Abstract
Natural sources have been important for the development of new active molecules for many years. Various small molecules with unique chemical skeleton and potent bioactivities were discovered through various sources like plants, marine products, and microorganisms, etc., which are considered as very important part of the nature. A number of potent antifungals have been originated from various natural sources. This account describes structure and activities of selected agents isolated from various natural sources.
Keywords: Antifungals, biological activities, chemical structure, natural sources
INTRODUCTION
In the immunological comprised patients single mold spore can initiate the deadly process. Patients on chemotherapy or immunosuppression, imposed for organ transplantation and those with chronic diseases such as AIDs, recurrent infection, cystic fibrosis, and diabetes are also at risk.[1,2] One of the factors aiding the spread of fungal disease has been the widespread use of broad-spectrum antibiotics, which eliminate or decrease the nonpathogenic bacterial populations that normally compete with fungi. Another has been the increased number of individuals with reduced immune responses caused by the acquired immunodeficiency syndrome or by the action of immunosuppressant drugs, or cancer chemotherapeutic agents. Besides their increasing frequency, fungal infections are still associated with an unacceptably high mortality, up to 40% in bloodstream infections caused by Candida albicans[3] and more than 50% in invasive aspergillosis.[4] The most commonly recognized causes of opportunistic invasive fungal infections (IFIs) traditionally are C. albicans, Cryptococcus neoformans, and Aspergillus fumigates.[5] Along with the widespread use of antifungal prophylaxis, the epidemiology of IFIs has shifted toward non-albicans Candida, non-fumigatus Aspergillus, opportunistic yeast-like fungi (e.g. Trichosporon and Rhodotorula spp.), zygomycetes, and hyaline moulds (e.g. Fusarium and Scedosporium spp.).[6] The limitations of current antifungal drugs, increased incidence of systemic fungal infections, and rapid development of drug resistance have highlighted the need for the discovery of new antifungal agents, preferably with novel mechanism of action.[7,8] While synthetic efforts remain the mainstream in antifungal drug discovery, as evidenced by the fact that 18 out of 23 antifungal drugs approved from 1980 to 2002 are synthetic (83% belongs to single azole class), unique antifungal natural products have demonstrated remarkable success in this regard.[9] For example, two major drug classes currently in use (amphotericin B, the gold standard and armamentarium, and the lipopeptide caspofungin) are the important antifungal drugs approved in recent years, are derived from natural products.[10] Natural products provide opportunities for new drug leads. This account describes structure and activities of selected agents isolated from various natural sources with the aim to examine the recent developments toward discovering novel antifungal drugs of natural origin. However, we have excluded certain compounds that are often termed “nuisance” compounds, because they show a variety of biological activities.
ANTIFUNGALS FROM NATURAL SOURCES
Phytochemical analysis of P. costaricense parts leads to the isolation and identification of δ-toco trienol, β-sitosterol, and other active constituents. Cinnamodial (1) and cinnamosmolide (2) were found to have high activity against Alternaria alternate (MIC=3.9μg/ml), C. albicans, and Wangeiella dermatides (MIC=15.6μg/ml). Compound (2) showed less potent antifungal activities than (1) but was more effective against C. albicans CNIA (MIC = 23.4μg/ml).[11]
Twentyfive natural products mainly halogenated furanones were isolated from the temperate red algae Delisea pulchra. The activity was found to be very strong against fungus P. oxalis for 18 compounds, 10 compounds (3-12) maintained strong activity at 0.1 μg/ml while two compounds 10 and 12 have shown activities comparable to miconazole [Figure 1].[12]
Figure 1.
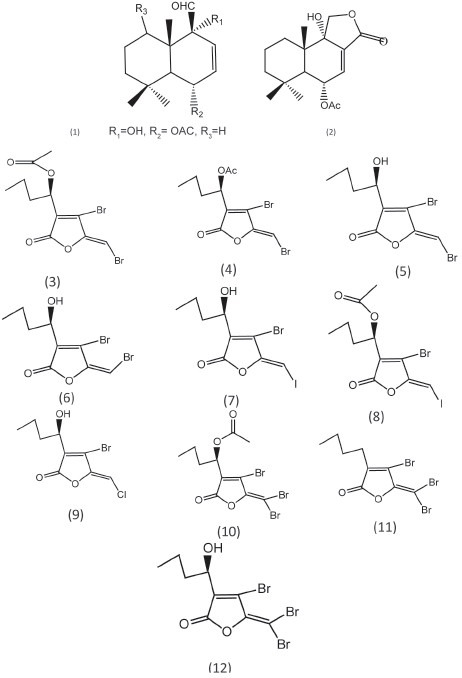
Halogenated furanones obtained from algae as antifungal agents
Carneic acids (13, 14)isolated as major constituents of the stromata of hypoxylon carneum have shown moderate antifungal activity against various fungal infections.[13] Hopeanolin (15), an unusual resveratral trimer with an ortho-quinone nucleus, from stem bark of Hopea exalata demonstrated very good antifungal activity [Figure 2]. This plant has six known stibenoids, shoremphinol (16), vaticanol (17), α-viniferin (18), pauciflorol A (19), vaticanol A (20), and trans-3,5,4’-trihydroxystilbene 2-C-glucoside (21) have shown antifungal activities in the MIC value range 0.1–22.5 μg/ml.[14] Two new macrolides, swinholides I (22) and hurghadolide A (23, 24) of which hurghadolide A possesses an unprecendent asymmetric 42-membered dilactone moiety were reported. Compounds 23 and 24 have been demonstrated to be active against C. albicans.[15]
Figure 2.
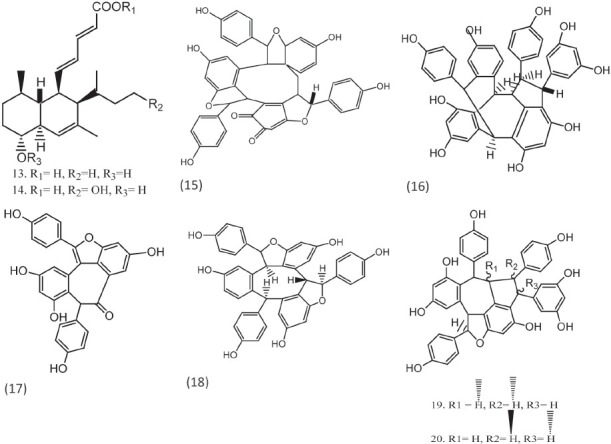
Compounds with quinone, furan nucleus
Marine-derived macrolides latrunculins A and B of the red sea sponge Negombata magnifica are the marine products. Latrunculin B (25) together with latrunculin T (26) was isolated and certain semisynthetic analogs (27, 28, 29, and 30) were reported to have potent activity against C. albicans, the presence of αβ lactones in the ring is responsible for the activities.[16]
Five new ent-labdane diterpenoids, 3-o- β d-glucopyranosyl-14,19-dideoxyandrographolide (31), 14-deoxy-17-hydroxy-andrographolide (32), 19-o-[ β-d-glucopyranosylandrographolide (33), 12-s-hydroxy andrographolide (34), and andrographatoside (35) were isolated from the aerial part of andrographis paniculata and all compounds were active against various fungal strains [Figure 3].[17]
Figure 3.
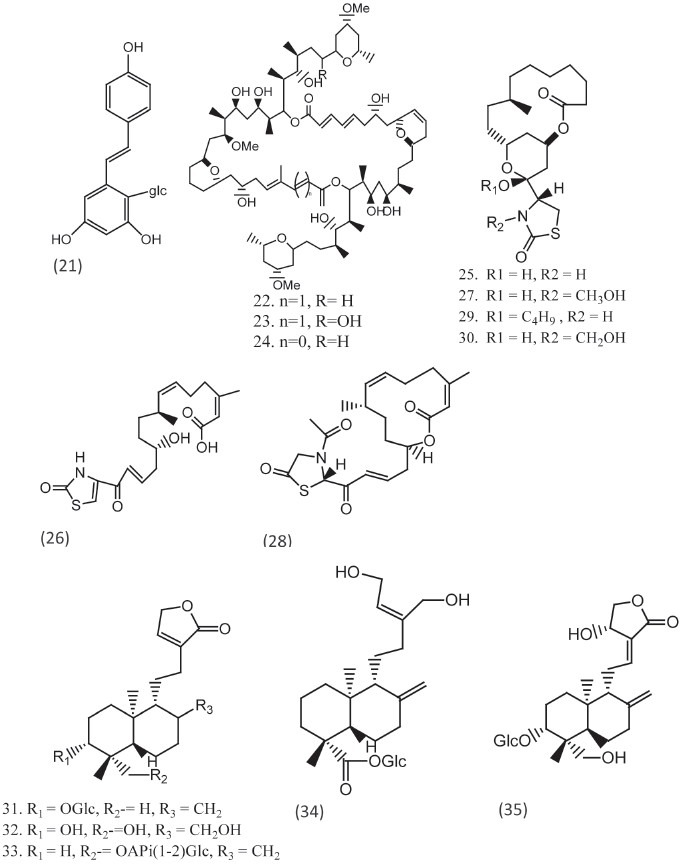
Compounds with thiazoles and macrolide structures
Three new N-methyl-4-hydroxy-2-pyridinone analogs, 6-epi-oxysporidinone (36), were isolated from the fungus Fusarium oxysporum (N17B). The compound showed selective fungistatic activity against Aspergillus fumigatus.[18] Four dialkylresorcinols (37-40) were isolated from the liquid culture of Pseudomonas sp. Ki19. These compounds were reported to inhibit A. fumigatus and Fusarium culmorum at 50 μg/ml.[19] Three linear sesquiterpene lactones, anthecotulide (41), hydroxyl anthecotulide (42), and acetoxyanthecotulide (43) were isolated from aerial parts of Anthemis auriculata.[20]
Two new azapilones named rotiorinols (44, 45) and stereoisomer (-)-rotiorin (46), and a known compound rubrotiorin (47) were isolated from the fungus Chaetorium cupreum CC3003. Compounds exhibited activity against C. albicans with IC50 values of 10.5, 16.7, 24.3, and 0.6, respectively [Figure 4].[21] Crysotriones A (48) and B (49), two new 2-acylcyclopentene-1,3-dione derivatives, were isolated from the fruiting bodied of the Basidiomycete Hygrophorus chrysodon found in mushrooms have shown activity against Fusarium verticillioides.[22] Two new peptaibols, septocylindrin A and septocylindrin B, were obtained from the fungus Septocylindrium sp.[23] A new marine -derived macrolides as neopeltolide (50) were reported to have activity against fungal pathogen C. albicans with MIC of 0.62 μg/ml.[24] The natural product dihydroferulic acid (DFA, 51) and the six synthesized derivatives of it were examined for antifungal activities. Test fungi included Saccharomyces cerevisiae, A. fumigatus, and A. flavus.[25] Six new minor dammarane triterpenoids (52, 53) were isolated from the roots of Gentiana rigescens. These are the glycosides and have shown antifungal activity against the pathogen Glomerella cingulata.[26]
Figure 4.
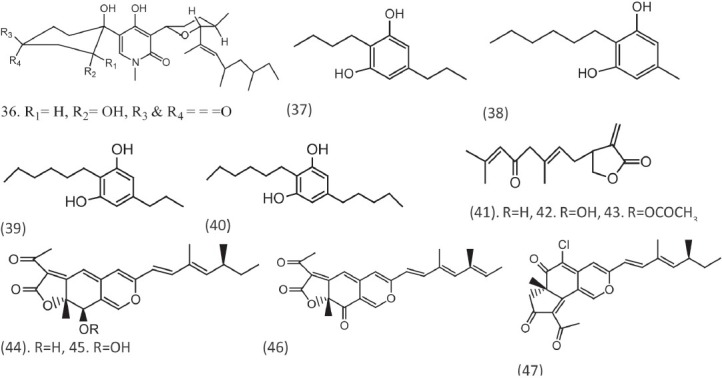
Compounds reported with antifungal activity from different chemical categories
Four solanapyrone analogs (solanapyrones, 54–57) were reported from an unidentified fungicolous fungus and showed activity against A. flavus and F. verticillioides, and C. albicans.[27] Two volatile benzaldehyde derivatives (58, 59) were isolated from a Basidiomycete Sacodontia crosea found to be active against various pathogenic fungi. The aerial part of Centaurea pullata resulted into compounds (60, 61) which is reported to have potent activity [Figure 5].[28]
Figure 5.
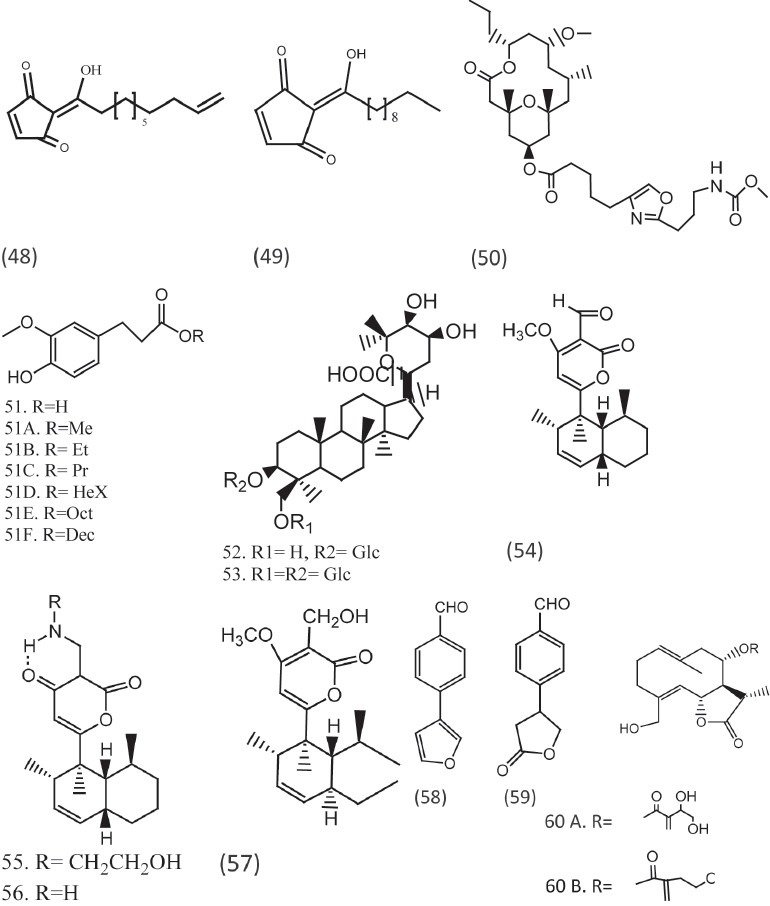
Compounds with remarkable antifungal activities
Leaves of Piper scutifolium yielded two new isobutyl amides, scutifolium A and scutifoliamide B, which were reported as fungistatics.[29] Compound phaeosphenone (62, 63) was isolated from Staphylococcus aureus and inhibited the growth of C. albicans with an MIC of 8 μg/ml.[30] 4-Terpenyl cannabinolate (64) which is isolated from Cannabis sativa have shown activity against C. albicans ATCC 90028 with an IC50 value of 8.5 μg/ml.[31] 1α,5α-Dioxy-11 α-hydroxyurs-12-en-3-one (65) and urasane triterpenoid (66) exhibited modest activity against E. coli, C. albicans with the MIC 31.9μM/L and 36.3μM/L, respectively.[32]
Pestafolide A (67), a new reduced spiro azaphilone derivative, and pestaphthalides A (68) and B (69) were isolated from Pestalotiopsis foedan. These compounds have shown activity against C. albicans (ATCC 10231), Geotrichum candidum, and A. fumigates (ATCC 10894).[33]
Lipophilic extracts of the stem bark of Buddleja globosa were found to have antifungal activity at 125 μg/ml against three dermatophytic fungal species Trichophyton rubrum, Tricophyton interdigitale, and Epidermophyton floccosum. The known compounds were the diterpene buddlejone (70), the bisditerpene maytenone, and the two sesquiterpenes buddledin A and buddledin B, while the novel compound was characterized as the diterpene deoxybuddlejone (71).[34]
From witches’ broom diseased bamboo, Phyllostachys bambusoides, N-p-coumaroylserotonin, and N-feruloylserotonin were isolated. N-p-Coumaroylserotonin possesses antifungal activity against Aciculosporium take, the causal agent of witches’ broom of bamboo. A new antifungal substance against rice blast fungus (Pyricularia oryzae), 5-(8’-Z-heptadecenyl) resorcinol, was isolated from etiolated rice seedlings together with a mixture of its homologs with C13, C15, and C17 saturated alkyl chains. Its structure was determined by 1NMR, 13C-NMR, and MS spectra. Its ED50 was 40μg/ml.[35]
Two antifungal terpenoids, 6-isopropyltropolone β-glucoside and 5-(3-hydroxy-3-methyl-trans-1-butenyl)-6-isopropyl-tropolone β-glucoside, named by us cupressotropolone A and B, respectively, were isolated from the bark of Italian cypress, in response to infection by the fungus Diplodia pinea f. sp. cupressi. These tropolone glucosides inhibited in vitro germination of spores of Diplodia pinea f. sp. cupressi, Seiridium cardinale, Alternaria alternate, and Verticillium dahliae.[36] The antifungal activity of Monilinia fructicola from some naturally occurring pterocarpans were reported with ED50 values around 2 × 10−5 μg/ml.[37]
Secondary metabolites were extracted from culture filtrates of three isolates of the mycoparasitic biocontrol agent Verticillium biguttatum. The two antifungal metabolites were 1-[2′,3′-dihydroxy-5′-(hydroxymethyl)phenyl]-3-methyl-but-2-ene (72) and 1-[2′-hydroxy-3′-methoxy-5′(hydroxymethyl)phenyl]-3-methyl-but-2-ene (72, 73). These new metabolites were named bigutol and methylbigutol, respectively. Low concentrations of each metabolite inhibited mycelial extension of Rhizoctonia solani (minimum inhibitory concentration 138 μg/ml). Production of these antifungals by V. biguttatum suggests that antibiosis may play a role during biocontrol by this mycoparasite, particularly of plant diseases caused by R. solani.[38] Four new phenalenone-type phytoalexins, named musanolones (74–77), have been isolated from infected rhizomes of banana plants (Musa acuminata; AAA cultivar Grand Nain). All the compounds have reported to have a strong inhibitory activity on the growth of the germination tube of F. oxysporum f. sp. cubense race 4.[39]
Bioassay-guided fractionation resulted in the isolation of four antifungal agents from the roots of Cudrania cochinchinensis. Two of these were new compounds, cudraxanthone S 13,56-tetrahydroxy-2-(1,1-dimethyl-2-propenyl)xanthone] (78) and cudraflavanone B (2′,4′,57-tetrahydroxy-6-prenylflavanone) (79). These compounds exhibited antifungal activities against C. neoformans, A. fumigatus, and A. nidulans (MICs , 2–8 μg/ml) [Figure 6].[40]
Figure 6.
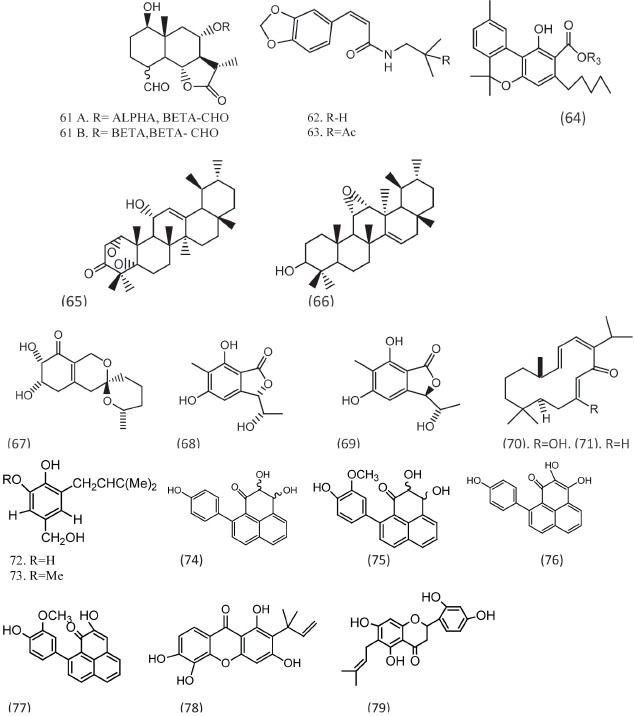
Compounds with good antifungal activities
Vitis vinifera antimicrobial peptide (Vv-AMP1), isolated from Vitis vinifera, shown to encode a plant defensin. Recombinant Vv-AMP1 showed nonmorphogenic antifungal activity against a broad spectrum of fungi, probably altering the membrane permeability of the fungal pathogens. Recombinant Vv-AMP1 was extremely heat-stable and showed strong antifungal activity against a broad spectrum of plant pathogenic fungi, with very high levels of activity against the wilting disease causing pathogens F. oxysporum and Verticillium dahliae. The Vv-AMP1 peptide did not induce morphological changes on the treated fungal hyphae, but instead strongly inhibited hyphal elongation. A propidium iodide uptake assay suggested that the inhibitory activity of Vv-AMP1 might be associated with altering the membrane permeability of the fungal membranes.[41,42]
Dalbergia is a genus of trees, shrubs, and woody climbers widely distributed in tropical and sub- tropical regions. It possesses good antimicrobial activity.[43] Terminalia chebula plant was also reported to have antifungal activity.[44] Entada abysinnica (Fabaceae) leaves used by herbalists from the Lake Victoria region, Kenya. E. abysinnica could be a rich source of antimicrobial agents, especially antifungals (zones of inhibition were between 9.00 and 14.10 mm) against C. albicans, Sa. typhi, and St. aureus.[45] Butea monosperma has been reported for their antimicrobial activities.[46] Nyctanthes arbortristis have also shown great potential for antifungal activity.[47]
CONCLUSION
A large number of metabolites from marine invertebrates, from plants, and from various other natural sources have been reported to inhibit the pathogenic fungi. These compounds represent a wide variety of structural classes ranging from cinnamodial sterols, furanones, quinines, and resorcinol to terpenoids in natural origin category. The spread of multidrug-resistant strains of fungus and the reduced number of drugs available make it necessary to discover new classes of antifungals and compounds that inhibit these resistant mechanisms. This has led to a search for therapeutic alternatives, particularly among medicinal plants and compounds isolated from them used for their empirically antifungal properties. In these natural sources, a series of molecules with antifungal activity against different strains of fungus have been found, which are of great importance to humans and plants. It is hoped that other chemists will embark on the search for potent antifungal agents.
ACKNOWLEDGMENTS
The authors are thankful to Head, School of Pharmacy to provide e-library facilities. The author Sudha Vengurlekar is thankful to Council for Scientific and Industrial Research (CSIR), New Delhi, India for providing senior research fellowship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Treyvaud Amiguet V, Petit P, Ta CA, Nuñez R, Sánchez-Vindas P, Alvarez LP, et al. Phytochemistry and antifungal properties of the newly discovered tree pleodendron costaricense. J Nat Prod. 2006;69:1005–9. doi: 10.1021/np0504863. [DOI] [PubMed] [Google Scholar]
- 2.Bossche HV, Koymans L. Review Article Cytochromes P450 in fungi. Mycoses. 1998;41:32. doi: 10.1111/j.1439-0507.1998.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 3.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Lupinacci J, et al. Caspofungin vs amphotericin B deoxycolatein in the the treatment of invasive candidiasis in neutropenic patients: A multi-centre, randomized, double-blind study. N Engl J Med. 2002;347:2020–9. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 4.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: Systematic review of the literature. Clin Infec Dis. 2001;32:358–66. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 5.Case CP, MacGowan AP, Brown NM, Reeves DS, Whitehead P, Felmingham D. Prophylactic oral fluconazole and candida fungaemia. Lancet. 1991;337:790. doi: 10.1016/0140-6736(91)91406-k. [DOI] [PubMed] [Google Scholar]
- 6.Winguard JR, Merz WG, Rinaldi MG, Jonhson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–77. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 7.Georgopapada NH, Walsh TJ. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–91. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontoyiannis DP, Mantadakis E, Smonis G. Systemic mycosis in immunocompromised host: An update in antifungal therapy. J Hosp Infec. 2003;53:243–58. doi: 10.1053/jhin.2002.1278. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drug over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 10.Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotricin B and Caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis. 2006;42:938–44. doi: 10.1086/500939. [DOI] [PubMed] [Google Scholar]
- 11.Amiguet VT, Petit P, Ta CA, Nunez R, Vindas PS, Alvarez LP, et al. Phytochemistry and antifungal properties of the newly discovered tree pleodendron. J Nat Prod. 2006;69:1005–09. doi: 10.1021/np0504863. [DOI] [PubMed] [Google Scholar]
- 12.Wright AD, de Nys R, Angerhofer CK, Pezzuto JM, Gurrath M. Biological activities and 3D qsar studies of a series of Delisea pulchra(cf.fimbriata) derived natural products. J Nat Prod. 2006;69:1180–7. doi: 10.1021/np050510c. [DOI] [PubMed] [Google Scholar]
- 13.Quang DN, Stadler M, Fournier J, Asakawa Y. Carneic acids A and B, chemotaxonomically significant antimicrobial agents from the xylariaceous ascomycete Hypoxylon carneum. J Nat Prod. 2006;69:1198–202. doi: 10.1021/np0602057. [DOI] [PubMed] [Google Scholar]
- 14.Ge HM, Huang B, Tan SH, Shi da H, Song YC, Tan RX. Bioactive oligostilbenoids from the stem bark of Hopea exalata. J Nat Prod. 2006;69:1800–2. doi: 10.1021/np060242y. [DOI] [PubMed] [Google Scholar]
- 15.Youssef DT, Moobery SL. Hurghadolide A and swinholide I, potent actin microfilament disrupters from the red sea sponge Theonella swinhoei. J Nat Prod. 2006;69:154–7. doi: 10.1021/np050404a. [DOI] [PubMed] [Google Scholar]
- 16.El Sayed KA, Youssef DT, Marchetti D. Bioactive natural and semisynthetic Latrunculins. J Nat Prod. 2006;69:219–23. doi: 10.1021/np050372r. [DOI] [PubMed] [Google Scholar]
- 17.Shen YH, Li RT, Xiao WL, Xu G, Lin ZW, Zhao QS, et al. ent-Labdane diterpenoids from Andrographis paniculata. J Nat Prod. 2006;69:319–22. doi: 10.1021/np050160u. [DOI] [PubMed] [Google Scholar]
- 18.Jayasinghe L, Abbas HK, Jacob MR, Herath WH, Nanayakkara NP. N-Methyl-4-hydroxy-2-pyridinone analogues from Fusarium oxysporum. J Nat Prod. 2006;69:439–42. doi: 10.1021/np050487v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohanka A, Levenfors J, Broberg A. Antimicrobial dialkylresorcinols from Pseudomonas sp.Ki19. J Nat Prod. 2006;69:654–7. doi: 10.1021/np0600595. [DOI] [PubMed] [Google Scholar]
- 20.Theodori R, Karioti A, Rancic A, Skaltsa H. Linear sesquiterpene lactones from Anthemis auriculata and their antibacterial activity. J Nat Prod. 2006;69:662–4. doi: 10.1021/np058084i. [DOI] [PubMed] [Google Scholar]
- 21.Kannokmedhakul S, Kanokmedhakul K, Nasomjai P, Louangsysouphanh S, Soytong K, Isobe M, et al. Antifungal azaphilones from the fungus Chaetomium cupreum CC3003. J Nat Prod. 2006;69:891–5. doi: 10.1021/np060051v. [DOI] [PubMed] [Google Scholar]
- 22.Gilardoni G, Clericuzio M, Tosi S, Zanoni G, Vidari G. Antifungal acyclopentenediones from fruiting bodies of hygrophorus chrysodon. J Nat Prod. 2007;70:137–9. doi: 10.1021/np060512c. [DOI] [PubMed] [Google Scholar]
- 23.Gassner NC, Tamble CM, Bock JE, Cotton N, White KN, Tenny K, et al. Accelerating the discovery of biologically active small molecules using a high-throughput yeast halo assay. J Nat Prod. 2007;70:383–90. doi: 10.1021/np060555t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright AE, Bothelo JC, Guzman E, Harmody D, Linley P, McCarthy PJ, et al. Neopeltolide a macrolide from a Lithistid sponge of the family Neopeltidate. J Nat Prod. 2007;70:412–6. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- 25.Beck JJ, Kim JH, Campbell BC, Chou SC. Fungicidal activities of dihydroferulic acid alkyl ester analogues. J Nat Prod. 2007;70:779–82. doi: 10.1021/np0606345. [DOI] [PubMed] [Google Scholar]
- 26.Xu M, Wang D, Zhang YJ, Yang CR. Dammarane from the roots of Gentiana rigescens. J Nat Prod. 2007;70:880–3. doi: 10.1021/np070012z. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt LE, Gloer JB, Wicklow DT. Solanapyrone analogues from a Hawaiian fungicolous fungas. J Nat Prod. 2007;70:1317–20. doi: 10.1021/np070251m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokubun T, Rozwadowski Z, Duddeck H. Benzaldehyde derivatives from sarcodontia crocea. J Nat Prod. 2007;70:1539–41. doi: 10.1021/np070305s. [DOI] [PubMed] [Google Scholar]
- 29.Djeddi S, Karioti A, Sokovic M, Stojkovic D, Seridi R, Skaltsa H. Minor sesquiterpene lactones from Centaurea pullata and their antimicrobial activity. J Nat Prod. 2007;70:1796–9. doi: 10.1021/np070125i. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Ondeyka JG, Zink DL, Basilio A, Vicente F, Collado J, et al. Isolation, structure and antibacterial activity of Phaeosphenone from a Phaeosphaeria sp. discovered by antisense strategy. J Nat Prod. 2008;71:1304–7. doi: 10.1021/np8001833. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed SA, Ross SA, Slade D, Radwan MM, Zulfiqar F, Matsumoto RR, et al. Cannabinoid Ester constituents from high potency Cannabis sativa. J Nat Prod. 2008;71:536–42. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JJ, Fei DQ, Chen SG, Gao K. Antimicrobial triterpenoids from Vladimiria muliensis. J Nat Prod. 2008;71:547–50. doi: 10.1021/np070483l. [DOI] [PubMed] [Google Scholar]
- 33.Ding G, Liu S, Guo L, Zhou Y, Che Y. Antifungal metabolites from the plant endophytic fungus Pesalotiopsis foedan. J Nat Prod. 2008;71:615–8. doi: 10.1021/np070590f. [DOI] [PubMed] [Google Scholar]
- 34.Mensah AY, Houghton PJ, Bloomfield S, Vlietinck A, Vanden Berghe D. Known and Novel Terpenes from Buddleja globosa displaying selective antifungal activity against dermatophytes. J Nat Prod. 2000;63:1210–3. doi: 10.1021/np0001023. [DOI] [PubMed] [Google Scholar]
- 35.Esumi SY, Hyakutake H, Kono Y, Sakurai A. Isolation of 5-(8’Z-heptadecenyl)-resorcinol from etiolated rice seedlings as an antifungal agent. Phytochemistry. 1996;41:1485–9. [Google Scholar]
- 36.Madar Z, Gottlieb HE, Cojocaru M, Riov J, Solel Z, Sztejnberg A. Antifungal terpenoids produced by cypress after infection by Diplodia pinea f. sp. cupressi. Phytochemistry. 1995;38:351–4. [Google Scholar]
- 37.Perrin DR, Cruickshank IAM. The Anti Fungal Activity of Pterocarpans Towards Monilinia-Fructicola Pisum-Sativum-D Trifolium-Pratense-D Disease Resistance. Phytochemistry. 1969;8:971–8. [Google Scholar]
- 38.Morris RA, Ewing DF, Whipps JM, Coley Smith JR. Antifungal hydroxymethyl-phenols from the mycoparasite Verticillium biguttatum. Phytochemistry. 1995;39:1043–8. [Google Scholar]
- 39.Luis JG, Quiiqones W, Echeverri F, Grillo TA, Kishi MP, Garcia FG, et al. Musanolones: Four 9-phenylphenalenones from rhizomes of musa acuminate. Phytochemistry. 1996;41:753–7. [Google Scholar]
- 40.Fukai T, Yonekawa M, Hou AJ, Nomura T, Sun HD, Uno J. Antifungal Agents from the Roots of Cudrania cochinchinensis against Candida, Cryptococcus, and Aspergillus Species. J Nat Prod. 2003;66:1118–20. doi: 10.1021/np030024u. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfield L, Claire-Lise M, Vidal M, Jose R. Transgenic Disease Resistance in Vitis vinifera: Potential Use and Screening. Am J Enol Vitic. 2010;61:348–57. [Google Scholar]
- 42.Vidal JR, Kikkert JR, Malnoy MA, Wallace PG, Barnard J, Reisch BI. Evaluation of transgenic ‘Chardonnay’ (Vitis vinifera) containing magainin genes for resistance to crown gall and powdery mildew. Transgenic Res. 2006;15:69–82. doi: 10.1007/s11248-005-4423-5. [DOI] [PubMed] [Google Scholar]
- 43.Neeru V, Manisha V, Sharma SK, Satish S. Chemistry and biological activities of the genus Dalbergia - A review. Phcog Rev. 2009;3:307–19. [Google Scholar]
- 44.Chattopadhyay RR, Bhattacharyya SK. PHCOG REV.: Plant Review Terminalia chebula: An update. Pharmacogn Rev. 2007;1:151–6. [Google Scholar]
- 45.Richard MM, John AO, Paul OO, Paul KM. Antifungal, antibacterial and antimycobacterial activity of Entada abysinnica Steudel ex A.Rich (Fabaceae) methanol extract. Phcog Res. 2010;2:163–8. doi: 10.4103/0974-8490.65511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burli DA, Khade AB. A Comprehensive review on Butea monosperma (Lam.) Kuntze. Pharmacogn Rev. 2007;1:333–7. [Google Scholar]
- 47.Sasmal D, Das S, Basu SP. Phytoconstituents and therapeutic potential of Nyctanthes arbortristis Linn. Pharmacogn Rev. 2007;1:344–9. [Google Scholar]


