Abstract
Fibroblast-like synoviocyte (FLS) invasiveness correlates with articular damage in rheumatoid arthritis (RA), yet little is known about its regulation. In this study we aimed to determine the role of the nuclear receptor liver X receptor (LXR) in FLS invasion. FLS were isolated from synovial tissues obtained from RA patients and from DA rats with pristane-induced arthritis. Invasion was tested on Matrigel-coated chambers in the presence of the LXR agonist T0901317, or control vehicle. FLS were cultured in the presence or absence of T0901317, and supernatants were used to quantify matrix metalloproteinase 1 (MMP-1), MMP-2, MMP-3, interleukin-6 (IL-6), tumor necrosis factor-α and C-X-C motif chemokine ligand 10 (CXCL10). Nuclear factor-κB (NF-κB) (p65) and Akt activation, actin cytoskeleton, cell morphology and lamellipodia formation were also determined. The LXR agonist T0901317 significantly reduced DA FLS invasion by 99% (P ≤ 0.001), and RA FLS invasion by 96% (P ≤ 0.001), compared with control. T0901317-induced suppression of invasion was associated with reduced production of activated MMP-2, IL-6 and CXCL10 by RA FLS, and with reduction of actin filament reorganization and reduced polarized formation of lamellipodia. T0901317 also prevented both IL-1β–induced and IL-6–induced FLS invasion. NF-κB (p65) and Akt activation were not significantly affected by T0901317. This is the first description of a role for LXR in the regulation of FLS invasion and in processes and pathways implicated both in invasion as well as in inflammatory responses. These findings provide a new rationale for considering LXR agonists as therapeutic agents aimed at reducing both inflammation and FLS-mediated invasion and destruction in RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects 0.5– 1% of the population and is associated with increased risk for joint deformities, disability, and reduced longevity (1,2). The RA synovial tissue is characterized by synovial hyperplasia, also called “pannus,” which is infiltrated with inflammatory cells. The RA synovial pannus produces proinflammatory cytokines, chemokines and proteases, and invades and destroys cartilage and bone (3). The fibroblast-like synoviocyte (FLS) has a central role in the formation of the RA synovial pannus and in joint destruction (3,4).
The in vitro invasive properties of FLS from patients with RA and from rats with pristane-induced arthritis (PIA) through collagen-rich Matrigel have been shown to correlate with radiographic erosive changes and with histological joint damage, respectively (5,6). Erosive changes and joint damage correlate with worse disease outcome, including increased risk for disability and for the development of deformities (7–9). Therefore, understanding of the processes and genes regulating FLS invasion has the potential to generate new targets for therapies aimed at reducing articular damage as well as improving disease outcome.
We have recently determined that synovial tissues from arthritic DA rats, a strain that develops severe and erosive PIA, have significantly reduced expression of nuclear receptors (NRs) compared with synovial tissues from rats with mild and nonerosive disease (10). Additionally, DA rats have reduced expression of NR target genes, suggesting not only reduced NR expression but also reduced NR activity. NRs are a family of ligand-activated transcription factors that regulate several cellular processes, including lipid metabolism, calcium metabolism and, importantly for arthritis, inflammatory responses (11). Members of the NR family include the vitamin D receptor (VDR), retinoid X receptor γ (RXRγ) and the liver X receptor α (LXRα), among others (12). Therefore, we hypothesized that the increased expression and activity of one or more NRs in synovial tissues might have a protective effect in maintaining inflammation-free and noninvasive synovial tissue and FLS (13).
LXRα can be stimulated by endogenous ligands such as oxysterols and forms heterodimers with RXRγ. The LXRα–RXRγ dimer binds to LXR-responsive elements located in the promoter of gene targets to modulate gene expression. LXRα is of particular interest for four additional reasons. First, LXR agonists have been shown to ameliorate several rodent models of inflammation and autoimmunity, such as lung inflammation (14), acute dermatitis (15), experimental allergic en-cephalomyelitis (EAE) (16) and murine lupus (17). Second, LXR agonists reduce macrophage expression of proinflammatory cytokines (15). Third, LXR agonists successfully treated collagen-induced arthritis (CIA) in mice, and reduced disease severity and articular damage via unknown mechanisms (18,19). And fourth, DA synovial tissues expressed increased levels of CYP7b1, which is an enzyme that inactivates oxysterols, the natural ligands and activators of LXR synthesized from cholesterol (13), suggesting reduced levels of activating ligands for LXRα in arthritis-susceptible DA synovial tissues.
In the present study we investigated the potential role of LXR in the regulation of the invasive properties of FLS from patients with RA and DA rats.
MATERIALS AND METHODS
Rats and the Induction of PIA
Arthritis-susceptible DA rats (DA/Hsd) (8–12 wks old) were housed in a specific pathogen-free environment. For the induction of PIA, DA rats received 150 μL of pristane by intradermal injection at the base of the tail (20). All rats developed PIA and were euthanized on d 21 after induction, and synovial tissues were collected from the ankle joints for the isolation of FLS. All animal work was approved by the Feinstein Institute’s institutional animal care and use committee.
RA Patients
Synovial tissues were obtained from RA patients who met the 1987 American College of Rheumatology criteria (21) and were undergoing an elective orthopedic surgery. All patients signed an informed consent form under a protocol approved by the Feinstein Institute’s institutional review board.
Isolation and Culture of FLS
FLS were isolated by use of enzymatic digestion from synovial tissues from DA rats and from RA patients, as previously described (5,22). Briefly, tissues were minced and incubated with a solution containing 0.15 mg/mL DNase, 0.15 mg/mL hyaluronidase (type I-S) and 1 mg/mL collagenase (type IA) (Sigma-Aldrich, St. Louis, MO, USA) in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) for 1 h at 37°C. Cells were washed and resuspended in complete medium containing DMEM with 10% fetal bovine serum (FBS), glutamine 30 ng/mL, amphotericin B 250 μg/mL and gentamycin 20 ng/mL (Invitrogen) for culture. All experiments were done with cells cultured for at least four passages (>95% FLS purity).
The T0901317 LXR Agonist
T0901317 (Cayman Chemicals, Ann Arbor, MI, USA) is a synthetic agonist that specifically binds and activates both LXRα and β at 1 μmol/L and 10 μmol/L concentrations (15,23). These concentrations were used in the present study. T0901317 was reconstituted in dimethyl sulfoxide (DMSO), and DMSO was used as vehicle control in all experiments.
MTT Survival Assay
FLS were plated in triplicate at 1 × 104 cells per well in 96-well flat-bottomed plates and allowed to adhere for 24 h in complete medium (described above). FLS were treated with T0901317 at 10 μmol/L or DMSO in serum-free medium for 24 h. Cell survival was determined by using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Millipore, Billerica, MA, USA) according to manufacturer’s instructions.
FLS Invasion Assay
We tested the effect of T0901317 on FLS invasion using an in vitro trans-well system through collagen-rich Matrigel (BD, Franklin Lakes, NJ, USA) as previously described (5,24). Briefly, 70–80% confluent FLS were harvested by trypsin-EDTA digestion. Then 2 × 104 cells were resuspended in 500 μL of serum-free DMEM and plated in the upper compartment of the Matrigel-coated inserts. T0901317 (final concentration of 1 μmol/L or 10 μmol/L) or the same amount of the control solvent DMSO was added to the upper chambers. The lower compartment was filled with complete media. After 24 h incubation at 37ºC the supernatant in the upper chamber was collected, and the upper surface of the insert was scraped with cotton swabs to remove noninvading cells and the Matrigel layer. The opposite side of the insert was stained with Crystal Violet (Sigma, Saint Louis, MO, USA) and the total number of cells that invaded through Matrigel was counted at 100× magnification. Experimental treatments were done in duplicate.
Quantitative Real-Time PCR
FLS RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Samples were digested with DNase (Qiagen) and eluted with 30 μL of RNase-free water. RNAs were quantified and assessed for purity with a NanoDrop spectrophotometer (Rockland, DE, USA). We used 200 ng of total RNA from each sample for cDNA synthesis with the Superscript III kit (Invitrogen).
Quantitative real-time PCR (qPCR) was done as previously described (5). Briefly, we used the Universal ProbeLibrary (Roche, Indianapolis, IN, USA). Probes were used at a final concentration of 250 nmol/L. We designed primers targeting rat and human genes with the Universal ProbeLibrary Assay Design Center (Roche), and used a 400-nmol/L concentration with Absolute Blue QPCR Master Mix (Thermo Scientific, Surrey, UK). Samples were run in duplicate on a Roche 480 qPCR thermocycler, and the means were used for analysis. Data were analyzed with LC480 software version 1.5 (Roche). Relative expression of all the genes was adjusted for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in each sample (ΔCt), and the ΔCt was used for t test analysis. A P value ≤0.01 was considered significant. Fold-differences were calculated with the 2−ΔΔCt method (25).
Zymography
FLS were cultured for 24 h in the upper chambers of the Matrigel-coated inserts as part of the invasion experiments. Supernatant from each upper chamber was collected and concentrated with Microcon YM30 columns (Millipore). Equal amounts of concentrated supernatant protein was mixed with Tris-glycine–sodium dodecyl sulfate sample buffer (Invitrogen), then loaded into a precasted zymogram gel (Invitrogen) and run for 90 min at 125 V, as previously described (5). Gels were then treated with renaturing buffer (Invitrogen), followed by overnight incubation in developing buffer (Invitrogen) at 37°C. Gels were stained with SimplyBlue Safe Stain (Invitrogen) for 1 h at room temperature and washed. Gelatin zymography was used to assess MMP-2, and casein zymography was used for MMP-3 (5,26).
Cytometric Bead–Based Cytokine Detection
Supernatants from the upper chambers of RA FLS invasion experiments were concentrated with Microcon columns (Millipore) and analyzed for total protein content using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). Concentrations of IL-6, IL-8, IL-10, TNFα, monocyte chemotactic protein-1 (MCP-1) and CXCL10 were determined with a bead-based detection system (Cytometric Bead Array, CBA, BD Bioscience, San Jose, CA, USA) according to the manufacturer’s protocol.
Western Blots
MMP-1
Equal volumes of the upper-chamber supernatants (as described in the zymography section) were loaded on a NuPAGE 10% Bis-Tris gel (Invitrogen) in the presence of 2-(N-morpholino) ethanesulfonic acid (MES) buffer (Invitrogen) and run under reducing conditions. Proteins were transferred overnight to a polyvinylidene difluoride (PVDF) membrane (Immobilion, Millipore). The membrane was then blocked with 5% blotting-grade nonfat dry milk (Bio-Rad, Hercules, CA, USA) and incubated with mouse monoclonal anti-MMP-1 antibody (Calbiochem, EMD, La Jolla, CA, USA). Horseradish peroxidase–conjugated anti-mouse IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the secondary antibody.
Akt and phospho-Akt
For detection of Akt, FLS were plated at 70–80% confluence in 6-well plates and allowed to adhere for 24 h. Cells were then serum starved for 24 h and pretreated with T0901317 10 μmol/L or DMSO (control) for 2 h, followed by 5-min culture with IL-1β 10 ng/mL. Total cell lysates were collected with radioimmunoprecipitation assay buffer (RIPA, Thermo Scientific) containing Halt Protease & Phosphatase inhibitor cocktail (Thermo Scientific). Protein content was determined with a BCA protein assay kit (Thermo Scientific) and the same amount of total proteins was loaded on a NuPAGE 10% Bis-Tris gel (Invitrogen) in the presence of MES buffer (Invitrogen) and in reducing conditions. Proteins were transferred to PVDF membranes, blocked with 5% nonfat milk, then probed with antibodies against the phosphorylated form of Akt (Cell Signaling, Danvers, MA, USA). Horseradish peroxidase–conjugated anti-mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) was used as secondary antibody for 1 h at room temperature in Tris-buffered saline with 0.1% Tween-20. Protein bands were detected by Amersham ECL plus (GE Healthcare, Buckinghamshire, UK) and visualized using X-OMAT Kodak film. Following the analyses of phospho-Akt, membranes were stripped and reprobed with a rabbit antibody against total Akt (Cell Signaling) and an anti-rabbit IgG (Amersham, GE Healthcare) used as secondary antibodies. Densitometry was done with Adobe Photoshop, version 7.0 (San Jose, CA, USA).
LXRα and LXRβ
DA FLS were stimulated and cultured under the same conditions described above for Akt in the presence or absence of DMSO (control), T0901317 10 μmol/L and IL-1β 10 ng/mL for 24 h, 48 h or 72 h, followed by cell lysis and protein extraction. A rabbit polyclonal antibody that recognizes both LXRα and LXRβ (Santa Cruz Biotechnology) was used, and a monoclonal antibody against human Vinculin (Sigma) was used as control.
p65 Nuclear Factor-κB Activity
DNA-binding activity of the nuclear factor-κB (NF-κB) protein p65 was quantified in nuclear extracts using the TransAM™ NF-κB (p65) (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, FLS were pretreated for 20 h with T0901317 10 μmol/L or DMSO and then stimulated with IL-1β 10 ng/mL for 30 min.
Immunofluorescence Microscopy
Confluent FLS (10–20%) were cultured on cover slips, starved overnight and then treated with either DMSO or T0901317 10 μmol/L in complete media for 20 min. FLS were then fixed with 4% formaldehyde (Ted Pella Inc., Redding, CA, USA) and permeabilized with phosphate-buffered saline (PBS)-Triton X-100 0.1%, and blocked with 5% nonfat milk. Cells were incubated with rabbit antibodies against phosphorylated focal adhesion kinase-1 (FAK) (Cell Signaling) for 1 h at room temperature, washed with PBS with 0.1% Triton X-100, and then incubated with TexRed-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min, and Phalloidin–fluorescein isothiocyanate (FITC) (Sigma-Aldrich) used to stain actin filaments. Stained and washed cells were mounted and observed under fluorescent microscopy (Zeiss Axiovert 200M with Zeiss software Axioversion 4.7).
Actin Cytoskeleton, Lamellipodia and Phospho-FAK Scoring System
To quantify the potential changes induced by T0901317 in FLS morphology, we recently developed a new scoring system (27), which includes a previously established actin scoring system (28), plus the addition of scores for the following major parameters of interest: (a) actin filament characteristics and distribution (0–3); (b) cell morphology (0 or 2); (c) lamellipodia location (0 or 2); and (d) distribution of phosphorylated FAK (0–2). The cell scoring ranged from zero to nine, and 20 cells per treatment group and per cell line were analyzed.
All supplementary materials are available online at www.molmed.org.
RESULTS
LXR is Expressed and Functional in Arthritic DA FLS
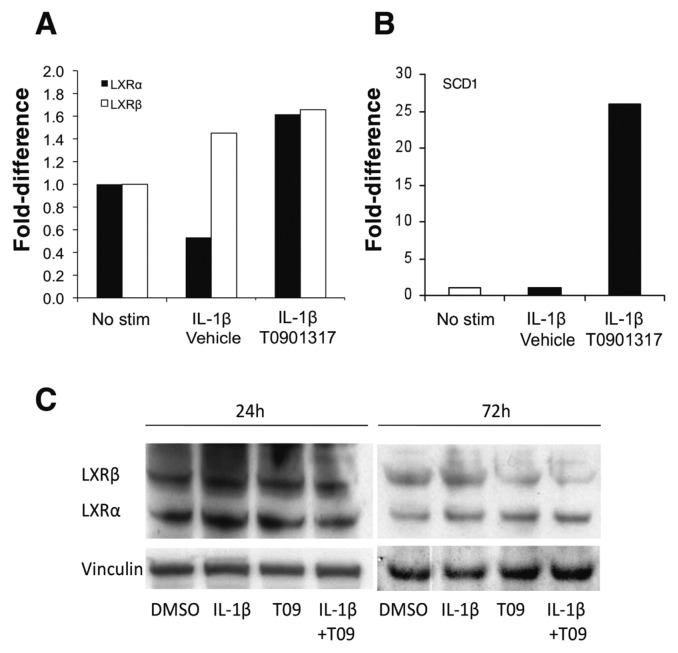
We have previously shown that LXRα and its targets are expressed in reduced levels in synovial tissues and in FLS from DA rats compared with arthritis-resistant rats (10). LXRβ is also expressed by DA synovial tissues and FLS, but levels were similar to those in arthritis-resistant rats, and therefore less likely to account for the differences in disease severity. We considered that proinflammatory cytokines expressed at increased levels in arthritic synovial tissues might reduce LXR expression and interfere with its transcriptional activity, potentially creating a state of reduced sensitivity to natural LXR ligands. To test this hypothesis, we used IL-1β, IL-6 and IL-17, all expressed at significantly increased levels in the synovial tissues of RA patients and DA rats (29,30). RA FLS were cultured in the presence or absence of IL-1β (10 ng/mL), IL-6 (10–100 ng/mL) or IL-17 (0.1–10 ng/mL) and T0901317 (10 μmol/L) for 24 h, followed by RNA extraction and qPCR for LXRα, LXRβ and the LXR-inducible gene stearoyl-CoA desaturase 1 (SCD1). IL-1β–treated FLS had a significantly reduced expression of LXRα but not LXRβ (Figure 1A). The IL-1β–induced reduction in LXRα levels was prevented by pre-treatment with T0901317. SCD1 levels were low in unstimulated FLS and remained low following IL-1β treatment (Figure 1B). However, treatment with T0901317 resulted in a 26-fold increase in the expression of SCD1, demonstrating that the receptors were responsive to the synthetic agonist T0901317.
Figure 1.
LXR and the LXR-inducible gene SCD1 are expressed in FLS. (A) LXRα and LXRβ expression in RA fibroblast–like synoviocytes is reduced by IL-1β (10 ng/mL) treatment. Pre-treatment with the LXR agonist T0901317 (10 μmol/L) prevented the IL-1β–induced reduction of LXRα (four FLS cell lines from different RA patients). Levels of LXRβ were not significantly affected by IL-1β treatment. (B) Levels of the LXR-inducible gene SCD1 were low at baseline and were not affected by IL-1β treatment. Pretreatment with the LXR agonist T0901317 significantly increased the expression of SCD1 by 26-fold, demonstrating that LXR is responsive to the synthetic agonist (#P = 0.0014, t test; n = 7). (C) Western blot showing that both LXRα and LXRβ proteins are expressed in FLS, but levels are not changed by IL-1β treatment in the presence or absence of T0901317 during 24-h and 72-h periods. Vinculin was used as the loading control for protein normalization (representative of experiments done with five different DA FLS cell lines). No stim, no stimulation.
LXRα and LXRβ proteins were expressed by FLS, but, unlike mRNA levels, protein levels did not change following IL-1β treatment in the presence or absence of T0901317 during 24–72-h experiments. These results suggest that these proteins have a long half-life, and that changes in their levels of protein did not explain the effect of T0901317 on FLS invasion over a 24-h period (Figure 1C).
IL-6 and IL-17 did not cause the same effects as IL-1β on LXR. IL-6 treatment increased LXRα expression by 3.5-fold, and IL-6 plus T0901317-treated FLS continued to have a 1.85-fold increase in LXRα expression compared with controls (Supplementary Figure S1A). IL-17 treatment did not affect the mRNA levels of LXRα, but the addition of T0901317 was still able to increase the expression of LXRα by 2.8-fold (Supplementary Figure S1B). LXRβ mRNA levels were not affected by IL-6 or IL-17 treatment (Supplementary Figures S1A, B).
The LXR Agonist T0901317 Significantly Reduced the Invasive Properties of FLS from DA Rats and from RA Patients
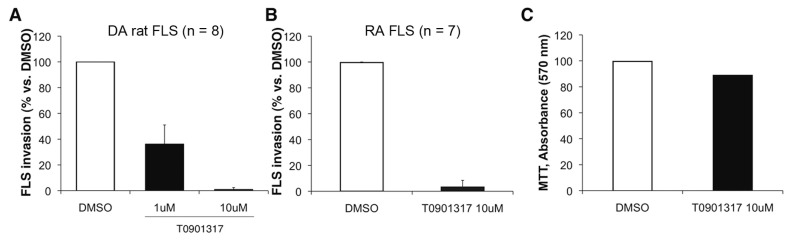
Treatment with T0901317 significantly reduced the number of invading FLS from arthritic DA rats (n = 8) in a dose-dependent manner by 64% (1 μmol/L) and 99% (10 μmol/L), compared with vehicle-treated controls (P < 0.001, Mann-Whitney test; Figure 2A). The numbers of invading FLS from patients with RA (n = 7) were also significantly reduced by 99% in the presence of T0901317 (10 μmol/L), compared with vehicle-treated controls (P ≤ 0.001, Mann-Whitney test; Figure 2B).
Figure 2.
The LXR agonist significantly reduced FLS invasion. (A) The synthetic LXR agonist T0901317 significantly reduced the number of invading DA FLS in a dose-dependent manner by 64% (1 μmol/L) and 99% (10 μmol/L) (P < 0.001, Mann-Whitney test; mean ± standard deviation [SD]; n = 8). (B) Numbers of invading FLS from patients with RA were also significantly reduced by 99% with T0901317 at 10 μmol/L, compared with vehicle-treated (DMSO) controls (P ≤ 0.001, Mann-Whitney test; mean ± SD; n = 7). (C) MTT assay showing that T0901317 10 μmol/L was not toxic and did not cause increased cell death over a 24 h period, the same period of time used for the invasion assays.
The MTT assay showed that T0901317 at 1 μmol/L or 10 μmol/L concentrations did not affect FLS numbers or viability during the same period of time used for the invasion experiments (24 h) (Figure 2C). Therefore, the reduced invasion of FLS treated with T0901317 was unlikely due to drug-induced cell toxicity.
T0901317 Reduced the Levels of Activated MMP-2 in RA FLS
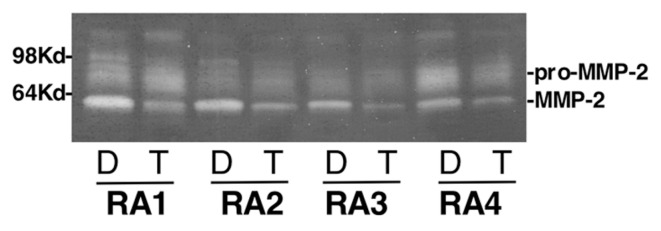
Supernatants of FLS cultured on Matrigel (upper compartment of the invasion chambers) in the presence or absence of T0901317 were analyzed on a gelatin Zymogram. T0901317 treatment significantly reduced levels of the active form of MMP-2 in supernatants of RA FLS (n = 6; Figure 3).
Figure 3.
Treatment with the LXR agonist reduces RA FLS production of active MMP-2. Supernatants from six different RA FLS cell lines (four are shown) cultured on Matrigel were used in a zymogram to determine the amount of MMP-2 in the presence or absence of the LXR agonist T0901317 10 μmol/L (D, DMSO; T, T0901317).
Levels of total and activated forms of MMP-1 and MMP-3 measured in the same FLS supernatants by Western blot and zymography, respectively, were not significantly affected by T0901317 (data not shown).
T0901317 Did Not Affect NF-κB (p65) Activation
DA FLS were stimulated with IL-1β (10 ng/mL) to induce increased NF-κB activation. However, pretreatment with T0901317 did not consistently reduce the activation of p65 (data not shown).
T0901317 Did Not Affect Akt Phosphorylation
FLS were treated with FBS 10% to induce Akt activation and phosphorylation. Pretreatment with T0901317 did not significantly reduce the phosphorylation of Akt (n = 3; data not shown).
Stimulation of LXR Inhibits FLS Morphological Changes Required for Polarized Migration and Invasion
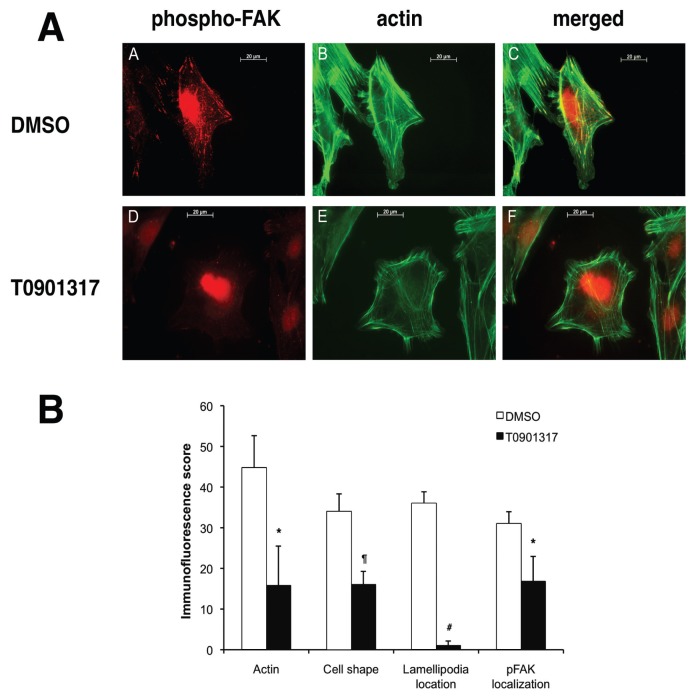
DA FLS treated with T0901317 had a significantly lower actin cytoskeleton score (64% reduction) compared with DMSO-treated controls (P < 0.05, t test; Figure 4B). T0901317-treated cells had fewer thick actin filaments compared with controls, and were more likely to have no thick filaments, suggesting an inhibition of actin reorganization. FLS treated with T0901317 were 50% less likely to have an elongated shape as typically seen in polarized and moving cells (P = 0.003, t test, Figure 4). Lamellipodia form in a polarized manner in migrating and invading cells, and colocalize with phospho-FAK (pFAK) (Figure 4A, panels A–C). T0901317-treated FLS had few polarized lamellipodia, with a score that was 97% lower than DMSO-treated cells, suggesting that these cells were not moving or prone to invading (P = 0.00005, t test, Figures 4A [panels D–F], B). The pFAK scores were also significantly lower in T0901317-treated cells, with a 45% reduction compared with DMSO (P = 0.03, t test, Figures 4A [panel D], B).
Figure 4.
Morphologic changes induced by T0901317 in FLS. (A) T0901317 prevents morphologic changes required for FLS invasion. Insets A–C: DA FLS treated with control vehicle (DMSO) have a linearized-fusiform shape, with thick and longitudinal actin fibers, polarized formation of lamellipodia, and phospho-FAK colocalization with lamellipodia. Insets D–F: T0901317 DA FLS have a round, nonfusiform shape, with thinner actin fibers, and with either no lamellipodia formation, or a nonpolarized formation of lamellipodialike structures. These lamellipodialike structures do not colocalize with phospho-FAK. (Red, phospho-FAK; green, phalloidin–FITC–actin staining; images are representative of four different FLS cell lines). (B) Immunofluorescence morphologic scoring of FLS. DA FLS cultured on glass slides in the presence or absence of T0901317 10 μmol/L were scored for actin cytoskeleton characteristics, cell shape, lamellipodia location and phospho-FAK localization (see Methods for details). T0901317 significantly reduced all parameters compared with control (*P < 0.05; #P = 0.00005; ¶P = 0.0036, t test; mean ± SD; n = 4).
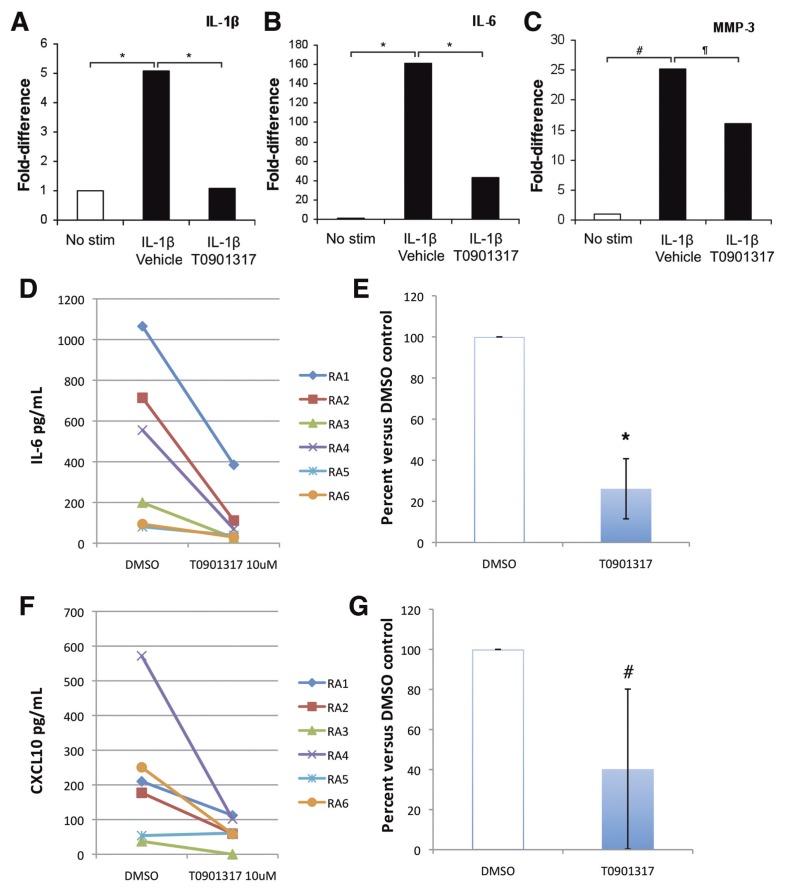
The LXR Agonist T0901317 Reduces the Expression of IL-1β, IL-6, CXCL10 and MMP-3
IL-1β, IL-6, and MMP-3 are expressed at increased levels in synovial tissues and FLS from arthritic DA rats and RA patients and are involved in the development of cartilage and bone erosions. Furthermore, both IL-1β and IL-6 are known to increase the synthesis of MMP-2, which was increased in RA FLS and was reduced by the T0901317, as shown above. Therefore, we examined the effect of the LXR agonist T0901317 on the mRNA expression of IL-1β, IL-6 and MMP-3. IL-1β stimulation of DA FLS for 24 h significantly increased the expression of IL-1β, IL-6 and MMP-3 by 5-fold, 161-fold and 25-fold, respectively (Figures 5A–C). Treatment with the LXR agonist T0901317 completely prevented the increase in mRNA levels of IL-1β, reduced IL-6 expression by 73%, and reduced MMP-3 expression by 36% (Figures 5A–C).
Figure 5.
The LXR agonist T0901317 reduces the expression of proinflammatory mediators and MMP-3 in DA and RA FLS. (A) Treatment of DA FLS with IL-1β 10 ng/mL caused a significant increase in the expression of IL-1β mRNA by 5-fold, which was completely prevented by T0901317. (B) IL-1β induced a 161-fold increase in mRNA levels of IL-6, and T0901317 reduced that to 43-fold, or a 73% reduction compared with IL-1β alone. (C) IL-1β induced a 25-fold increase in the expression of MMP-3, and T0901317 reduced that to 16-fold, compared with control, or a 36% reduction. (D, E) Levels of IL-6 in supernatants of RA FLS cultured overnight on Matrigel were significantly reduced by 74% with T0901317 treatment. (F, G) Levels of CXCL10 in supernatants of RA FLS cultured overnight on Matrigel were significantly reduced by 60% with T0901317 treatment. *P ≤ 0.0005; #P = 0.004; ¶P = 0.01 (t test). No stim, no stimulation.
Next, supernatants from RA FLS cultured without serum on Matrigel (upper compartment of the invasion chambers) were used to quantify IL-6, IL-8, IL-10, MCP-1, TNFα and CXCL10 protein levels. These culture supernatants were chosen because they provide a direct correlation with the invasion studies. Similar to results from the invasion studies, treatment with the LXR agonist T0901317 significantly reduced levels of IL-6 by 74% (P = 0.0000002, Figures 5D, E) and levels of CXCL10 by 60% (P = 0.004, Figures 5F, G), respectively. MCP-1, IL-10 and TNFα were nearly undetectable. Levels of IL-8 were not significantly changed by T0901317 (data not shown). Therefore, our results suggest that the LXR agonist affects mRNA expression and protein synthesis of key mediators of both inflammatory responses in arthritis, as well as mediators of FLS invasion and articular damage.
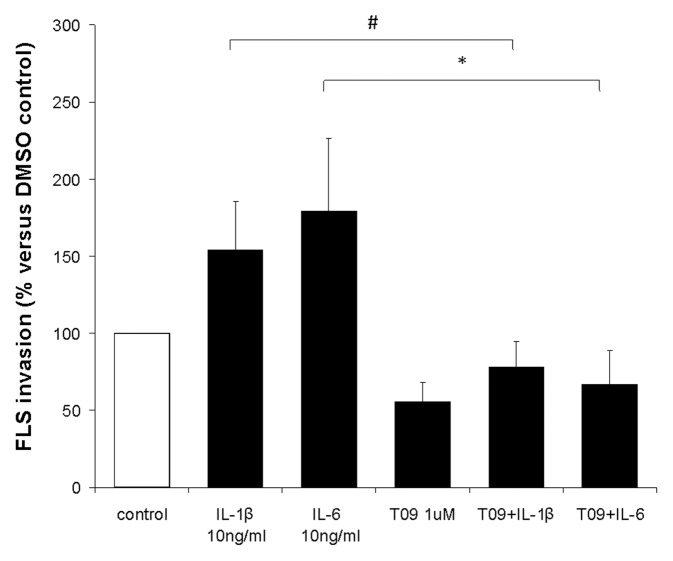
LXR Stimulation with T0901317 Prevents IL-1β and IL-6–Induced FLS Invasion
Although IL-1β had been previously shown to regulate FLS invasion, the IL-6 effect was mostly inferred from its effects on cancer cells and in increasing FLS production of MMPs. Our results showing that the LXR agonist T0901317 decreased the expression of both IL-1β and IL-6 raised the possibility that the LXR-mediated suppression of FLS invasion could be indirectly mediated by reducing levels of these cytokines. To test this possibility, we conducted invasion experiments with DA FLS (five different FLS cell lines) treated with IL-1β (10 ng/mL) or IL-6 (10 ng/mL) in the presence or absence of T0901317 (Figure 6). Both IL-1β and IL-6 treatment induced a significant increase in FLS invasion by 154% and 179%, respectively. This is the first time that IL-6 was actually shown to increase FLS invasion.
Figure 6.
The LXR agonist T0901317 blocks IL-1β– and IL-6–induced FLS invasion. Treatment of DA FLS with IL-1β (10 ng/mL) or IL-6 (10 ng/mL) significantly increased cell invasion by 154% and 179%, respectively, compared with controls (white bar). Treatment with T0901317 (1 μmol/L) prevented the cytokine-induced increased invasion, suggesting that LXR interferes with IL-1β and IL-6 activity. T0901317 treatment reduced FLS invasion to levels below the control group, suggesting that factors other than IL-1β, IL-6 and their receptors are involved in the LXR-mediated suppression of invasion (#P = 0.022; *P = 0.017; mean ± standard error of the mean; t test; n = 5 different FLS cell lines).
Treatment with T0901317 completely prevented IL-1β and IL-6–induced increases in FLS invasion, suggesting that LXR interferes with signaling or effector pathways regulated by the receptors for these cytokines (Figure 6). T0901317-treated cells were less invasive than controls, suggesting that, in addition to interfering with IL-1β and IL-6 activity, T0901317 also interferes with other invasion-mediating processes.
Although the LXR agonist reduced IL-1β and IL-6 expression, our results demonstrated that its effect on invasion was not dependent on the suppression of cytokine expression as it retained its invasion-suppressing effect despite cytokine treatment/supplementation.
DISCUSSION
Synovial tissue hyperplasia and tissue invasion and destruction of cartilage and bone are characteristic findings of RA (3), and FLS have a central role on these processes (4). Cartilage, bone and ultimately joint destruction correlate with disease severity and with increased risk for deformities and disability, yet little is known about the genes regulating these processes. Previous studies have shown that the in vitro invasive properties of FLS through collagen-rich Matrigel correlate with histologic damage in rats with PIA (5), and with radiographic erosions and damage in patients with RA (6). Therefore, this model has direct clinical correlation and relevance, and we consider it an important and useful strategy to understand the processes and genes regulating FLS invasion, and to identify new potential targets for therapies aimed at preserving joint architecture and reducing articular damage.
We have previously determined that the expression of the antiinflammatory NR LXRα is decreased in synovial tissues from arthritic rats (10). In the present study we show that IL-1β can reduce the expression of LXRα in FLS, providing a new explanation for the reduced expression and activity of LXRα in vivo.
Treatment with the LXRα agonist T0901317 significantly reduced the invasive properties of FLS from arthritic rats and from patients with RA by more than 90%. Our results demonstrate that the LXR-mediated suppression of invasion involves: (a) reducing the production of the active form of MMP-2, a protease implicated in FLS invasion (31) and radiographic damage in RA (32); (b) reducing levels of MMP-3, another protease implicated in RA joint damage (33) and FLS invasion (24);and (c) interfering with actin cytoskeleton reorganization, with FLS ability to form lamellipodia in a polarized manner and with colocalization of phospho-FAK with lamellipodia, all required for cell mobility and invasion (22,31,34,35).
The LXR agonist T0901317 also reduced the expression of cytokines central to RA pathogenesis and joint damage such as IL-1β (36–38) and IL-6 (39,40). IL-1β and IL-6 increase the production of several MMPs (41), and IL-1β directly increases FLS and synovial tissue invasion and destruction of cartilage (37–38). Furthermore, the synovial levels of IL-1β are predictive of joint damage progression in RA (42). IL-6 had not been previously studied in the regulation of FLS invasion, but it was a reasonable possibility given its proinvasive and prometastatic effects in cancers (43). This is the first study to show that IL-6 increases the invasive properties of RA FLS. Therefore, our results suggest that IL-1β and IL-6 may have mutually suppressive and antagonistic effects against LXRα, and that LXR agonists are capable of preventing the increased FLS invasiveness induced by these cytokines.
CXCL10 levels are increased in RA (44,45), and antibodies targeting this chemokine ameliorated disease activity in patients (46) and dramatically reduced cartilage and bone damage in rodent arthritis (47). We have recently determined that in addition to its chemotactic activity for CXCR3-positive mast cells and T cells, CXCL10 increases invasion in an autocrine and paracrine manner in FLS from arthritic rats and patients with RA (27). Therefore, the reduction in levels of CXCL10 induced by the LXR agonist T0901317 provides yet another mechanism to reduce FLS invasion.
LXR has a known direct effect on gene expression via its binding to LXR-responsive elements that might explain part of our observations. Yet, there is some suggestion from the literature that LXR has nongenomic effects, potentially interfering with signaling pathways such as NF-κB in brain tissues (48) and Akt in prostate cancer cells (49). We studied the effect of T0901317 on FLS NF-κB (p65) and Akt activation, but, similarly to other investigators (14), we did not observe consistent suppression, suggesting that some of the LXR nongenomic effects are cell or tissue specific.
LXR agonists have been successfully used by two different groups to reduce susceptibility, disease severity and articular damage in collagen-induced arthritis in mice (18,19), although a third group unexpectedly found opposite results (50). In addition to suppressive effects on FLS invasion and cytokine/chemokine and MMP production described here, others have reported that LXR agonists suppress the differentiation of Th17 T cells (16), which are central to the pathogenesis of RA and other autoimmune diseases. LXR agonists also significantly improved another Th17-mediated disease, EAE, which is a model of multiple sclerosis (16). Our results combined with other data reported in the literature suggest that the use of LXR agonists may provide a beneficial effect that involves interference with processes mediating both FLS invasion and articular damage, as well as regulation of proinflammatory mediators and the development of pathogenic Th17 cells.
CONCLUSION
In the present study we determined that LXR is functional and responsive in DA FLS and in RA FLS, and treatment with a synthetic agonist significantly reduces FLS invasion. Our observations provide a new mechanistic explanation for the disease-ameliorating and joint-protecting effects of LXR agonists, including the inhibition of IL-1β– and IL-6–induced effects on FLS, and provide a new rationale for considering clinical trials with this group of drugs in patients with RA.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank M Keogh and C Mason of the Feinstein Institute’s Tissue Donation Program for their assistance in obtaining RA synovial tissues. Funding was provided by the National Institutes of Health grants R01-AR46213, R01-AR052439 (NIAMS) and R01-AI54348 (NIAID) to P Gulko.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Gossec L, et al. Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study. Ann Rheum Dis. 2004;63:675–80. doi: 10.1136/ard.2003.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe F, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Ladner U, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laragione T, Brenner M, Mello A, Symons M, Gulko PS. The arthritis severity locus Cia5d is a novel genetic regulator of the invasive properties of synovial fibroblasts. Arthritis Rheum. 2008;58:2296–306. doi: 10.1002/art.23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolboom TC, et al. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:1999–2002. doi: 10.1002/art.21118. [DOI] [PubMed] [Google Scholar]
- 7.van Zeben D, Hazes JM, Zwinderman AH, Vandenbroucke JP, Breedveld FC. Factors predicting outcome of rheumatoid arthritis: results of a followup study. J Rheumatol. 1993;20:1288–96. [PubMed] [Google Scholar]
- 8.Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–17. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Drossaers-Bakker KW, et al. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum. 1999;42:1854–60. doi: 10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Linge CP, Li W, Gulko PS. Increased synovial expression of nuclear receptors correlates with protection in pristane-induced arthritis: a possible novel genetically regulated homeostatic mechanism. Arthritis Rheum. 2011;63:2918–29. doi: 10.1002/art.30507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Wan YJ. Nuclear receptors and inflammatory diseases. Exp Biol Med (Maywood) 2008;233:496–506. doi: 10.3181/0708-MR-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–3. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 13.Brenner M, et al. Identification of two new arthritis severity loci that regulate levels of autoantibodies, IL-1beta and joint damage. Arthritis Rheum. 2012;64:1369–78. doi: 10.1002/art.33468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birrell MA, et al. Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses. J Biol Chem. 2007;282:31882–90. doi: 10.1074/jbc.M703278200. [DOI] [PubMed] [Google Scholar]
- 15.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 16.Cui G, et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–70. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chintalacharuvu SR, Sandusky GE, Burris TP, Burmer GC, Nagpal S. Liver X receptor is a therapeutic target in collagen-induced arthritis. Arthritis Rheum. 2007;56:1365–7. doi: 10.1002/art.22528. [DOI] [PubMed] [Google Scholar]
- 19.Park MC, Kwon YJ, Chung SJ, Park YB, Lee SK. Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatology. 2010;49:882–90. doi: 10.1093/rheumatology/keq007. [DOI] [PubMed] [Google Scholar]
- 20.Vingsbo C, et al. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med. 2010;16:352–8. doi: 10.2119/molmed.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolboom TC, et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61:975–80. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, et al. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1beta and TNF-alpha. Mediators Inflamm. 2000;9:155–60. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laragione T, Brenner M, Sherry B, Gulko PS. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion in rheumatoid arthritis. Arthritis Rheum. 2011;63:3274–83. doi: 10.1002/art.30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verderame M, Alcorta D, Egnor M, Smith K, Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci U S A. 1980;77:6624–8. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner M, Laragione T, Mello A, Gulko PS. Cia25 on rat chromosome 12 regulates severity of autoimmune arthritis induced with pristane and with collagen. Ann Rheum Dis. 2007;66:952–7. doi: 10.1136/ard.2006.066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins E, Brenner M, Laragione T, Gulko PS. Synovial expression of Th17-related and cancer-associated genes is regulated by the arthritis severity locus Cia10. Genes Immun. 2012;13:221–3. doi: 10.1038/gene.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laragione T, Brenner M, Li W, Gulko PS. Cia5d regulates a new fibroblast-like synoviocyte invasion-associated gene expression signature. Arthritis Res Ther. 2008;10:R92. doi: 10.1186/ar2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldbach-Mansky R, et al. Active synovial matrix metalloproteinase-2 is associated with radiographic erosions in patients with early synovitis. Arthritis Res. 2000;2:145–53. doi: 10.1186/ar79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunnane G, Fitzgerald O, Beeton C, Cawston TE, Bresnihan B. Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arthritis Rheum. 2001;44:2263–74. doi: 10.1002/1529-0131(200110)44:10<2263::aid-art389>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–11. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Tilghman RW, et al. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J Cell Sci. 2005;118:2613–23. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–9. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Neidhart M, Gay RE, Gay S. Anti-interleukin-1 and anti-CD44 interventions producing significant inhibition of cartilage destruction in an in vitro model of cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2000;43:1719–28. doi: 10.1002/1529-0131(200008)43:8<1719::AID-ANR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Joosten LA, et al. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–55. [PubMed] [Google Scholar]
- 39.Nishimoto N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasai M, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Ito A, Itoh Y, Sasaguri Y, Morimatsu M, Mori Y. Effects of interleukin-6 on the metabolism of connective tissue components in rheumatoid synovial fibroblasts. Arthritis Rheum. 1992;35:1197–201. doi: 10.1002/art.1780351012. [DOI] [PubMed] [Google Scholar]
- 42.Kirkham BW, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–31. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 43.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–55. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanaoka R, et al. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther. 2003;5:R74–81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuan WP, et al. CXCL 9 and CXCL 10 as Sensitive markers of disease activity in patients with rheumatoid arthritis. J Rheumatol. 2010;37:257–64. doi: 10.3899/jrheum.090769. [DOI] [PubMed] [Google Scholar]
- 46.Yellin M, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2009;60(Suppl 10):414. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 47.Salomon I, et al. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol. 2002;169:2685–93. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- 48.Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global is-chemic brain injury by reduction of nuclear factor-kappaB. Neuroscience. 2010;166:1101–9. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pommier AJ, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–23. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 50.Asquith DL, et al. Liver X receptor agonism promotes articular inflammation in murine collagen-induced arthritis. Arthritis Rheum. 2009;60:2655–65. doi: 10.1002/art.24717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.