GA solution was added to one of the petridishes and allowed to grow for 6 days then the cells of GA treated gametophyte were undertaken and it has been observed that the gametophyte, endoplasmic reticulum increases and the cytoplasm gets densely filled up with it. Ribosomes, golgi bodies, mitochondria and chloroplast also increases in size.[18]
SCIENTIFIC CLASSIFICATION
Scientific classification[19] of L. flexuosum is presented in Table 1.
Table 1.
Scientific classification

DISTRIBUTIONAL RANGE
Asia temperate: China, Guangdon, Guangxi, Guizhou, Hainan, Yunnan.
Asia tropical: Indian subcontinent (India, Srilanka), Indo-China (Indonesia, Thailand), Malaysia (Indonesia, Malaysia, Papua New Guinea, Phillippines).
Australia: Northern territory, Queensland, Western Australia [Figure 3].[9]
Figure 3.
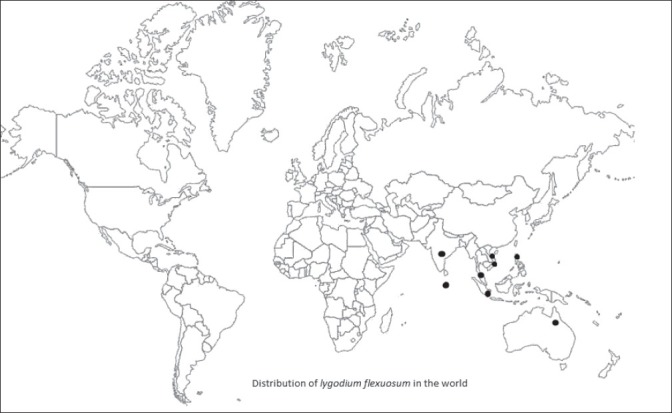
Distribution of lygodium flexuosum in the world
In India it is found in Dehradun, Kumaon, Shahjanpur, Gorakhpur, throughout the plains in Bengal up to 5000 feet, both the sides of Madras state up to 4000 feet and Kerela.[20] It is also found at low altitudes (below 1000 m) especially common in bhabar forest. In the forest around Dehradun Dryopteris cochleata with dimorphicfronds is concipious near Rishikesh and Dehradun. L. flexuosum is growing very well in the forest of Gumaniwala and Clemantown on the forest floor and Neelkhanth road near Barrage Rishikesh. In Madhya Pradesh it is found in Annupur, Bastar, Betul, Bilaspur, Chhindwara, Damoh, Gwalior, Hoshangabad, Indore, Khandwa, Mandla, Raigarh, Raipur, Sidhi, Shivpuri and Panna.[21,22,23]
ECOLOGY
L. flexuosum is the sole genus in the family Lygodiaceae, though it is included in the family Schizaceae by some botanist.[23] It occurs on mangrove and had tree-dominated habitat subdivision is petridophyte and had life form of cryptophytes category.[24,25] It is commonly epiphytically grows on moss-covered tree trunks, branches a lithophytes on shady boulders along with moss and in Thailand its habitat is in abundance.[26]
ETHNOPHARMACOLOGY
From the past decades this plant is used as an expectorant. Fresh roots are boiled with mustard oil and used in external applications for rheumatism, sprains, scabies, eczema and cut wounds, they are reported to be particularly useful for carbuncles. Stems may be used for tying rice sheaves.[20,27] L. flexuosum is an important medicinal plant as some of the scholars of Indian System of Medicine reported that the plant may be ‘Rudra Jata’, an intermediate drug in classical text of Ayurveda and its medicinal properties have been reported from all the parts of the plant. The rhizome and root is ethnomedicinally useful in the treatment of jaundice. The leaf paste is applied all over the body for 7 days to cure jaundice by Kadar tribes of South Western Ghats of India. The root is used in jaundice and stomach pain by Rabha, Oraon and Mech tribes in Jalpaiguri district of West Bengal, India. L. flexuosum extract had antiproliferative and apoptotic activity in both cancer cells. L. flexuosum n-hexane extracts which is responsible for the possible hepatoprotective action.[28–30] This fern reported to exhibit antiferility activity.[31,32] In China it is used as an expectorant. Bidi made of root is smoked. Fresh root is boiled with mustard oil are used for massage, powder of whole plant is taken.[33,34] Extract of the rhizome of L. flexuosum in India is used to cure gonorrhea. The ash of plant is used for treating herpes this plant is used to feed domestic animals to treat foot and mouth diseases. During the time of scarcity, Chitwan people use their knowledge of wild plants to obtain vegetable for sustenance they collect tender plant and its parts from the common land. This fern is used as fodder and forage resources of common land in Western Chitwan.[35,36] Regarding the conservation aspects Lodhas of West Bengal believe bhut raj (L. flexuosum) to be adobe of gods.[37] There are 60 to 79 plant species growing in the plantation and out of these 70% are palatable and can be used as forages for the rearing of cattle. Palatable species are those that can be termed as grazable by the cattle and L. flexuosum is the fern which is palatable.[38–40] it is used in the manufacturing of basket, hats, bags and other fancy articles.[41] It is also used to reduce inflammation and acts as panacea for wounds, treat ulcer, various respiratory diseases, general disorders, muscles sprains and it also had the potential to act as the pain killer. It also causes U- to-C-RNA editing and sometimes exceeds the conventional RNA editing.[42–44]
PHYTOCHEMICAL STUDIES
Identification of antheridiogens in L. flexuosum
Antheridiogens were found in two species of Lygodiaceae family that is L. flexuosum and Lygodium circinnatum fern these were analyzed by gas chromatography-mass spectrometry (GC-MS). In L. flexuosum, GA73-Me was also identified as a major antheridiogen with X2 being detected as a minor one. The total antheridium formation activity in the culture medium of 7-week old prothallia of L. flexuosum was more than 1000 times higher than that of Lygodium japonicum. On the other hand the response of gametophytes of the former two Lygodium ferns to GA73-Me was more than 100 times lower than that of L. japonicum.[45]
Biosynthesis of GA73 methyl ester in Lygodium ferns
It is a principal antheridiogens in Lygodium ferns was investegated from the methanol extract of prothallia of Lygodium ferns GA25, GA73, GA73-Me, GA88-Me and a few unknown GA73 derivatives were detected by GC-MS. Because the presence of GA25 suggests that GA24, a direct precursor of GA25 may also be present in Lygodium ferns and the feeding experiments were used to investigate the possibility that GA24 is a precursor of GA73-Me. In L. flexuosum ferns [2H2] GA24 was converted into [2H2] GA73-Me and a trace amount of [2H2] GA9-Me, whereas [2H3] GA9 was converted into [2H3] GA9-Me and [2H3] monohydroxy derivatives and had been identified by GC-MS from the culture medium of Lygodium ferns and the results suggest that GA73-Me is biosynthesized from GA24 via GA73 their neither GA9 or GA9-Me, though the possibilities also suggest that GA73-Me is biosynthesized from 9,15- cyclo-GA9(GA103), [2H2]GA103 was not converted into [2H2] GA73-Me.[46,47]
L. flexuosum is a rich source alkaloidal constituent it also constitute of flavanoids, saponins and cumarins. A new triterpene ester, an anthraquinone has been found in this plant. Lygodinolide was isolated from the methanolic extract of L. flexuosum. It has been found to contain a new compound characterized as O-P-coumaryl dryocrassol, besides drycrassol itself, tectoquinone, kaempferol, kaempferol-3-β-D-glucoside, β-sitosterol and stigmasterol [Figure 4]. In Lygodiaceae ferns, antheridiogens were reported to be GA-related compounds. Antheridic acid is a major antheridiogen in four Anemia ferns. The new heterocyclic spiro-terpenoid niveulone which is found to be similar to the compound lygodinolide and niveulone was isolated from the cultural fluid of the ascomycete Dasyscyphus niveus and its chemical structure and relative configuration were determined by spectroscopic techniques.[48–51]
Figure 4(a-h).
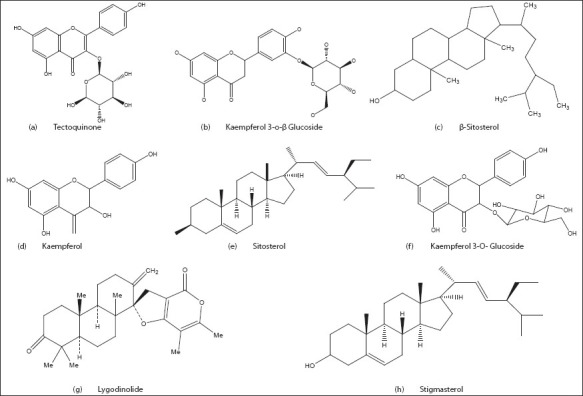
Structures of the chemical compounds found in Lygodium flexuosum
PHARMACOLOGICAL PROPERTIES
Hepatoprotective effects
Rat buated with extract of Lygodium flexuosum after establishment of carbon tetrachloride induces liver injury showed significantly protection of the liver as evidence by AST, ALT, LDH and MDA Levels. Phytochemical study yielded saponins, triterpenes, sterol and bitter principles which could explain the possible hepatoprotective actions. Hepatic glutathione levels were significantly increased by the treatment with the extract in both the experimental groups. Histopathological changes induced by CCl4 were also significantly reduced by the extract treatment in preventive and curative groups. Treatment with the n-hexane extract reduced the mRNA levels of proinflammatory cytokinens, growth factor and other signaling molecules which are involved in hepatic fibrosis. The expression levels of tumor necrosis factor, interleukin, transforming growth factor, procollagen-I, procollagen-III and the tissue inhibitor of metalloproteinase-I were elevated during CCl4 administration and reduced the levels to normal by the treatment with extract treatment. D-galactosamine-induced liver injury showed complete protection of liver as evidenced from normal AST, ALT and LDH levels, hepatic GSH and MDA levels and also by normal histological index of liver in treated rats.[24] Rats pretreated with L. flexuosum prevent the elevation of serum AST, ALT, LDH and liver lipid peroxides in ccl4 treated rats which show it is responsible for hepatoprotective action.[28,30]
Antiploriferative apoptotic effect
L. flexuosum extract has antiproliferative and apopotic activity in cancer cells and has inhibitory role in TNF-α induces NF-α B activation in PLC/PRF/5 cells confirming it potential as a chemopreventive agents. L. flexuosum extract inhibited the cell viability and induced apoptosis in hepatoma cells in a concentration-dependent manner as evidenced by apoptotic changes such as flipping of phosphatidly serine, cleavage of PARP. Cell cycle analysis showed the sub-G1 apoptotic population in cells treated with higher concentration of the extract. When activated with exogenous TNF-α in transfected hepatoma cells it was observed that NF-κB-dependent gene expression was inhibited by treatment with L. flexuosum extract in PCL/PRF/5 cells dose-dependently.[29]
Antifertility activity
The alcoholic extract of the L. flexuosum (Linn.) Sw. used by a tribal population in Maharashtra showed antifertility activity in rats, mice and rabbits.[31,32]
Antibacterial activity of L. flexuosum
The study was conducted to evaluate the antibacterial potential of different parts of L. flexuosum. Antibacterial activity evaluated by disc diffusion method toward MTCC strains. It was observed that the rhizome of the plant possessed maximum antibacterial activity compared to petiole and leaf. Rhizome extract were found to be more effective against Gram-positive bacteria like M. luteus and S. aureus compared to Gram negative. Rhizome extract possessed antibacterial principles soluble in methanol and acetone that could regulate growth and multiplication of tested bacteria species.[52,53]
Antiogenic effect of L. flexuosum against N-nitrosodiethylamine-induced hepatotoxicty in rats
Antiogenic effect of L. flexuosum was evaluated in wistar rats intoxicated with N-nitrosodiethylamine (NDEA) in preventive and curative models. In preventive groups, NDEA was administered for 20 weeks. Rats intoxicated with NDEA had elevated the levels of serum gamma-GT, AST, ALT, LDH and hepatic MDA and decreased the level hepatic GSH. L. flexuosum extract treatment significantly reduced the levels of Ang-1 and Ang-2 and Tie-2 mRNA in rat liver is evidenced by RT-PCR. L. flexuosum extract at a dose of 200 mg/kg effectively reversed the hepatotoxicity induced by N-nitrosodiethlyamine in both preventive and curative model.[54]
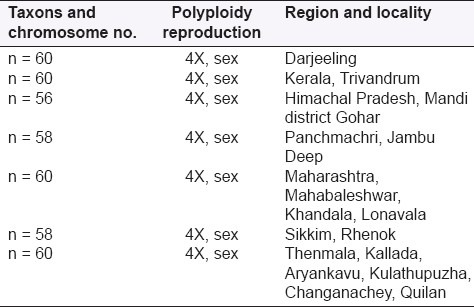
TAXONS AND CHROMOSOME NUMBER
Taxons and chromosome[55] numbers are presented in Table 2.
Table 2.
Taxons and chromosomes number
FEDERAL REGULATION
Lygodium genus is considered as weeds: L. circinnatum, L. flexuosum, L. japonicum, L. microphyllum and L. polymorphum. So the federal regulation had amended the noxious weed regulation by adding L. flexuosum to the list of terrestrial noxious weeds this is necessary to prevent the artificial spreading of this weed into the United States. Fertile Lygodium leaves contain reproductive structures filled with spores that can become wind borne and spread the fern into uninfested areas. Florida currently has a lygodium management plan, which was released in 2006, and an active control program in place. The states of Alabama, North Carolina, South Carolina and Vermont, incorporate the federal noxious weed list by reference into their State noxious weed lists and included L. flexuosum as state noxious weeds. L. flexuosum is a vine-like fern that spreads by rhizomes and by climbing over other lowland vegetation. L. flexuosum are noxious weeds that posed a serious threat to U.S. agriculture and the environment. Accordingly, APHIS issued a Federal Import Quarantine Order on May 30 (2008), that immediately restricted the importation from all countries of any part of L. flexuosum capable of propagation, including nursery stock, spores and leaves (fronds), unless authorized by a PPQ permit for specified research in containment. This interim rule is intended to codify provisions of the existing federal order that prevent the introduction into or spread of L. flexuosum within the United States. L. flexuosum is a weed of rice, plantation crops and natural lowland vegetation in eastern Asia and it is not known to occur in the United States. This robust species spreads by rhizomes and by climbing over other vegetation. Like, it has the potential to cause serious environmental and economic damage in the southernmost areas of the United States.[56] Plant protection and quarantine is issuing a federal import quarantine orders restricting the importation of L. flexuosum. The restriction applies to any parts capable of propagation, including spores and leaves (fronds) of these climbing ferns. The purpose and goal of their federal regulation is to prevent the entry from all foreign countries into the united states of two harmful noxious weeds L. fexuosum and L. microphllum. This federal order is issued pursuant to section 412(a) of the plant protection act of June 20 (2000), as amended 7 U.S.C 7712(a), which authorizes the secretary of agriculture to prohibit or restrict the importation or entry of any plant, plant part, noxious weed or articles if the secretary determines that the prohibition or restriction is necessary to prevent the entry of a plant pest or noxious weed into united states.[57–61] In the United States L. flexuosum comes under the category of 15 highest ranking species showing the greatest invasive potential.[62]
Newly described moth attacks invasive ferns
The search of natural enemies of an invasive weed that threatens Florida's wetlands has led to a stem boring moth that attacks ferns the moth, Siamusotima aranea and was found in Thailand in stems of a native fern L. flexuosum by USDA-ARS scientist. It was discovered in the stems of L. lexuosum during the exploration for biological control agents. In South Africa lygodium ferns were known as an ancient group of ferns. The paucity of natural enemies associated with lygodium. Larva and pupa on the stem-boring pyralid have been collected from L. flexuosum in Thailand. Adults of the Thai moth have white eggs with dark lines resembling the legs of a spider, which it may mimic. Taxonomic studies of the moth, tentatively called Ambia new species. Neostrombaceous albicomus is the herbivores collected on Lygodium species in Malaysia, Thailand, Singapore and Vietnam.[63–65]
Types of colonization by Arbuscular mycorrhizal fungi
Types of colonization[66,67] is presented in Table 3.
Table 3.
Types of colonization by Arbuscular mycorrhizal fungi

CONCLUSIONS
In the present review we have made an attempt to congregate the botanical, pharmacological, ethno- pharmacological, pharmacological and toxicological information on L. flexuosum, a medicinal herb used in the Indian system of medicine. Survey of literature revealed the presence of lygodinolide, alkaloids, cumarins, flavonoids and saponins in this plant. Research on lygodinolde has gained a special attention in recent times as several of them have shown promising activities like antifertility, wound healing, eczema and hepatoprotective, but most of the compounds are too toxic to be clinically used when taken in large amount. This plant is also used in bidi and the bidi made of root is smoked. Fresh roots are boiled with mustard oils and used for massage. In lygodiaceae ferns, antheridiogens were reported to be GA-related compounds. Antheridic acid is a major antheridiogen in four Anemia ferns. It is used to cure ulcer, eczema, cut and wound. It is also used in treating ailments like dysmennorrhea, jaundice, sprains and rheumatism. Now days, this had proven to be one of the most useful drug in the treatment of wounds. It consist of chemical constitutes like tectoquinone, kaempferol, kaempferol-3-β-D-glucoside, β-sitosterol and stigmasterol. It has been found to contain a new compound characterized as O-P-coumaryl dryocrassol, besides drycrassol itself. L. flexuosum is an important medicinal plant as some of the scholars of Indian System of Medicine reported that the plant may be ‘Rudra Jata’, an intermediate drug in classical text of Ayurveda and its medicinal properties have been reported from all the parts of the plant. L. flexuosum extract had antiproliferative and apoptotic activity in both cancer cells. L. flexuosum n-hexane extracts which is responsible for the possible hepatoprotective action. This review will definitely help for the researchers as well as practitioners, dealing with this plant.
ACKNOWLEDGMENT
Authors are thankful to the Prof R. M. Dubey, Vice Chancellor, IFTM University, Moradabad (UP), for providing necessary facilities and their constant encouragement during entire study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kirtikar KR, Basu BD. Indian Medicinal Plants. Dehradun: National Book Distributions; 2005. p. 1. [Google Scholar]
- 2.Kokate CK, Purhoit AP, Gokhale SB. Text book of Pharmacognosy. 36th ed. Pune: New Delhi: Nirali Prakashan; 2006. p. 1. [Google Scholar]
- 3.Mukherjee Pulok K. Quality control of herbal drugs. Kolkata: Business Horizons Pharmaceutical; 2002. p. 2. (39, 98). [Google Scholar]
- 4.Rangari VD. Pharmacognosy and Phytochemistry. 2nd ed. Nashik: Career Publications; 2008. p. 7. [Google Scholar]
- 5.Evans WC. Saunders. 14th ed. Singapore: Harcourt Brace Asia; 1997. Text book of Pharmacognosy; p. 329. (298-9). [Google Scholar]
- 6.Medicinal ferns of India by National botanic gardens. Lucknow, India. Bulletin no. 29. 1959:4–5. [Google Scholar]
- 7.Federal noxious weed disseminules of the U.S. [Last accessed on 2011]. Available from: http://itp.lucidcentral.org/id/fnw/key/FNW_Seeds/Media/Html/fact_sheets/Lygodium_fl exuosum.htm .
- 8.Bayer AG. In: Important crops of the world and their weeds. Leverkusen, Bayer AG, editors. Vol. 2. Weinheim (Federal Republic of Germany): VCH Verlagsgesellschaft mbH; 1992. p. 1682. [Google Scholar]
- 9.USDA. Germplasm information network (GRIN) ARS National genetic resources program. [Last accessed on 2011 Feb 2]. Available from: http://www.ars-grin.gov/npgs/searchgrin.html .
- 10.Barnes DE. Common weeds of Malaysia and their control. Kuala Lumpur, Malaysia: Ancom Berhad; 1992. p. 349. [Google Scholar]
- 11.Missouri Botanic Garden, W3TROPICOS database. [Last accessed on 2000]. Available from: http://mobot.mobot.org?w3t/Search/vast.html .
- 12.Perumal G. Ethanomedical use of petridophyte from kolli hills, namakkal district, Tamil Nadu, India. Ethnobotanical Leaflets. 2014;14:161–72. [Google Scholar]
- 13.Flora of Australia online. Lygodium flexuosum (L.) Sw., J. Bot. (Schrader) 1800(2): 106 (1801) [Last accessed on 2011 Feb 2]. Available from: http://www.anbg.gov.au/abrs/online-resources/fl ora/stddisplay.xsql?pnid=56755 .
- 14.Manchester SR, Zavada MS. Lygodium foliage with intact sorophores from the ecocene of Wyoming. J Bot Gaz. 1987;148:392–9. [Google Scholar]
- 15.Muller RJ. Shoot morphology of the climbing fern Lygodium (Schizaeaceae), General organography, leaf, initiation and branching. J Bot Gaz. 1982;143:319–30. [Google Scholar]
- 16.Bhambie S, Rao CGP. Studies in Pteridophytes IX. Root apex organisation in some pteridophytes. Proc Indian Nail Sci Acad. 1972;39B(2):150–156. [Google Scholar]
- 17.Raghavan V, Clark HS. A comparative study of cell division patterns during germination of spores of Anemia, Lygodium and Mohria. J Am Bot. 1980;7:655–63. [Google Scholar]
- 18.Effect of Gibberellic acid on the ultra structure morphology of the cells of Lygodium flexuosum(L.) Sw. Letter to the editor. 1977;46:85. [Google Scholar]
- 19.Vietnam plant data centre. [Last accessed on 2011]. Available from: http://www.botanyvn.com/cnt.asp?param=edir&v=Lygodium%20fl exuosum&list=species&lg=en .
- 20.New Delhi: CSSIR; Anonymous, “The Wealth of India, Raw Materials”, Publication and Information Directorate; pp. 199–200. [Google Scholar]
- 21.Joshi P, Pande HC, Pande PC. “Ferns of central Himalayas –I (Chamoli and Rudraprayag) 2002:201–4. [Google Scholar]
- 22.Singh S, Dixit RD, Sahu TR. Ethanomedical uses of petridophytes of Amarkantak, Madhya Pradesh. J Indian Tradit Knowl. 2005;4:392–5. [Google Scholar]
- 23.Lygodium information, article and encyclopedia resource at ireferenceca. [Last accessed on 2011]. Available from: http://www.ireference.ca/search/Lygodium/
- 24.Wills M, Zerbe S, Breitung W. Habitat survey, mapping and assessment in the Mai Po nature reserve. Hong Kong (China): Archiv Natur Lands; 2006. pp. 53–69. [Google Scholar]
- 25.Manhas RK, Mukesh GK, Deepa K. Plant diversity of a fresh water swamps of Doon valley, India. J Am Sci. 2009;5:1–7. [Google Scholar]
- 26.Boonkerd T. Pertidophyte flora of thong Pha Phum National Park, Kanchan aburi province, Thailand. J Nat Hist Chulalaongkorn Univ. 2006;6:17–30. [Google Scholar]
- 27.Chopra RN, Nayar SL. Glossary of Indian medicinal plants. Vol. 1. New Delhi: NISCAIR Press; 2006. p. 158. [Google Scholar]
- 28.Wills PJ, Asha VV. Protective effect of Lygodium fl exuosum (L.) Sw. (Lygodiaceae) against D-galactosamine induced liver injury in rats. J Ethnopharmacol. 2006;108:116–23. doi: 10.1016/j.jep.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Wills PJ, Asha VV. Chemopreventive action of Lygodium flexuosum extracts in human hepatoma PLC/PRF/5 and Hep 3B cells. J Ethnopharmacol. 2009;122:294–303. doi: 10.1016/j.jep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Wills PJ, Asha VV. Protective effect of Lygodium fl exuosum (L.) Sw. extract against carbon tetrachloride- induced acute liver injury in rats. J Ethnopharmacol. 2006;108:320–6. doi: 10.1016/j.jep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Gaitonde BB, Mahajan RT. Antifertility activity of Lygodium flexuosum. Indian J Med Res. 1980;12:597–604. [PubMed] [Google Scholar]
- 32.Mahajan RT, Gaitonde BB, Bade VB. Preliminary Studies on Antifertility activity of L Lygodium flexuosum in Albino Rats. Bull Haffkine. 1979;3:12–5. [Google Scholar]
- 33.Banerjee RD, Sen SP. Antibiotic activity of petridophytes. J Econ Bot. 1980;34:284–98. [Google Scholar]
- 34.Kamble SY, Patil SR, Sawant PS. Studies on plants used in traditional medicine by Bhilla tribe of Maharashtra. J Indian Tradit Knowl. 2010;9:591–8. [Google Scholar]
- 35.Samant SS, Pant S. Diversity, distribution pattern and conservation status of the plant used in liver diseases, ailments in Indian Himalayan region. J Mt Sci. 2006;3:28–47. [Google Scholar]
- 36.Dangol DR. Traditional uses of plants of common land and habitat in western Chitwan, Nepal. J Inst Agric Anim Sci. 2008;29:71–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Jain SK, Kapoor SC. Divine botany universal and useful but under explored traditions. J Indian Tradit Knowl. 2007;6:534–9. [Google Scholar]
- 38.Pride D. Symbiosis: Cattle rearing in oil palm plantations. Palm Oil Truth Foundation. [Last accessed on 2011]. Available from: http://www.palmoiltruthfoundation.com .
- 39.Jalaludin S, Halim RA. Development of the livestock industry in Malaysia, regional workshop on area wide integration of crop livestock activites. Bangkok, Thailand: FAO regional office; 1998. [Google Scholar]
- 40.Latif J, Mamat MN. A financial study of cattle integration in palm oil plantation. Oil Palm Inds Econ J. 2002;2:34–44. [Google Scholar]
- 41.Bayani NS. Non-Wood Forest Products in Asia-Philippines. FAO corporate document repository, Forestry Department, Philippines. [Last accessed on 2011]. Available from: http://www.fao.org/docrep/X5334e/x5334e09.htm .
- 42.Shrivastava K. Importance of ferns in human medicines. Ethnobotanical Leaflets. 2007;11:231–4. [Google Scholar]
- 43.Baltrushes N. Senior Honor's Thesis. California, Berkely: Department of Integrative Biology; 2005. Medical ethanobotany phytochemistry and bioactivity of the ferns of Moorea French Polynesia. [Google Scholar]
- 44.Volkmar U. Exploring non-coding mitochondrial DNA sequence in bryophytes molecular evolution. Dissertation, submitted to Rheinischen Friedrich-Wilhelms-Universität Bonn. 2010 [Google Scholar]
- 45.Yamauchi T, Oyama N, Yamane H, Murofushi N, Schraudolf H, Pour M, et al. Identification of antheridiogens in lygodium flexuosum and circinnatum. Plant Physiol. 1996;111:741–5. doi: 10.1104/pp.111.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamauchi T, Oyama N, Yamane H, Murofushi N, Schraudolf H, Pour M, et al. Biosynthesis of GA73 methyl ester in lygodium ferns. Plant Physiol. 1997;113:773–8. doi: 10.1104/pp.113.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamane H. Fern Antheridiogens. Int Rev Cytol. 1998;184:1–6. [Google Scholar]
- 48.Achari B, Basu K, Saha CR, Prakashi SC. A New Triterpene Ester, an Anthraquinone and Other Constituents of the Fern Lygodium flexuosum. J Planta Med. 1986;52:329–30. doi: 10.1055/s-2007-969169. [DOI] [PubMed] [Google Scholar]
- 49.Basudev A. Structure of Lygodinolide: A novel spiro furan prehydro phenanthrene derivatives from Lygodium flexuosum. J Org Chem. 1990;55:4977–8. [Google Scholar]
- 50.Rao S. Pharmacognostical studies on Lygodium flexuosum. J Pharm Res. 2010;3:1976–8. [Google Scholar]
- 51.Rojas de la Parra V, Mierau V, Anke T, Sterner O. Niveulone, a hetrocyclic spiro terpenoid from th ascomycete dasyscyphus niveus. J Antibiot (Tokyo) 2006;59:57–60. [Google Scholar]
- 52.Anishmon VS, Toji T. In vitro antibacterial activity of Lygodium flexuosum. Nig J Nat Prod Med. 2005;9:46–7. [Google Scholar]
- 53.Banerjee RD, Sen SP. Antibiotic activity of petridophytes. Econ Bot. 1980;34:284–98. [Google Scholar]
- 54.Wills PJ, Suresh V, Arun M, Asha VV. Antiangiogenic effect of Lygodium flexuosum against N-nitrosodiethylamine-induced hepatotoxicity in rats. Chem Biol Interact. 2006;164:25–38. doi: 10.1016/j.cbi.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Bir SS, Verma SC. Chromosome's atlas of the Indian petridophytes. 1951;2009:84. [Google Scholar]
- 56.Federal import quarantine order of climbing ferns Lygodium flexuosum and Lygodium microphyllum. Bugwood blog center for invasive species and ecosystem health. [Last accessed on 2011]. Available from: http://bugwood.blogspot.com/2008/06/federal-import-quarantineorder-for.html .
- 57.Federal Register. National Veterinary Accreditation Program. Animal and plant health inspection services USDA. 2009;74:64998–65012. [Google Scholar]
- 58.International organization for biological control nearctic regional section, Canada. IOBC-NRS Newsletter. 2005;27:1–6. [Google Scholar]
- 59.News from Southern weed science society. [Last accessed on 2011];2008 3(12):1–10. Available from: http://www.swss.ws/Newsletter/2008_August.pdf . [Google Scholar]
- 60.Washington report. 2008. Jun, [Last accessed on 2011]. Available from: http://www.wssa.net/WSSA/SciPolicy/JUNE%202008%20DSP%20Report.pdf .
- 61.2008 WSSA committee report to members. Summary for 2008 and action plan for 2009. [Last accessed on 2011]. Available from: http://www.docstoc.com/docs/40584357/2008-WSSA-Committee-Report-to-Members-Summary-for-2008 .
- 62.Chris P. Weed risk assessment – An attempt to predict future invasive weeds of U.S.A. [Google Scholar]
- 63.Goolsby JA, Purcell FM, Wright T. Weedland weed of U.S. Biocontrol down under, U.S.D.A-ARS. Queensland: Australian Biological Control Laboratory; 2001. pp. 1–54. [Google Scholar]
- 64.Pemberton RW, Goolsby JA, Wright T. Old world climbing fern. United States: Biological control of invasive plants in eastern United States; 2002. p. 413. [Google Scholar]
- 65.Solis MA, Yen S-H, Goolsby JH, Wright T, Pemberton R, Winotai A, et al. Siamusotima aranea, a new stem-boring musotimine (Lepidoptera : Crambidae) from Thailand feeding on Lygodium flexuosum (Schizaeaceae) Ann Entomol Soc Am. 2005;98:887–95. [Google Scholar]
- 66.Ferriter A. A report from the Florida's exotic pest plant council's lygodium task force, Florida. 1st ed. Florida: 2001. Lygodium management plan for Florida; pp. 1–59. [Google Scholar]
- 67.Khade SW, Rodrigues BF. Arbuscular Mycorrhizal fungi associated with some petridophytes from western ghat region Goa. J Trop Ecol. 2002;43:251–6. [Google Scholar]


