Abstract
World Health Organization (WHO) has recommended that traditional health and folk medicine systems are proved to be more effective in health problems worldwide. Trianthema portulacastrum Linn. is a herb used in Ayurvedic medicine. The principal constituent of T. portulacastrum Linn. is ecdysterone and the other constituents are trianthenol, 3-acetylaleuritolic acid, 5,2’-dihydroxy-7-methoxy-6,8-dimethylflavone, leptorumol, 3,4-dimethoxy cinnamic acid, 5-hydroxy-2-methoxybenzaldehyde, p-methoxybenzoic acid, and beta cyanin. Different parts of Trianthema portulacastrum Linn. are traditionally used as analgesic, stomachic, laxative, treatment of blood disease, anemia, inflammation, and night blindness. Laboratory investigations on extracts of the plant have demonstrated significant pharmacological activities, such as antioxidant, diuretic, analgesic, hepatoprotective, and anticarcinogenic. This article compiles all updated information related to T. portulacastrum Linn. Scientifically proved activities are co-related with traditional concepts. Scientific evidence exists with respect to their major and minor constituents. The novelty and applicability of T. portulacastrum are hidden. Such things should be overcome through modern scientific concepts.
Keywords: Azoaceae, phytochemistry, pharmacological properties, Trianthema portulacastrum, traditional medicine
INTRODUCTION
Ever since his existence on this planet, man has had to depend on nature for sustenance and survival. Plant-based medicines have been used by mankind since time immemorial. According to the report of World Health Organization (WHO), over 80% of the world population relies on the traditional system of medicine, largely plant based, to meet their primary health care.[1] India is one of the nations blessed with a rich heritage of traditional medical systems and rich biodiversity to complement the herbal needs of the treatment administered by these traditional medical systems. The recognized Indian systems of Medicine are Ayurveda, Siddha, and Unani, which use herbs and minerals in the formulations,[2] but it is mandatory to prove traditional concepts scientifically in the laboratory. Over the last few years, researchers have aimed at identification and validating plant-derived substances for the treatment of various diseases. Interestingly, it is estimated that more than 25% of modern medicines are directly or indirectly derived from plants.[3] The global demand for herbal medicine is not only large, but also growing. Nowadays, collection of medicinal plants from forest is very difficult due to government's forest policy, rapid loss of diversity of plants, natural habitats, traditional community life, cultural diversity, and knowledge of medicinal plants. To solve this problem weeds may provide medicines with low cost, more potential, and without adverse side effects.[4]
Bishkhapra (Trianthema portulacastrum Linn.), belonging to the family Aizoaceae, is one of the common weed, which has enormous traditional uses against diseases and some bioactive compounds have been isolated from this weed.[1]
Botanical synonym [Table 1]
Table 1.
Synonyms of T. portulacastrum in different systems of medicines[5]

T. monogyna Linn.[5]
Eng. Horse Purslane
Hindi Sabuni, santhi, Vishakhapara, Lal-sabuni, Svet-sabuni
Sanskrit Shvetapunarnava, Chiratika, Dhanapatra, Shvetamula, Upothaki
Tamil Sharunnai, Shavalai
Telgu Ambatimadhu, Atikamamidi, Galijeru
Bombay Svetapunarnava
Punjab Bishkapra, itsit
Beng. Gadabani
Kan. Muchchugoni
Mal. Sharunnau
Madras Mukkarattai
Marathi Pundharighetntuli
Taxonomical classification[8]
Kingdom - Plantae (Plants)
Sub Kingdom - Ttracheobionta (Vascular plants)
Superdivision - Spermatophyta (Seed plants)
Division - Magnoliophyta (Flowering plants)
Class - Magnoliopsida (Dicotyledons)
Subclass - Caryophyllidae
Order - Caryophyllales (Herbaceous and fleshy)
Family - Aizoaceae (Fig-marigold family)
Genus - Trianthema L. (Trianthema)
Species - Trianthema portulacastrum
Occurrence and distribution
It is an exotic weed and a native of tropical America. It is growing throughout most tropical countries, such as Baluchistan, Ceylon, and India.[4] It is now naturalized throughout India in cultivated fields, river beds, waste ground, etc.[2] Its infestation is very common in various agricultural and vegetable crops, such as mustard, maize, pigeon pea, mung bean, potato, onion, cotton, soybean, pearl millet, and sugarcane, especially during the rainy seasons.[8–10]
Two forms are reported to occur in this species; a red-colored form known as Lal Sabuni, in which the stem, leaf-margin, and flours are red; a green-colored form known as Svet Sabuni, which has a green stem and white flowers.[5]
Propagation and cultivation
This is not cultivated commercially, but it is found throughout India as a tropical problematic terrestrial weed by virtue of its infestation in plains, river beds, and in wastelands. It also grows automatically in cultivated fields with agriculture and vegetable crops, especially in the rainy seasons.
Part used as medicine[4][Figure 1–3]
Figure 1.

Whole plant of Trianthema portulacastrum L.
Figure 3.

Leaf lower side
Figure 2.

Leaf upper side
Whole plant
Roots
Leaves
MACROSCOPIC DESCRIPTION
Root: Thin, slender, tapering, and tortuous, with lateral branching fibrous root, 5–15 cm in length; 0.3–2.5 cm in diameter, light yellow externally, creamish white internally, fractures fibrous.
Stem: Cylindrical, dichotomously branched, prostrate or trailing, somewhat glabrous, at places reddish tinted, nodes swollen, fresh stem succulent.
Flower: Small, solitary, sessile, pinkish, nearly concealed by the pouch of the petiole, calyx tube scarious, thin, stamens 10–15, ovary superior, sessile, style single papillose, shorter than the stamens [Figure 4].
Fruit: Fruit capsule is 3–5 mm long, almost concealed in the petiolar pouch, slightly concave, upper beak-like part at the time of dehiscence, carrying 2–3 seeds, lower cup-like part enclosing 2–5 or more seeds, fracture fibrous [Figure 5]. Seeds are reniform, dull black, rough, muriculate.[1,4,8]
Figure 4.

Flower
Figure 5.

Seeds
MICROSCOPIC DESCRIPTION
Root
The TS of root is almost circular in outline. The outer most layer of the cork is somewhat obliterated followed by 3–4 rows of closely arranged thin-walled cubical to tangentially elongated rectangular cells. Distinct cork is not evident. Underneath these lines a narrow zone of cortex, composed of fairly large, oblong or polygonal or tangentially elongated thin-walled parenchymatous cells. In TS of younger roots, 2 phloem bundles embedded in the central wood are seen, which gradually form a complete ring around the double-shaped xylem, 2–3 layers of parenchymatous cells are present in between each ring of xylem and phloem. Alternate zone of xylem and phloem are found due to anomalous secondary growth and their numbers vary according to the age of the plant. Xylem consists of vessels of various sizes, tracheids, and xylem parenchyma and phloem consists of sieve tubes, companion cells, and phloem parenchyma. Rosette and rhomboidal crystals of calcium oxalate are present in parenchyma of phloem and cortical region. Traces of lateral branches are also observed at some places [Figure 6].
Figure 6.
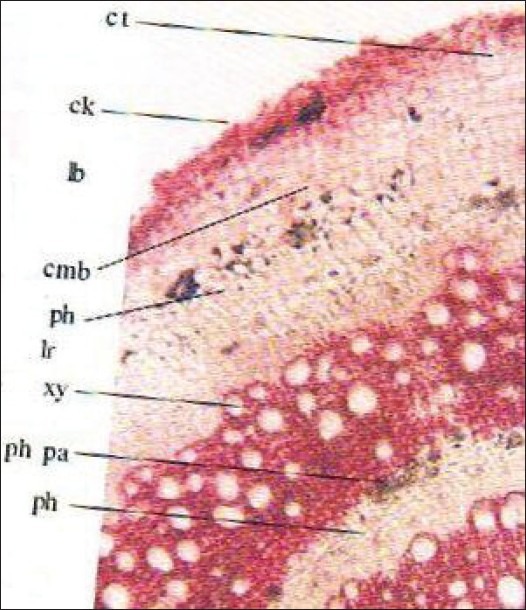
TS of root, Cmb: Cambium, lb: Lateral branch, Ck: Cork, lr: Lateral root, Ct: Cortex, ph: Phloem, E: Epidermis, ph pa: Phloem parenchyma, End: Endodermis, pi: Pith, Id: Crystal idioblast, pr: Pericycle, T: Trichome
Stem
TS of stem is almost circular in outline, epidermis covered by thin cuticle, trichomes are usually unicellular but sometimes bicellular trichomes also present. Cortex collenchymatous, 8-10 cells broad. Endodermis well-developed pericycle distinct in the form of sclerenchymatous patches; phloem comprises sieve tubes, companion cells, and phloem parenchyma, cambium indistinct. Xylem consists of vessels, tracheids, fibers, and xylem parenchyma. Vessels are either solitary or in radial rows of 3–7. Pith is well developed and parenchymatous, starch grain and rosette crystals of calcium oxalate are present in cortical as well as in pith region [Figure 7].
Figure 7.
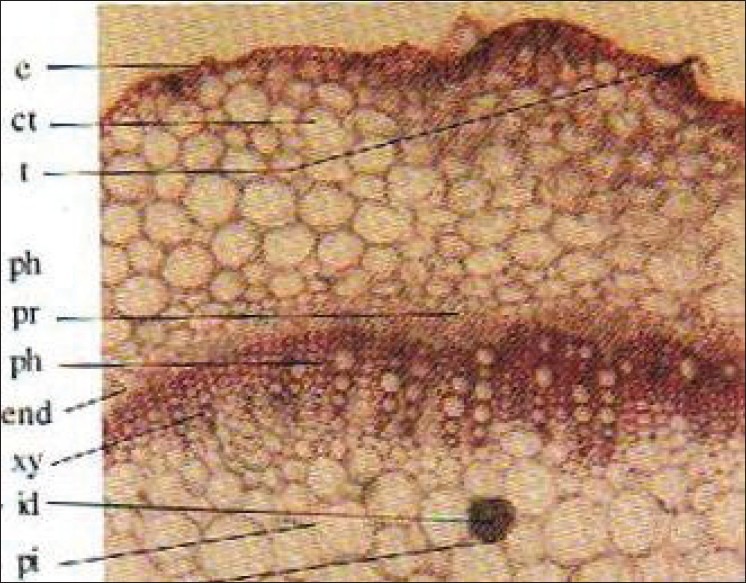
TS of stem Cmb: Cambium, lb: Lateral branch, Ck: Cork, lr: Lateral root, Ct: Cortex ph: Phloem, E: Epidermis, ph pa: Phloem parenchyma, End: Endodermis, pi: Pith, Id: Crystal idioblast, pr: Pericycle, T: Trichome
Leaf
TS of the leaf passing through midrib shows slight depression on the upper side and broad elevation on the lower side, a layer of parenchymatous hypodermis, becoming two celled over the meristele lines underneath this, palisade continuous over the meristele. In midrib the number of vascular bundles varies from 1 to 3. The meristele consisting of xylem in row and inconspicuous phloem with arc of large parenchymatous cells toward the upper side and 2- to 4-celled pericyclic fibres toward the lower side. A single-layered upper and lower epidermis covered by thin slightly striated cuticle interrupted by uni- to multicellular, uniseriate trichomes and stomata. Some trichomes are balloon-shaped mostly found on the margins. The epidermis consists of straight-walled cells with faint cuticular striation and anisocytic to paracytic stomata on both upper and lower surfaces [Figure 8].
Figure 8.
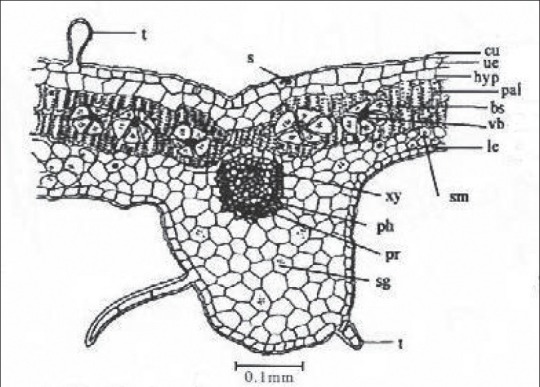
TS of leaf passing through midrib, bs- Bundle sheath, cr- Crystal, Cu- Cuticle, hyp- Hypodermis, Le-Lower epidermis, pal- Palisade, Pbs- Peripheral bundle sheath, ph- Phloem, Ph- Pericycle, r- Raphid, S- Stomata, sg- Starch grains, T- Trichome, sm- Spongy mesophyll, Ue- Upper epidermis, vb- Vascular bundle, Xy- Xylem
TS passing through lamina show single-layered hypodermis, 2–3 layered palisade and 3–5 loosely arranged spongy parenchymatous cells. Vascular bundles abundant in palisade region surrounded by large parenchymatous sheath. Rosette crystals, idioblast with raphides of calcium oxalate and small oval-shaped starch grains are also found in mesophyll region [Figure 9].
Figure 9.
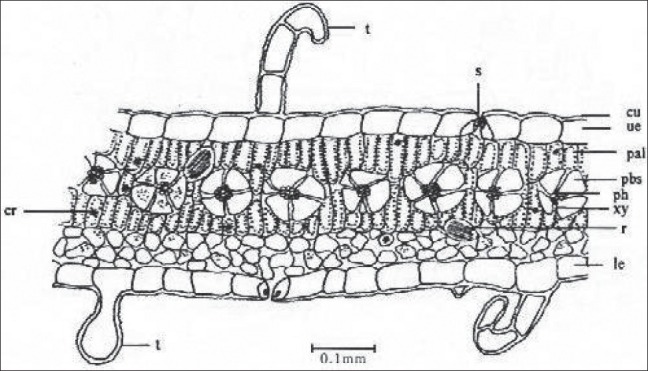
TS of leaf passing through lamina bs- Bundle sheath, cr- Crystal, Cu- Cuticle, hyp- Hypodermis, Le-Lower epidermis, pal- Palisade, Pbs- Peripheral bundle sheath, ph- Phloem, Ph- Pericycle, r- Raphid, S- Stomata, sg- Starch grains, T- Trichome, sm- Spongy mesophyll, Ue- Upper epidermis, vb- Vascular bundle, Xy- Xylem
Powder
Yellowish green. Shows starch grains, fragments of leaves with epidermal cells of paracytic and anisocytic stomata, unicellular balloon-shaped and multicellular, uniseriate trichomes, fibres, tracheids, vessels, with spiral scalariform and reticulate secondary wall thickenings. Idioblast with raphides of calcium oxalate of leaf, idioblast with single rosette crystal of calcium oxalate of stem, rosette, prismatic crystals of calcium oxalate, fragments of cork, and pollen grains [Figure 10].[1]
Figure 10.
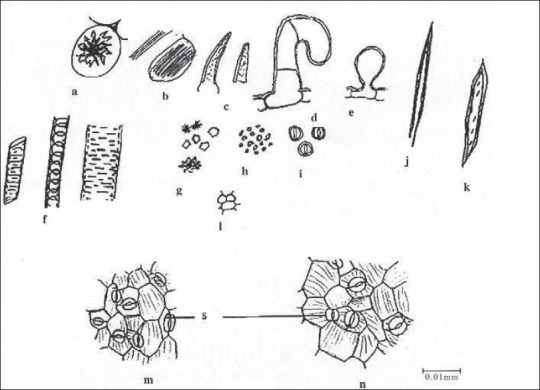
Powder Microscopy, a- Idioblast, b - Idioblast with raphides, c- Warty trichome, d - Multicellular trichome, e- Unicellular trichome balloon shape, f- Vessels, g- Crystals, h- Starch grains, i- Pollen grains, j- Fibre, k-Tracheid, l- Cork cells, m- Upper epidermal cells, n- Lowerer epidermal cells, s- Stomata
PHYTOCONSTITUENTS
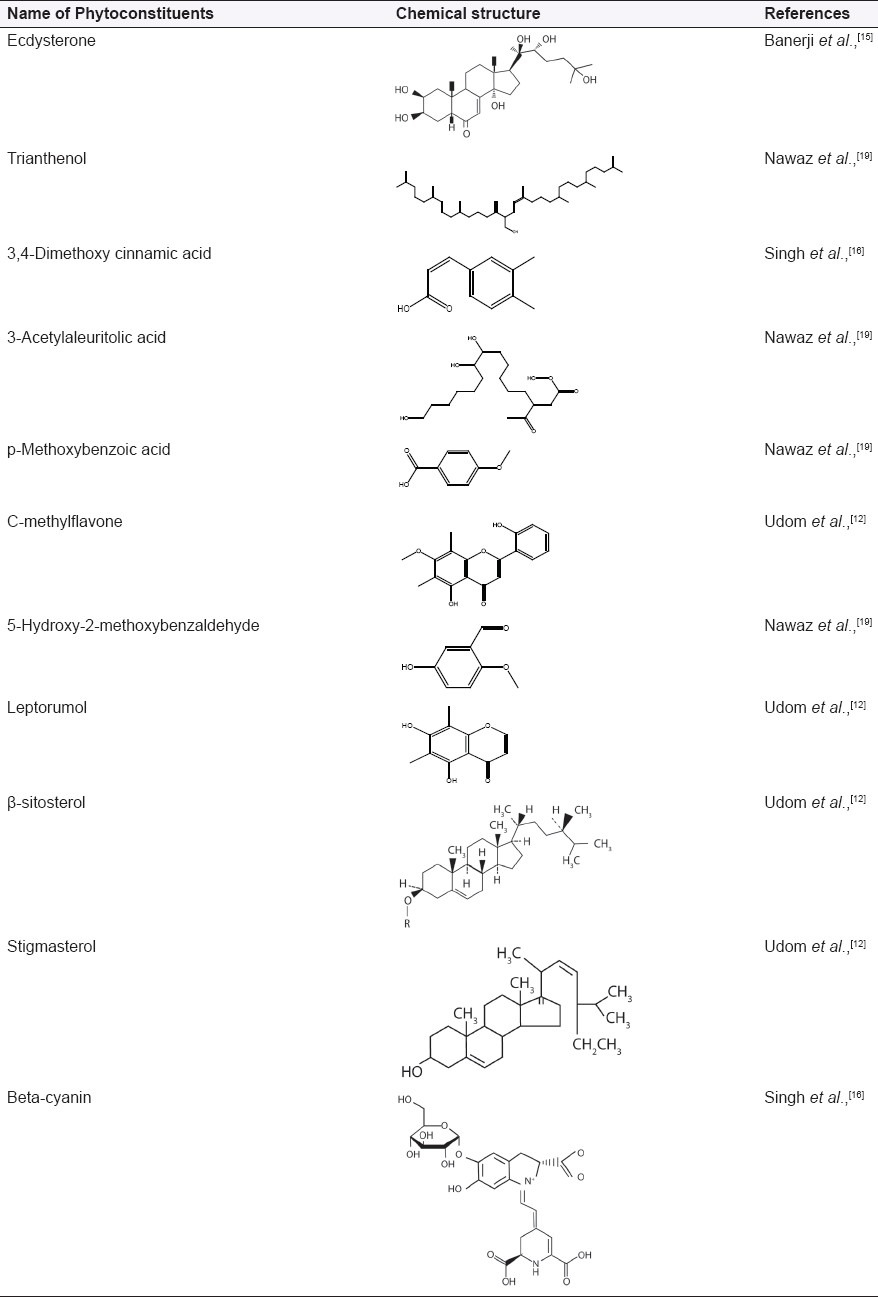
Photochemical screening has revealed the presence of steroids, flavonoid, fats, terpenes, carbohydrates, tannins, and alkaloids. Phytochemical constituents in the various parts of the plant are very significant.[6] The methanolic extract was screened for the presence of various phytoconstituents, such as steroids, alkaloids, terpenoids, glycosides, flavonoids, phenolic compounds, and carbohydrates [Table 2].[11]
Table 2.
Various chemical constituents of T. portulacastrum
Phytochemistry
Extraction of air-dried plant with dichloromethane has led to the isolation of a new flavonoid, 5,2’-dihydroxy-7-methoxy-6,8-dimethylflavone (C-methylflavone), along with 5,7-dihydroxy-6,8-dimethylchromone (leptorumol), which has been previously reported from a fern species.[12]
The red and white flowers contain an alkaloid trianthemine, also punarnavine.[5]
The plant contains nicotinic acid (Vitamin B3), ascorbic acid (Vitamin C).[13] The mineral profile of T. portulacastrum as calcium (0.3%), magnesium (0.2%), iron (50 ppm), copper (8 ppm), zinc (30.0 ppm), and manganese (50 ppm), whereas the phosphorus content at 0.13% ± 0.1% and crude protein 1.5% ± 1.2% [Table 1].[14]
The plant is rich in phosphorous and iron but poor in calcium. The high content of oxalate affects the assimilation of calcium. Carotene (2.3 mg/100 g) has also been reported.[13]
Chromatography of dried plant with methylene chloride on silica yielded long chain esters; a mixture of C14, C16, C18, C20, and C22 long chain alcohols; β-sitosterol, stigmasterol, and their β-glucopyranosides.[12]
A tetraterpenoid named trianthenol has been isolated from the chloroform extract of the plant. Its structure was established as 15-hydroxymethyl-2,6,10,18,22,26,30-heptamethyl-14-methylene-17-hentriacontene on the basis of spectroscopic data, including high resolution mass and two-dimensional Nuclear Magnetic Resonance (NMR) techniques.[12]
Ecdysterone has been isolated from the whole plant; yield in g/kg is 0.1. Ecdysterone is the most widely occurring phytoecdysone.[15] Phytoecdysteroids are a plant steroids related in structure to the invertebrate steroid hormone 20-hydroxyecdysone. Typically, they are C27, C28, or C29 compounds possessing a 14α-hydroxy-7-en-6-one chromophore and A/B-cis ring fusion (5β-H).[16] Ecdysterone biogenesis may be increased by the use of various phytohormones and different sucrose levels in seedling callus culture of T. portulacastrum.[17]
Hydrocarbons from the surface wax of the fresh leaves of plant have been isolated and characterized and their relative distribution determined through gas liquid chromatography studies. The considerable occurrence of branched chain hydrocarbons may be an indication of the characteristics of lower plants based on taxonomy.[16]
Beta-cyanin and 3,4-dimethoxy cinnamic acid also have been reported from T. portulacastrum and other aizoaceae family.[18]
Four new compounds first time reported from T. portulacastrum as 5-hydroxy-2-methoxy benzaldehyde, 3-acetyl aleuritolic acid, p-methoxy benzoic acid, and p-propoxy benzoic acid.[12]
ETHNOMEDICINAL USES
The plant is alexiteric, analgesic, stomachic, laxative, alterative; cures “Kapha,” bronchitis, heart diseases of the blood, anemia, inflammations, “Vata,” piles and ascites.[4] The plant has been used in the indigenous system of medicine for the obstruction of the liver asthma, amenorrhea, dropsy, edema, ascites, and beri-beri. A decoction of the herb is used as a vermifuge and is useful in rheumatism; it is also an antidote to alcoholic person.[5]
Root: Antipyretic, analgesic, spasmolytic, deobstruent, and anti-inflammatory. The ayurvedic pharmacopoeia of India recommended in diseases of liver and spleen, anemia, and edema.[13] The root applied to the eye cures corneal ulcers, itching, dimness of sight, and night blindness.[4]
The root is cathartic and abortifacient with irritant properties. An infusion of the roots is administered in jaundice, stranguary, and dropsy.[2,13]
The powdered bitter and nauseous root is given in combination with ginger as a cathartic.
In the Philippine Islands, the powdered root is given as a cathartic.[4]
Leaves: Used as diuretic in edema and dropsy. A decoction of the herb is used as an antidote to alcoholic poison.[13] Leaves have been reported to be diuretic, and therefore useful in the treatment of edema and ascites.[2,13]
PHARMACOLOGICAL/BIOLOGICAL ACTIVITIES
Antifungal activity
Tetraterpenoid trianthenol and chloroform extract of plant both showed antifungal activity. When these were subjected to in vitro fungicidal bioassay against a number of human and plant pathogens, the percentage inhibition of the crude extract and trianthenol was found to be moderate in comparison to respective standard drugs.[19]
Chemosterilant/molting hormone activity
Ecdysterone is the most widely occurring phytoecdysone, is also obtained from whole plant of T. portulacastrum as a major chemical constituent. The compound and its analogs have potential use as chemosterilants and because they stimulate protein synthesis not only in insects but also in mammals. The bioassay for the molting hormone activity was carried out by modifying the “Chilo dipping” technique. In the present work, Musca domestica (housefly) larvae were used, which give quicker response compared with Chile. The isolated abdomens of housefly larvae were dipped in the crude extracts of the plants. The molting hormone activity was shown by the formation of a pupanum. For quantitative experiments, the test solutions were injected into the isolated larval abdomens. A dose of 0.01 μg of ecdysterone gave a full pupation response.[20] There are possibilities of utilizing insect molting hormone as third-generation insecticide.[21]
Analgesic activity/antinociceptive activity
The ethanol extract of T. portulacastrum (whole plant) was evaluated by acetic acid-induced writhing and hot plate methods to assess analgesic activity. It was found that the extract caused an inhibition on the writhing response induced by acetic acid in a dose-dependent manner. A dose of 250 mg/kg extract and Aspirin could block the writhing response by 50.92% and 67.68% (P < 0.001), respectively. It was also indicated that the extract showed significant antinociceptive action in hot plate reaction time method in mice. This effect was comparable to that of standard drug Aspirin-treated controls, suggesting the central activity of extract.[6]
Antihyperglycemic activity/hypoglycemic
The methanolic extract of T. portulacastrum whole plant produced significant antihyperglycemic activity against streptozotocin (STZ)-induced diabetic rats, which are comparable to glibenclamide (a standard oral hypoglycemic agent). The 100 and 200 mg/kg suspension of methanolic extract produced a significant antihyperglycemic effect (P < 0.05) after 1 h following administration and this antihyperglycemic effect was more pronounced after 4 h of treatment in STZ-induced diabetic rats.[18]
The methanolic extract of whole plant was administered for 7 days to normal and alloxan-induced diabetes rats at a dose of 100, 200, and 300 mg/kg. The extract produced significant reduction (P < 0.001) in blood glucose in normal and diabetic rats as compared with standard oral hypoglycemic agent, glibenclamide.[22]
Hypolipidemic activity
The methanolic extract of whole plant produced a dose-dependent hypolipidemic activity in rats. Diabetes mellitus associated with lipid metabolism is improperly regulated. A dose of 100, 200, and 300 mg/kg showed beneficial effects on the lipid profile in normal as well as alloxan-induced diabetic rats at the end of the treatment period, that is, 7 days.[22] The hypolipidemic drugs have attended considerable attention because of their potential to prevent cardiovascular disease by retarding the accelerated atherosclerosis in hyperlipidemic individuals.[17]
Anticarcinogenic activity
The study clearly demonstrates that the anticarcinogenic property of T. portulacastrum L. is very promising. The chloroform fraction of overground part of T. portulacastrum has emerged as the most active fraction inhibiting chemically induced rat hepatocarcinogenesis. Hepatocarcinogenesis was induced by the potent carcinogen diethylnitrosoamine (DENA). The chloroform extract of T. portulacastrum has proved significant in reducing nodule incidence by as much as 25% as compared to 100% in the DENA-treated group. Most of the antineoplastic agents available in the market produce many toxic side effects, such as nephrotoxicity, cirrhosis, and so on, but since the control as well as the experimental animals indulged in normal food and water intake, the extract may be taken to be nontoxic.[23]
Hepatoprotective activity
The ethanolic leaves extract of T. portulacastrum L. (Aizoaceae) showed a significant dose-dependent (100 mg and 200 mg/kg p.o.) hepatoprotective protective effect against two well-known hepatotoxins, namely, paracetamol- and thioacetamide-induced hepatotoxicity in albino rats. The degree of protection was measured by using biochemical parameters, such as serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), bilirubin (BRN), and total protein (TP). The plant extract completely prevented the toxic effects of paracetamol (acetaminophen) and thioacetamide on the above serum parameters.[24]
The studies also indicate that the ethanolic leaves extract of T. portulacastrum is a potent hepatoprotectant as silymarin. The hepatoprotective effect of ethanolic leaves extract of T. portulacastrum studied on aflatoxin B1 (AFB1)-induced hepatic damage in a rat model and compared with silymarin, a well-known standard hepatoprotectant. Pretreatment with T. portulacastrum (100 mg/kg/p.o.) and silymarin (100 mg/kg /p.o.) for 7 days reverted the condition to near normal.[25]
An ethanolic extract of T. portulacastrum (ETP) gives a significant protection against acute and chronic CCl4-induced hepatocellular injury in mice. ETP of the plant (excluding roots) protects against acute liver injury induced by alcohol and CCl4 in mice by modulating hepatic lipid peroxidation and glutathione (GSH) level,[26] and by restoration of enzymes of the plasma membrane, microsomal, lysosomal, and cytoplasmic fractions of hepatic tissue.[27] Moreover, CCl4-mediated hepatic lipid peroxidation and activities of the related antioxidative enzyme levels were significantly inhibited by ETP.[27] Marked protection was given by ETP as reflected by the hematological status, hematopoietic system and plasma protein levels in mice during CCl4 poisoning.[28]
A study by Sarkar et al. provides a strong evidence that ETP can offer protection against the induction of chromosomal aberrations, DNA-chain break and sugar-base damage in liver, induced by either chronic or a single acute dose of CCl4. The biological and molecular response of ETP suggests the underlying molecular mechanisms of the promising antihepatotoxic activity of T. portulacastrum.[11]
Antioxidant activity
The ethanolic leaves extract of T. portulacastrum L. showed the antioxidant activity in relation to hepatotoxins, paracetamol, and thioacetamide in rats. The levels of antioxidant enzymes, namely glutathione reductase (GSH-R), glutathione-S-transferase (GST), glutathione peroxidase (GPX), superoxide dismutrase (SOD), and catalase (CAT), were decreased significantly in toxicants-treated rats when compared with those of normal control animals. Thus antioxidant capacity of the liver decreased leading to the generation of lipid peroxides resulting in liver damage. But treatment with 100 mg and 200 mg/kg p.o. ethanolic leaves extract of the plant increases the activity of SOD and CAT and it scavenges free radicals and reduces hepatic damage. So it might be concluded that the hepatoprotective action of extract is due to its antioxidant activity.[11]
Glomerulosclerosis
The methanolic extract of T. portulacastrum L. with 100 and 200 mg/kg, b.w. produced a protection against atherosclerotic diet or CCT (4% cholesterol, 1% cholic acid, and 0.5% thiouracil) diet-induced glomerulosclerosis and hepatic damage by reducing serum lipid levels, AST, ALT (aspartate and alanine transaminases), and creatinine levels in rats.[29]
AYURVEDIC PROPERTIES
Rasa (Taste) – Tikta (bitter)
Virya (Potency) – Ushan (hot)
Dosa Nidan (Treatment) – Kapha, Vata
Dosage
Leaves - 2–5 gm powder
Decoction - 20–30 gm of the drug
Use of plant drug as adulterant/substitute
Trianthema species are used as adulterant of the Boerhavia species (Punarnavaa). T. portulacastrum is wrongly equated with Shveta-punarnavaa or Rakta-punarnavaa. Lal sabuni is adulterated with Boerhavia diffusa, svet sabuni with white-flowered species, B. verticillata (B. erecta L., B. punarnava).[13]
Nutritive value
T. portulacastrum, a common farm weed in tropical countries, contains 21.5% ± 1.2% crude proteins, similar to Lucerne with relatively low structural carbohydrate. The mineral profile of T. portulacastrum was well above the critical level as far as calcium (0.3%), magnesium (0.2%), iron (50 ppm), copper (8 ppm), zinc (30.0 ppm), and manganese (50 ppm) are concerned, whereas the phosphorus content at 0.13% ± 0.1% was below the critical level recommended. Degradability studies in rumen stimulation technique (RUSITEC) revealed that nearly half of the dry matter in T. portulacastrum was soluble and degradable, whereas 69.9% of the nitrogen was insoluble but degradable. The digestible rumen degradable nitrogen and digestible undegradable nitrogen values were 1.2% and 1.4%, respectively, with the total absorbable nitrogen value of 2.5%. A study revealed that supplementation of digestible organic matter to the extent of 14.9% and phosphorus to the extent of 0.2% was suggested as a tool to exploit the full potential nutritive value of T. portulacastrum as a fodder.[14]
Toxicity assessment
Oral acute toxicity studied in mice and rats using various doses of methanolic extract of dried whole plant. No mortality was recorded till 4000 mg/kg, hence the extracts were found to be safe up to the dose levels of 4000 mg/kg.[17] Ethanolic extract of dried leaves administered intraperitoneally to albino Swiss mice to either sex; none of the mice exhibited any abnormal behavioral responses.[6]
Popularity of trianthema portulacastrum over time
Graph shows, how interesting is the plant T. portulacastrum. Plots of numbers of papers mentioning T. portulacastrum (filled column histogram and left hand axis scale) and line of best fit, 1926-2006.
Plots of a proportional microindex, derived from numbers of papers mentioning T. portulacastrum as a proportion of the total number of papers published for that year (broken line frequency polygon and right hand scale) and line of best fit, 1926–2006.[30]
CONCLUSION
This plant has sometimes been identified with Shvetapunarnava. But recent investigations have, however, shown that the drug known in Ayurveda as Shvetapunarnava is not a species of Trianthema. Thus care should be taken to prevent misidentification and adulteration. It is believed that information as presented in a glance in this review on its phytochemistry and various biological properties of the extract and the constituents confirm the therapeutic value of the plant and might provide incentive for proper evaluation of the plant in medicine. Test conducted in mice and rats for analgesic, hepatoprotective, and antifungal activities have shown significant results without adverse side effects. As it is a weed, widely available in all the seasons, drastic conditions and ease of collection in low cost, the plant could serve as a “lead” for the development of novel agents having good efficacy in various disorders in the future. Moreover, there are possibilities of utilizing insect molting hormone, Ecdysterone as third-generation insecticide.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vol. 1. New Delhi: Indian Council of Medical Research; 2003. Anonymous. Quality Standards of Indian Medicinal Plants; pp. 261–70. [Google Scholar]
- 2.Kumar A, Saluja AK, Shah UD, Mayavanshi AV. Pharmacological potential of Albizzia lebbneck: A review. Pharmacogn Rev. 2007;1:171–4. [Google Scholar]
- 3.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 4.Kirtikar KR, Basu BD. Indian Medicinal Plants. Vol. 2. Allabahad: Lalit Mohen Basu; 1975. pp. 1180–1. [Google Scholar]
- 5.Vol. 10. New Delhi: Council of Industrial and Scientific Rearch; 1976. Wealth of India (Raw Materials) p. 281. [Google Scholar]
- 6.Shanmugam SK, Bama S, Kiruthiga N, Kumar RS, Sivakumar T, Dhanabal P. Investigation of analgesic activity of leaves part of the Trianthema portulacastrum (L) in standard experimental animal models. Int J Green Pharm. 2007;1:39–41. [Google Scholar]
- 7.Vocks R. Tropical forest healers and habitat preference. Eco Bot. 1996;50:381–400. [Google Scholar]
- 8. [Last accessed on 2010 Mar 20]. Available from: http://www.plants.usdav.gov/
- 9.Balyan RS, Bhan VM. Emergence, growth and reproduction of horse purslane (Trianthema portulacastrum) as influenced by environmental conditions. Weed Science. 1986;34:516–9. [Google Scholar]
- 10.Randhawa MA, Khan MA, Khan NH. Influence of Trianthema portulacastrum infestation and plant spacing on the yield and quality of maize grain. Int J Agric Biol. 2009;11:225–7. [Google Scholar]
- 11.Sarkar A, Pradhan S, Mukhopadhyay I, Bose SK, Roy S, Chatterjee M. Inhibition of early DNA-damage and chromosomal aberrations by Trianthema portulacastrum L.in carbon tetrachloride-induced mouse liver damage. Cell Biol Int. 1999;23:703–800. doi: 10.1006/cbir.1999.0439. [DOI] [PubMed] [Google Scholar]
- 12.Udom K, Nattapol W-I, Santi TP, Warinthorn C, Gysorn V, Jim S, et al. A C-methylflavone from Trianthema portulacastrum. Phytochemistry. 1997;44:719–22. [Google Scholar]
- 13.Khare CP. Indian Medicinal Plants, an Illustrated Dictionary. Berlin/Heidelberg: Springer-Verlag; 2006. p. 96. 668. [Google Scholar]
- 14.Bharathidhasan S, Ganesh Babu NS, Balakrishnan V. In vitro Evaluation of the Nutritive Value of Trianthema portulacastrum as a Source of Fodder for Ruminants. Malays J Nutr. 2007;13:179–87. [PubMed] [Google Scholar]
- 15.Banerji A, Chintalwar GJ, Joshi NK, Chadha MS. Isolation of ecdysterone from Indian plants. Phytochemistry. 1971;10:2225–6. [Google Scholar]
- 16.Singh BP, Singh RP, Jha OP. Flavonoids of some Aizoaceae and Molluginaceae of Bhagalpur. Biol Bull India. 1982;4:157–63. [Google Scholar]
- 17.Tripathi KD. Essentials of Medical Pharmacology. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2004. p. 575. [Google Scholar]
- 18.Sundera A, Shyam RG, Bharath A, Rajeshwara Y. Antihyperglycemic Activity of Trianthema Portulacastrum Plant in Streptozotocin Induced Diabetic Rats. Pharmacologyonline. 2009;1:1006–11. [Google Scholar]
- 19.Nawaz HR, Malik A, Ali MS. Trianthenol: An antifungal tetrapenoid from Trianthema portulacastrum (Aizoaceae) Phytochemistry. 2001;56:99–102. doi: 10.1016/s0031-9422(00)00270-3. [DOI] [PubMed] [Google Scholar]
- 20.Dinan L. Phytoecdysteroids: Biological aspects. Phytochemistry. 2001;57:325–39. doi: 10.1016/s0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 21.Ravishankar GA, Mehta AR. Control of ecdysterone biogenesis in tissue culture of Trianthem portulactrum. J Nat Prod. 1979;42:152–8. doi: 10.1021/np50002a003. [DOI] [PubMed] [Google Scholar]
- 22.Anreddy RN, Porika M, Yellu NR, Devsrakonda RK. Hypoglycemic and hypolipidemic activities of Trianthema portulacastrum Linn.Plant in normal and alloxan induced diabetic rats. Int J Pharmacol. 2010;6:129–33. [Google Scholar]
- 23.Bhattacharya S, Chatterjee M. Protective role of Trianthema portulacastrum against diethylnitrosamine induced experimental hepatocarcinogenesis. Cancer Lett. 1998;129:7–13. doi: 10.1016/s0304-3835(98)00085-8. [DOI] [PubMed] [Google Scholar]
- 24.Kumar G, Banu GS, Pappa PV, Sundararajan M, Pandian MR. Hepatoprotective activity of Trianthema portulacastrum L.against paracetamol and thioacetamide intoxication in albino rats. J Ethnopharmacol. 2004;92:37–40. doi: 10.1016/j.jep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Banu GS, Kumar G, Murugesan AG. Ethanolic leaves extract of Trianthema portulacastrum L.Ameliorates aflatoxin b1 induced hepatic damage in rats. Indian J Clin Biochem. 2009;24:250–6. doi: 10.1007/s12291-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal A, Bishayee A, Chatterjee M. Trianthema portulacastrum affords antihepatotoxic activity against carbon tetrachloride-induced chronic liver damage in mice: Reflection in subcellular levels. Phytother Res. 1997;11:216–21. [Google Scholar]
- 27.Mandal A, Bandyopadhyay S, Chatterjee M. Trianthema portulacastrum L. reverses hepatic lipid peroxidation, glutathione status and activities of related antioxidant enzymes in carbon tetrachloride-induced chronic liver damage in mice. Phytomedicine. 1997;4:239–44. doi: 10.1016/S0944-7113(97)80074-8. [DOI] [PubMed] [Google Scholar]
- 28.Mandal A, Karmakar R, Bandyopadhyay S, Chatterjee M. Antihepatotoxic potential of Trianthema portulacastrum against carbon tetrachloride-induced chronic hepatocellular injury in mice: Reflection in hematological, histological and biochemical characteristics. Arch Pharm Res. 1998;21:223–30. doi: 10.1007/BF02975279. [DOI] [PubMed] [Google Scholar]
- 29.Shyam Sunder A, Reddy RN, Rajeshwar Y, Kiran G, Devkota KP, Baburao B. Protective effect of methanolic extract of Trianthema portulacastrum in atherosclerotic diet induced renal and hepatic changes in rats. Der Pharm Lett. 2010;2:540–5. [Google Scholar]
- 30. [Last accessed on 2010 Jul 10]. Available from: http://www.newcrops.Uq.edu.au/listing/species_pages_T/Trianthema_ portulacastrum.htm .


