Abstract
Heat shock proteins (HSP) have long been considered intracellular chaperones that possess housekeeping and cytoprotective functions. Consequently, HSP overexpression was proposed as a potential therapy for neurodegenerative diseases characterized by the accumulation or aggregation of abnormal proteins. Recently, the discovery that cells release HSP with the capacity to trigger proinflammatory as well as immunoregulatory responses has focused attention on investigating the role of HSP in chronic inflammatory autoimmune diseases such as multiple sclerosis (MS). To date, the most relevant HSP is the inducible Hsp70, which exhibits both cytoprotectant and immunoregulatory functions. Several studies have presented contradictory evidence concerning the involvement of Hsp70 in MS or experimental autoimmune encephalomyelitis (EAE), the MS animal model. In this review, we dissect the functions of Hsp70 and discuss the controversial data concerning the role of Hsp70 in MS and EAE.
INTRODUCTION
Heat shock proteins (HSP) are a group of phylogenetically conserved proteins found in all prokaryotic and eukaryotic cells. These proteins are named according to their molecular weight, which range from 17 kDa to more than 100 kDa, and are classified into six families, namely the HSP100, HSP90, HSP70, HSP60, HSP40 and the small HSP families. In general, HSP expression can be constitutive or inducible, depending on cell conditions. Under physiological conditions, HSP exert housekeeping functions and act as molecular chaperones that assist in the proper folding of newly synthesized proteins. Moreover, HSP also play an important role in preventing protein aggregation, degrading unstable and misfolded proteins and transporting proteins between cellular compartments (1–5). Under conditions of stress, such as heat shock, inducible HSP are highly up-regulated by heat shock factors (HSF), which are generated as part of the heat shock response (HSR), to maintain cellular homeostasis and to develop cell survival functions. The term “HSP” originated following their discovery in 1962 by Ritossa in response to thermal stress in Drosophila (6); however, other stress conditions, including nutrient deprivation, irradiation, hypoxia, heavy metals, oxidative and toxic stress, infections and exposure to inflammatory cytokines, also induce HSP expression (7–11). Therefore, HSP are also called stress proteins.
HSP70, the most conserved of the HSP families, includes the cytosolic and nuclear constitutive Hsc70 (Hsp73) and the stress-inducible Hsp70 (Hsp72) proteins, the endoplasmic reticulum (ER) Bip (Grp78), and the mitochondrial mt-Hsp70 (Grp75) protein (12–14). Hsp70 has been the subject of particularly extensive studies because it exhibits different functions in accordance with its location; intracellular Hsp70 exerts cytoprotective functions as a chaperone protein, whereas extracellular Hsp70 exerts immunomodulatory functions that trigger immunological responses (acting as danger signals) as well as tolerance responses.
Multiple sclerosis (MS) is the most common chronic inflammatory disease associated with the demyelination of the central nervous system (CNS). Although the etiology of MS is not fully understood, current evidence suggests a critical role for the immune system in the pathogenesis of the disease. Autoreactive myelin-specific T cells and antibodies are present within MS plaques, and multiple mechanisms of myelin immune-mediated injury have been described in MS patients (reviewed in [15,16]).
The cytoprotection of CNS cells (mainly oligodendrocytes and neurons) and the immunomodulation of T-cell responses against myelin antigens are critical approaches in MS therapy. Recently, several reports have implicated HSP, mainly Hsp60 and −70, in different human autoimmune diseases, including arthritis and type I diabetes mellitus, and their beneficial effect in Alzheimer’s and Parkinson’s diseases has been suggested (reviewed in [17–20]). In this review, we dissect the findings associated with Hsp70 in an attempt to better understand the role of this protein in MS, a neurodegenerative autoimmune disease.
MS PATHOGENESIS
MS is a chronic and progressive neurodegenerative disease that is deemed to affect more than 2.1 million people worldwide (21). MS is considered an immune-mediated disease characterized by the presence of inflammatory demyelinating lesions in the CNS. Because of the destruction of the myelin sheath occurring in MS, the nerve action potential is disrupted, which leads to the apparition of neurological disability such as blurred vision, muscle weakness and spasm as well as motor symptoms. Disease onset usually occurs in young adults between 20 and 40 years of age, and affects women more frequently. Four clinical forms can be distinguished based on clinical onset and disease progression: Relapsing-Remitting (RRMS), the most frequent clinical form affecting approximately 80% to 85% of MS patients; Secondary Progressive (SPMS); Primary Progressive (PPMS); and Progressive Relapsing (PRMS) (16,22).
MS is a complex neurological disorder conditioned by both genetic and environmental factors including gender, sexual hormones, ethnic origin, geographical latitude of residence, smoking, pathogen exposure and vitamin D levels (23–27). The most important genes conferring susceptibility to MS (although with a weak effect) are the MCH class II (HLA-DRB1*1501 allele), and the interleukin (IL)-2 and IL-7 receptor genes, which are involved in the immune response (28,29).
Pathological hallmarks of MS include areas of focal demyelination characterized by inflammation, gliosis and both oligodendrocyte and neuronal loss (30). Based on the presence of inflammatory infiltrates in the CNS as well as on the data accumulated from experimental autoimmune encephalomyelitis (EAE) and its animal model (31), MS is considered a T-cell mediated autoimmune disorder, although its precise etiology is not yet fully comprehended. EAE is induced by immunizing susceptible animals with myelin-derived proteins (or peptides) such as proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG) or myelin basic protein (MBP). Animals with EAE reproduce the major clinical and histopathological features of MS and it has been demonstrated that EAE can be transferred adoptively in healthy animals by injecting myelin-specific CD4+ T cells (32–34). Taking into account the data gathered over the years, it is believed that a complex combination of genetic and environmental factors induces the activation of myelin-specific peripheral T cells in MS patients. Some mechanisms such as molecular mimicry (cross-reaction between pathogen-reactive T-cell epitopes and myelin antigens) (35) or deficient immunoregulatory control (failure of regulatory T-cell function) (36) have been proposed as trigger factors, although this issue is still controversial. As a consequence of T-cell activation, inflammatory cytokines such as IFN-γ, TNF-α, IL-1β and IL-6 are secreted, inducing the expression of adhesion molecules in endothelial cells from the blood vessels, thus driving lymphocyte transmigration across the blood brain barrier (BBB) (37). Once within the CNS, myelin-specific CD4+ T cells are reactivated, initiating a complex inflammatory response that also includes CD8+ T cells, B cells, antibodies, natural killer (NK) cells, complement and other soluble factors produced by innate immune cells that culminate in the injury of axons and oligodendrocytes (15,38).
Moreover, spontaneous remyelination occurs in MS. In this light, both in MS and EAE, axons with thinner myelin sheaths are found. Unfortunately, this remyelinating process is often insufficient to bring about functional recovery. Therefore, remyelination failure is considered another critical aspect in MS, and, consequently, new therapies might be approached not only to block the immune response but also to promote neuroprotection and neuroregeneration. In this sense, Hsp70 represents a promising putative target molecule for MS treatment since it is involved in the immune response and has neuroprotective functions (discussed below).
THE ROLE OF HSP IN NEUROPROTECTION
HSP play two neuroprotective roles. On the one hand, HSP prevent protein aggregation and misfolding through their chaperone activity, and on the other hand they can induce antiapoptotic mechanisms (Table 1). HSP not only exhibit housekeeping functions but also have an essential function in promoting cell survival following stressful or harmful conditions. Following conditions of cellular stress, the accumulation of unfolded or misfolded proteins triggers an HSR that promotes HSP expression with the purpose of refolding these proteins to their native state or, if that is not possible, transferring the proteins to the degradative pathway (reviewed in [39–41]). On the basis of the idea that the accumulation of abnormal protein aggregates is a common histopathological hallmark contributing to neuronal degeneration, the role of HSP in chronic neurodegenerative diseases has been extensively studied. In this context, the induction of Hsp70 expression in the CNS, mainly in reactive astrocytes, oligodendrocytes and microglia (42–45), following conditions of stress has been described. In addition, Hsp70 is considered a powerful antiapoptotic protein because it inhibits multiple steps of programmed cell-death signaling in both intrinsic and extrinsic pathways (reviewed in [46–49]).
Table 1.
Intracellular and extracellular Hsp70 functions.
| Location | Functions | References |
|---|---|---|
| Intracellular | Cytoprotection | |
| Chaperon function: Hsp70 assists proper protein folding and cellular compartment translocation and avoids protein aggregation | 1–5,39–41 | |
| Apoptosis inhibition: Hsp70 blocks multiple steps of both intrinsic and extrinsic apoptotic pathways | 46–49 | |
| Extracellular | Immune response mediator | |
| Antigen adjuvant: Hsp70 binds peptides and misfolded proteins creating immune reactive complexes | 91–93 | |
| Danger signal: Hsp70 induces APC maturation and triggers innate immune response by TLR interaction | 76,81–90 |
The interconnection between the cytoprotective and antiapoptotic mechanisms remains unknown. Following stress or injury, two responses are triggered: the cytoprotective stress response, which induces HSP production, and the apoptotic pathway, which leads to cell death. Hsp70 exhibits a high affinity for hydrophobic peptides, and stress-induced, newly generated Hsp70 binds to misfolded proteins. If the levels of the newly generated Hsp70 (and its cochaperone Hsp40) successfully achieve protein refolding, the excess Hsp70 or the Hsp70 released after protein refolding will then interact (with lower affinity) with different apoptotic mediators to prevent cell death. Conversely, stress conditions that cause serious cellular damage will exceed the capacity of Hsp70 to refold proteins; thus, the apoptotic pathway will not be arrested, and this will lead to cell death. Consequently, insufficient induction or production of Hsp70 and its cochaperones will cause the aggregation of misfolded proteins and apoptotic cell death typical of neurodegenerative diseases. Accordingly, the overexpression of Hsp70 in neuronal cell cultures and mouse models of neurodegenerative diseases has demonstrated the beneficial effect of Hsp70 in ameliorating the severity of disease phenotypes by reducing the number and size of inclusions and the accumulation of disease-causing proteins. Hsp70 overexpression prevents the intracellular aggregation of amyloid peptides (50,51), tau protein (52), huntingtin (53) and α-synuclein fibril formation (54).
Neuroprotective Role of Hsp70 in MS
Studies of ischemic, hyperthermic and excitotoxic preconditioning have been conducted to investigate the cytoprotective role of HSP in neurodegenerative diseases. These studies involved provoking a sub-lethal insult (preconditioning) followed by a second, more severe, stimulus. The preconditioning treatment is associated with a reduction in neuron and glial cell loss. Because preconditioning principally induces the expression of Hsp70, the neuroprotective effects were linked to Hsp70 induction. Similarly, heat shock preconditioning in rodents, prior to EAE induction, leads to Hsp70 upregulation; consequently, the incidence of disease and neuronal damage are reduced and clinical signs show improvement (55,56).
However, contrary to previous beliefs, apart from the cytoprotective functions of HSP as intracellular proteins, HSP are also released into the extracellular milieu, where they trigger a wide variety of effects. In the CNS, glial cells produce and release HSP, including Hsc70 and Hsp70, which are rapidly absorbed by neurons. Because neurons express high levels of Hsc70, whereas Hsp70 is poorly induced after stress conditions (57), the glial-to-axon transfer of Hsp70 has been suggested as a compensatory neuroprotective maneuver in which glial cells protect adjacent neurons from acute stress or injury (58–61). Consequently, in light of this observation, the supply of exogenous Hsp70 into the CNS is a potential therapeutic strategy to reduce neuronal death in neurodegenerative diseases.
In MS, the immune response in the CNS leads to inflammatory and oxidative conditions that induce the overexpression of most HSP (including Hsp70) in the CNS lesions of MS patients and in the EAE animal model of the MS disease (62–65). Because Hsp70 provides neuroprotection from ischemia-induced cell death (66,67), the overexpression of this protein in MS lesions might protect CNS cells against the inflammatory environment that is typical of the disease (63,68–70). De Souza et al. reported that the stimulation of human glial cells with proinflammatory cytokines, such as IL-1, IFN-γ and TNF-α, induces Hsp70 expression, predominantly in oligodendrocytes (71). Taken together, the data suggest that the inflammatory phase occurring at the initial stages of MS/EAE acts as a preconditioning stimulus to induce Hsp70 expression as well as Hsp70 release by glial cells to protect neurons in the subsequent neurodegenerative phase. Therefore, a failure to induce Hsp70 or the insufficient production of HSP in the CNS is a possible determining factor for MS development.
Data accrued over the years have demonstrated the cytoprotective functions of both intra- and extracellular HSP in the CNS. As a result, therapeutic strategies focusing on HSP upregulation have been proposed for different neuropathologies that generally are characterized by misfolded protein aggregations. However, this characterization does not seem to apply to MS. The immune reaction that occurs in MS is thought to trigger a process of neurodegenerative damage that leads to clinical signs. In this scenario, extracellular HSP, namely Hsp70, might actually be harmful rather than beneficial. While the release of Hsp70 in Alzheimer’s and Parkinson’s diseases leads to a reduction in misfolded proteins, in MS, Hsp70 exacerbates the immune response by acting as an adjuvant for myelin peptides and as a proinflammatory cytokine (72). The mechanism by which Hsp70 promotes immune reactions is discussed in detail below.
THE ROLE OF HSP IN IMMUNOMODULATION
HSP exhibit immunomodulatory functions that further promote immune responses by acting as proinflammatory cytokines and by mediating regulatory immune responses (Table 1). Understanding the mechanisms of HSP involvement in the balance of autoimmunity versus tolerance is essential for the establishment of HSP as potential therapeutic tools for the treatment of different autoimmune diseases and cancer.
Cellular Response
To initiate a T-cell immune response, immunostimulatory antigen presentation by professional antigen presenting cells (APC), mainly dendritic cells (DC), but also monocytes and activated B cells, is required. Depending on the environmental signals present during APC maturation, T cell:APC interaction triggers either tolerance or immune responses. The literature describes two different types of HSP-APC interactions that trigger innate or adaptive immune responses based on the presence or absence of antigen binding (reviewed in [73–75]). Findings from in vitro studies have shown that HSP, including Hsp70, Hsp90, gp96 (ER Hsp90) and calreticulin, are released from necrotic cells (although not by apoptotic cells) after trauma or severe stress, and these HSP act as danger signals, promoting DC maturation and exerting immunostimulatory functions (76). Additionally, several studies have demonstrated that Hsp70 also can be released by glial cells and peripheral blood mononuclear cells (PBMC) under acute psychological stress conditions or following physical exercise (77–79). In this regard, Bausero et al. demonstrated that the proinflammatory environment mediated by IFN-γ, but not by the antiinflammatory cytokine TGF-β1, induces the active release of Hsp70 (80).
In contrast to the cytoprotective or homeostatic functions mediated by intra-cellular Hsp70, extracellular Hsp70 triggers both innate and adaptive immune responses. In innate immune responses, extracellular HSP, including Hsp60, Hsp70 and gp96, derived from pathogens or host cells, exert cytokinelike effects on APC maturation. In the case of Hsp70, the innate immune response is triggered through interaction with the toll-like receptor (TLR)-2, TLR-4 and CD14 costimulatory signaling (81). In addition to the activation and maturation of DC, Hsp70 acts as a chemoattractant and elicits the cytolytic effects of NK cells by mediating the interaction with CD94 (82–84). At the same time, released Hsp70 leads to the activation of the NF-κB transcription factor, which induces proinflammatory cytokine production (IL-12, IL-1β, IL-6, TNF-α, and GM-CSF) (76,85,86), chemokine secretion (MCP-1, RANTES, and MIP-1α) (87), and nitric oxide (NO) production (88) by macrophages and/or DC. Finally, extra-cellular Hsp70 enhances the expression of CD83, CD86 and CD40 as well as the major histocompatibility complex (MHC) class II on DC and the migration of these cells to draining lymph nodes (76,89,90), thus priming adaptive immune responses. Considering these findings, it has been hypothesized that conditions of acute stress induce the release of Hsp70, which acts as a danger signal to prepare the immune system against a likely pathogen challenge.
Based on their activity as chaperones, the HSP released into the milieu can act as adjuvants that bind immunogenic peptides. In the adaptive immune response, HSP-antigen complexes are internalized efficiently via endocytosis mediated by CD40, CD91 (α2-macroglobulin receptor) or LOX-1 scavenger receptors. Thus, the complexes are processed in a proteasome-dependent manner in the cytosol and are presented via MHC class I or MHC class II molecules, which then induces the activation of antigen-specific CD8+ or CD4+ T-cell responses, respectively (91–93). Because HSP-antigen complexes can induce the adaptive immune response, Hsp70 has been used as an adjuvant in vaccinations to enhance the immunogenicity of tumor antigens (94,95).
Chaperokine, a term coined by Asea, describes the dual role of HSP as chaperones and cytokines. However, the cytokine effect of HSP, including Hsp70, has been questioned based on the endotoxin contamination of recombinant HSP that are used in experimental procedures. It has been speculated that the cytokine response induced by Hsp70 is triggered by LPS or other contaminating microbial compounds and not Hsp70 itself (96,97). Although this possibility cannot be excluded, studies have shown that highly purified HSP do activate DC and monocytes in vitro (88).
A significant upregulation of Hsp70 expression has been detected in CNS lesions from MS patients (63,64,68). Interestingly, Cwiklinska et al. detected the presence of Hsp70- MBP and Hsp70- PLP complexes in the brain tissue from MS patients. MBP and PLP have been proposed as possible target autoantigens in MS. Hsp70 binds MBP and PLP peptides to create highly immunogenic complexes, which are processed by APC and presented via MHC II, thus stimulating specific CD4+ T-cell responses in animals with EAE (98–100). Galazka et al. reported that SJL and C57BL/6 mice immunized with PLP139–151 or MOG35–55 peptides, respectively, presented Hsp70 complexed with a number of peptides (Hsp70-pc) generated in the CNS during the inflammatory phase of EAE. The preimmunization of mice with these Hsp70-pc complexes before the induction of EAE results in a tolerogenic reaction mediated by NK cells in SJL but not in C57BL/6 mice. It appears that in splenocytes from SJL mice, Hsp70-pc upregulates the expression of the H60 molecule, a ligand of the activating NK cell receptor NKG2D. However, the level of H60 is not increased in C57BL/6 splenocytes (101,102). By contrast, when compared with their littermates, C57BL/6 Hsp70-deficient (Hsp70KO) mice presented a milder form of EAE characterized by a light initial acute episode followed by a complete remission. This effect has been associated with poor MOG-stimulating CD4+ T-cell proliferation that fails to cause clinical signs (103). These data suggest that Hsp70 upregulation may increase susceptibility to autoimmune demyelinating disease.
There is a subpopulation of clonally expanded γδ T cells that respond to Hsp70 within demyelinated brain lesions of relapsing-remitting MS patients (104). γδ T cells possess potent cytotoxic activity and can kill oligodendrocytes in vitro (105). In addition, γδ T cells as well as CD4+ T and NK cells produce large quantities of IL-17 (106), a proinflammatory cytokine that is involved in EAE and MS pathogenesis as well as in other autoimmune diseases (107,108).
Together, these findings demonstrate that aberrant/deregulated Hsp70 expression may be involved in the pathogenesis of MS and in other chronic inflammatory diseases by two possible mechanisms: exacerbating or contributing to the chronic inflammatory environment and/or eliciting the presentation of myelin autoantigens generated because of tissue injury and cell death. These hypotheses are supported by a recent study demonstrating the aberrant overexpression of Hsp70 following heat shock or inflammatory stress in PBMC from MS patients (109).
Molecular Mimicry
HSP are the immunodominant antigens of many bacteria and are highly conserved proteins expressed in all prokaryote and eukaryote organisms. In particular, Hsp70 demonstrates a 60% to 78% homology among eukaryotic cells, and there is a 40% to 60% homology between eukaryotic Hsp70 and E. coli DnaK (9,110). The most immunogenic bacterial HSP are Hsp60 and Hsp70, which are considered harmful antigens capable of linking infection and autoimmunity. T cells and antibodies generated against microbial HSP may target self-HSP, either through the recognition of conserved epitopes or via cross-reactivity (molecular mimicry), thus leading to tissue inflammation (111,112). The responses induced by molecular mimicry between bacterial and mammalian Hsp60 and Hsp70 have been implicated in the pathogenesis of some autoimmune and inflammatory diseases, including type-1 diabetes (113), atherosclerosis (114), arthritis (115) and MS (116–119), and elevated levels of extracellular HSP and anti-self HSP antibodies have been found in patients with these diseases. However, contrary to expectations, in several studies using animal models of arthritis (120,121), diabetes and MS (122,123) as well as in clinical trials (124), preimmunization with bacterial gp96, Hsp60 or Hsp70 abrogated the subsequently induced inflammatory disease. A study by Wieten et al. demonstrated that the spread of the regulatory capacity mediated by mycobacterial Hsp70 in proteoglycan-induced arthritis (PGIA), a chronic and relapsing T-cell-mediated murine model of arthritis, is dependent on IL-10 production and on specific regulatory self-HSP-cross-reactive T cells (125). The high degree of phylogenetic similarity between Hsp60 and Hsp70 as well as the presence of Hsp60 and Hsp70 in the peripheral circulation of healthy individuals (126,127) indicates that immune responses to HSP are highly controlled and regulated. However, the adult T-cell repertoire includes self-HSP-reactive T cells, which have escaped to central tolerance, thus traveling to the periphery where they are thought to play an important role in maintaining immune homeostasis. In light of this hypothesis, a number of mechanisms have been proposed. For example, under conditions of stress, nonprofessional APC may upregulate HSP expression and present self-HSP to T cells in the absence of costimulatory molecules, thus inducing anergy of self-HSP reactive T cells. It is possible that anergic self-HSP T cells have regulatory functions, either via the production of IL-10 or the induction of immunomodulatory APC (18). In an alternative mechanism, HSP-autoreactive T cells may be activated by the HSP from commensal bacteria in the gut. However, the tolerizing environment of the gut mucosa could lead to the production of self-HSP regulatory T cells (18). In a third possible mechanism, the low-affinity interaction of self-HSP peptides with self-HSP reactive T cells might generate a Th2 (IL-4 producing)–, Th3 (TGFβ producing)– or Tr1 (IL-10 producing)–mediated regulatory response (128).
Humoral Response
A number of studies have demonstrated an increased level of circulating Hsp60 and Hsp70 in hypertension, atherosclerosis and renal disease (129–131). In addition to the cytokinelike function, extracellular Hsp70 as well as membrane Hsp70 may be a target for antibodies. Because soluble Hsp70 and anti-Hsp70 antibodies (126) have been found in the peripheral circulation of healthy individuals, these anti-HSP antibodies may act in an immunoregulatory capacity to remove excess extracellular Hsp70 following the resolution of a stress situation. The presence of Hsp70 antibodies in the CSF and serum of MS patients was reported for the first time by Birnbaum and Kotilinek in 1993 (reviewed in [132]). Although there were no differences in the serum levels of these antibodies in MS patients compared with healthy donors (133), it is possible that the humoral immune response to extracellular Hsp70 is increased in the CNS of MS patients. In this regard, Chiba et al. found high levels of CSF IgG autoantibodies against Hsp70 in MS patients compared with patients with motor neuron diseases (MND) (119). High levels of Hsp27, Hsp60 or Hsp90 autoantibodies were not detected in these patients. However, despite investigation into the function of anti-Hsp70 antibodies in MS (134), the effect of these antibodies remains to be elucidated.
HSP GENETIC STUDIES
Genetic factors contribute to the development of MS. The MHC class II locus, the HLA-DRB1*1501 allele in particular, is the most important locus associated with susceptibility to developing MS (28). Interestingly, all three genes encoding Hsp70 (HSPA1A, HSPA1B, and HSP-HOM) are located within the MHC locus, namely in the MHC class III cluster close to the MHC II region (135). Similar to the Hsp70 gene in humans, the Hsp70 genes in other species, including the goat, mouse and rat also are located in the MHC region (reviewed in [136]), suggesting a possible relationship between Hsp70 and the altered immune response occurring in MS.
Analyses of Hsp70 genes have revealed a high degree of phylogenetic conservation. For example, HSPA1A and HSPA1B are 99% identical (137). Similarly, polymorphism in Hsp70 genes is low; therefore, polymorphisms found in coding regions lead to silent mutations (reviewed in [136]), suggesting that Hsp70 plays an essential role in cell physiology and in maintaining homeostasis following stress conditions. The relationship between different Hsp70 gene polymorphisms and MS has been investigated, and an effect on MS susceptibility/protection or an association with clinical data association was not established for different patient populations (138–140). Nevertheless, controversial differences between MS patients and healthy controls have been found in gene expression studies. Whereas earlier genetic studies showed HSPA1A overexpression in MS lesions (64,65,68), more recently HSPA1A expression was shown to be downregulated in the PBMC of MS patients (141–143). Histological studies in MS and EAE tissues have confirmed HSPA1A up-regulation in CNS lesions; however, surprisingly, analysis of the baseline expression of Hsp70 genes in PBMC revealed no differences among MS patients, healthy controls or patients with rheumatoid arthritis. However, an aberrant overexpression of Hsp70 protein was detected in MS patients following an inflammatory event or thermal stress (109). These findings suggest that the role of Hsp70 could be context-dependent; thus, in the CNS, Hsp70 could be neuroprotective, whereas in PBMC, it could be proinflammatory. Further studies are needed to elucidate how these functions are interconnected in MS.
THERAPEUTIC POTENTIAL OF HSP
The beneficial effect of Hsp70 overexpression in reducing protein inclusions or aggregates suggests that the pharmacological induction of Hsp70 is a potential and feasible therapeutic approach for different neurodegenerative diseases, such as Parkinson’s or Alzheimer’s disease. In this light, a number of compounds have been shown to induce HSP expression. Arimoclomol, an experimental drug that acts as an HSP coinducer, is in Phase II clinical trials for amyotrophic lateral sclerosis treatment (144,145). This drug has been shown to prolong the lifespan of the superoxide dismutase 1 (SOD1) enzyme in mice even when treatment was initiated after symptom onset (146,147). Inhibitors of Hsp90, such as geldamycin, lead to HSF1 release, which induces the expression of Hsp70 and other chaperones. In EAE, Hsp70 induction associated with geldamycin administration suppresses the glial inflammatory response and ameliorated EAE (148). Nonsteroidal antiinflammatory drugs (NSAID), such as sodium salicylate, directly activate HSF1 (149). In addition, NSAID are especially interesting owing to their dual cytoprotectant (inducing HSP expression) and antiinflammatory roles (150). The main problem associated with NSAID treatment is that a high concentration is required to achieve therapeutic levels in the CNS owing to the low capacity of these drugs for crossing the BBB (151). Finally, celastrol and triptolide are medicinal plant-derived antiinflammatory substances that have been used for centuries in traditional medicine to treat inflammatory and auto-immune diseases. The therapeutic effect of these compounds has been associated with the inhibition of NF-κB activation mediated by Hsp70 upregulation. Triptolide, when administrated therapeutically or as a prophylactic, is a potent suppressor of EAE by inducing Hsp70 expression, which in turn avoids NF-κB activation, thus attenuating the proinflammatory response (152–154). On the other hand, at high concentrations, triptolide also exerts an antiproliferative effect, promoting apoptosis mediated by Hsp70 downregulation (155,156). However, the toxicity and water-insolubility of triptolide reduce the clinical feasibility of this drug. Triptolide derivates have been designed and currently are being tested for cancer treatments (reviewed in [157]).
CONCLUSION
The pleiotropic functions of Hsp70, combined with the complexity of MS pathogenesis, make it difficult to elucidate the exact roles that Hsp70 plays in the disease. Hsp70 is associated with neurodegeneration and immunological deregulation in MS (Table 2 and Figure 1). A large number of studies have demonstrated that Hsp70 is overexpressed in the CNS of MS patients, and this phenomenon has been associated with a neuroprotective function of Hsp70 in neurons and oligodendrocytes in an inflammatory environment. However, when the overexpression of Hsp70 fails to prevent cell death, high quantities of Hsp70 are released into the milieu, which indirectly promotes or exacerbates an immunological response mediated by its cytokinelike property as well as its myelin-peptide adjuvant capacity.
Table 2.
Putative Hsp70 roles in MS pathogenesis.
| Proposed Hsp70 roles in MS | References | |
|---|---|---|
| CNS | Cytoprotection of neurons and oligodendrocytes after inflammatory stress conditions (cytokines, ROS, free radicals, etc.) | 55,63,64,68–71 |
| Immune system | ||
| APC | Maturation (MHC and costimulatory molecules expression) | 76,89,90 |
| Proinflammatory cytokine secretion triggered by Hsp70-TLR interaction (innate response) | 76,85–88 | |
| Presentation of MBP-Hsp70 and PLP-Hsp70 complexes by MHC-I or -II molecules (adaptive response) | 98–100 | |
| T cells | Generation of Hsp70-specific T cells by cross-reactivity with bacterial and endogenous Hsp70 | 116–119 |
| Induction of adaptive immune responses against MBP-Hsp70 and PLP-Hsp70 complexes presented by APC | 98–100 | |
| B cells | Anti-Hsp70 humoral response (anti-Hsp70 antibodies) | 119,132–134 |
| γδ T cells | Death of oligodendrocytes mediated by activation of γδ T cell cytotoxicity | 104,105 |
| NK cells | Immunoregulation mediated by NKG2D receptor | 101,102 |
ROS, reactive oxygen species.
Figure 1.
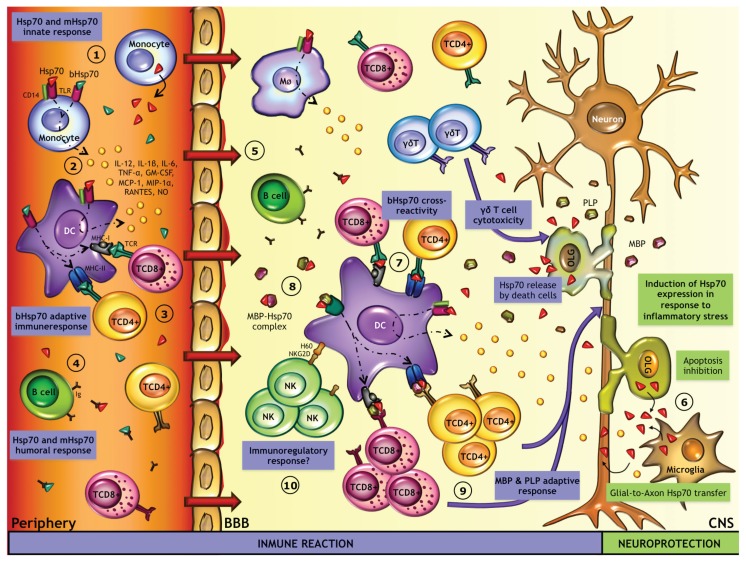
Proposed model of the role of Hsp70 in MS pathogenesis. After a stressful insult elicited by infectious pathogens, Hsp70 expression is induced and released by immune cells in the periphery (1). Acting as an alarm signal, endogenous Hsp70 as well as bacterial Hsp70 (bHsp70) may engage cell-surface signaling receptors, such as CD14, TLR2 and TLR4, to induce cytokines (IL-12, IL-1β, IL-6, TNF-α, and GM-CSF), chemokines (MCP1, MIP1α, and RANTES) and NO production by DC and/or monocytes (2). On the other hand, bHsp70 is processed intracellularly and is presented by MHC-I and MHC-II molecules in APC, leading to the generation of bHsp70-specific CD8+ and CD4+ T cells (3) and the production of anti-bHsp70 antibodies by B lymphocytes (4). Despite the presence of the BBB, leukocytes enter into the CNS (5). In the CNS, the inflammatory environment triggers Hsp70 expression by CNS cells (6). Because of the high degree of homology between bacterial and eukaryotic Hsp70, bHsp70-specific lymphocytes cross-react against released endogenous Hsp70, causing an (auto)immune response against self-Hsp70 (7). In addition, myelin peptides, such as PLP and MBP, generated during myelin destruction, are chaperoned by Hsp70. Hsp70-PLP and Hsp70-MBP complexes are recognized by APC (8), triggering an adaptive immune response against the PLP and MBP peptides (9). Finally, Hsp70 might exhibit an immunoregulatory function mediated by NK cells (10); however, the mechanisms are still unclear.
Conversely, in PBMC from MS patients, an aberrant upregulation of Hsp70 has been detected after heat shock or LPS stimulation. In a variety of tumor cells, high levels of Hsp70 have been associated with an increased tumorigenic potential and a negative prognostic value that is related to the powerful Hsp70 antiapoptotic function. Because MS is characterized by a deregulated immunological response, a strong increase in Hsp70 expression in PBMC could make them more pathogenic (that is, resistant to regulatory cell action).
Hsp70 has been shown to induce not only proinflammatory but also immunoregulatory responses. However, the mechanisms underlying the antiinflammatory capacity of Hsp70 are poorly understood. At present, it is unclear if the roles of Hsp70 in MS are beneficial or harmful, and more studies are needed to clarify the mechanisms.
ACKNOWLEDGMENTS
We thank Joseph A Graells for his help with editing the language in the manuscript. This study was supported by Red Española de Esclerosis Múltiple (REEM) (RD07/0060) from the Fondo de Investigación Sanitaria (FIS), Ministry of Science and Innovation, Spain; and the Ajuts per donar Suport als Grups de Recerca de Catalunya (2009 SGR 0793) from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya, Spain. CE is partially supported by the Miguel Servet program (CP07/00146) from the FIS, Ministry of Science and Innovation, Spain.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Flynn GC, Chappell TG, Rothman JE. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989;245:385–90. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–4. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 3.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 4.Murakami H, Pain D, Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988;107:2051–7. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992;12:2186–92. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritossa FA. New puffing pattern induced by temperature shock and DNP in Drosophila. Experentia. 1962;18:571–3. [Google Scholar]
- 7.Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–22. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riabowol KT, Mizzen LA, Welch WJ. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988;242:433–6. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- 9.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 10.Welch WJ. Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci. 1993;339:327–33. doi: 10.1098/rstb.1993.0031. [DOI] [PubMed] [Google Scholar]
- 11.Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–71. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 12.Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455–9. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–8. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 16.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 17.Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–93. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–30. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 19.Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2001;5:267–87. doi: 10.1517/14728222.5.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011;2011;618127 doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National MS Society. About MS: who gets MS. [Internet]? [cited 2012 Aug 27]. Available from: http://www.nationalmssociety.org/about-multiple-sclerosis/what-weknow-about-ms/who-gets-ms/index.aspx.
- 22.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 23.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 24.Sundstrom P, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 2004;62:2277–82. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 25.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 26.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194:7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Handel AE, et al. Smoking and multiple sclerosis: an updated meta-analysis. PLoS One. 2011;6:e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–10. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 29.Hafler DA, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–62. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 30.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 31.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 32.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2− T lymphocytes. J Immunol. 1981;127:1420–3. [PubMed] [Google Scholar]
- 33.Mokhtarian F, McFarlin DE, Raine CS. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984;309:356–8. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- 34.van der Veen RC, Trotter JL, Clark HB, Kapp JA. The adoptive transfer of chronic relapsing experimental allergic encephalomyelitis with lymph node cells sensitized to myelin proteolipid protein. J Neuroimmunol. 1989;21:183–91. doi: 10.1016/0165-5728(89)90174-4. [DOI] [PubMed] [Google Scholar]
- 35.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dore-Duffy P, Washington R, Dragovic L. Expression of endothelial cell activation antigens in microvessels from patients with multiple sclerosis. Adv Exp Med Biol. 1993;331:243–8. doi: 10.1007/978-1-4615-2920-0_38. [DOI] [PubMed] [Google Scholar]
- 38.Comabella M, Khoury SJ. Immunopathogenesis of multiple sclerosis. Clin Immunol. 2012;142:2–8. doi: 10.1016/j.clim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 40.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 42.Freedman MS, Buu NN, Ruijs TC, Williams K, Antel JP. Differential expression of heat shock proteins by human glial cells. J Neuroimmunol. 1992;41:231–8. doi: 10.1016/0165-5728(92)90074-u. [DOI] [PubMed] [Google Scholar]
- 43.Satoh J, Yamamura T, Kunishita T, Tabira T. Heterogeneous induction of 72-kDa heat shock protein (HSP72) in cultured mouse oligodendrocytes and astrocytes. Brain Res. 1992;573:37–43. doi: 10.1016/0006-8993(92)90111-l. [DOI] [PubMed] [Google Scholar]
- 44.Satoh J, Kim SU. HSP72 induction by heat stress in human neurons and glial cells in culture. Brain Res. 1994;653:243–50. doi: 10.1016/0006-8993(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 45.Foster JA, Brown IR. Differential induction of heat shock mRNA in oligodendrocytes, microglia, and astrocytes following hyperthermia. Brain Res. Mo Brain Resl. 1997;45:207–18. doi: 10.1016/s0169-328x(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 46.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–18. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 47.Benn SC, Woolf CJ. Adult neuron survival strategies—slamming on the brakes. Nat Rev Neurosci. 2004;5:686–700. doi: 10.1038/nrn1477. [DOI] [PubMed] [Google Scholar]
- 48.Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–51. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 49.Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1:53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–91. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 51.Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–6. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dou F, et al. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 2003;100:721–6. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muchowski PJ, et al. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–6. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–8. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 55.Heneka MT, et al. The heat shock response reduces myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in mice. J Neurochem. 2001;77:568–79. doi: 10.1046/j.1471-4159.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JF, Huang R, Xu J, Jin SJ, Yang YJ. Protective effects of heat shock preconditioning on the experimental autoimmune encephalomyelitis rats [in Chinese] Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:563–6. [PubMed] [Google Scholar]
- 57.Brown IR. Expression of heat shock genes (hsp70) in the mammalian nervous system. Results Probl Cell Differ. 1991;17:217–29. doi: 10.1007/978-3-540-46712-0_15. [DOI] [PubMed] [Google Scholar]
- 58.Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363:161–4. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- 59.Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–66. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 60.Sheller RA, Smyers ME, Grossfeld RM, Ballinger ML, Bittner GD. Heat-shock proteins in axoplasm: high constitutive levels and transfer of inducible isoforms from glia. J Comp Neurol. 1998;396:1–11. doi: 10.1002/(sici)1096-9861(19980622)396:1<1::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 61.Guzhova I, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 62.Gao YL, Brosnan CF, Raine CS. Experimental autoimmune encephalomyelitis. Qualitative and semiquantitative differences in heat shock protein 60 expression in the central nervous system. J Immunol. 1995;154:3548–56. [PubMed] [Google Scholar]
- 63.Aquino DA, Klipfel AA, Brosnan CF, Norton WT. The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J Neurochem. 1993;61:1340–8. doi: 10.1111/j.1471-4159.1993.tb13627.x. [DOI] [PubMed] [Google Scholar]
- 64.Aquino DA, et al. Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997;56:664–72. [PubMed] [Google Scholar]
- 65.Chabas D, et al. The influence of the proinflammatory cytokine, osteopontin, on auto-immune demyelinating disease. Science. 2001;294:1731–5. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 66.Rajdev S, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–91. [PubMed] [Google Scholar]
- 67.Hoehn B, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–9. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Stadelmann C, et al. Tissue preconditioning may explain concentric lesions in Balo’s type of multiple sclerosis. Brain. 2005;128:979–87. doi: 10.1093/brain/awh457. [DOI] [PubMed] [Google Scholar]
- 69.Brosnan CF, Battistini L, Gao YL, Raine CS, Aquino DA. Heat shock proteins and multiple sclerosis: a review. J Neuropathol Exp Neurol. 1996;55:389–402. doi: 10.1097/00005072-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Bajramovic JJ, et al. Differential expression of stress proteins in human adult astrocytes in response to cytokines. J Neuroimmunol. 2000;106:14–22. doi: 10.1016/s0165-5728(99)00260-x. [DOI] [PubMed] [Google Scholar]
- 71.D’Souza SD, Antel JP, Freedman MS. Cytokine induction of heat shock protein expression in human oligodendrocytes: an interleukin-1-mediated mechanism. J Neuroimmunol. 1994;50:17–24. doi: 10.1016/0165-5728(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 72.Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia. 2005;21:457–71. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- 73.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–51. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 74.Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- 75.Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–37. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- 76.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 77.Hunter-Lavin C, et al. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–7. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 78.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–8. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 79.Lancaster GI, et al. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–80. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–12. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 82.Multhoff G, et al. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–36. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 83.Gross C, et al. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8:348–60. doi: 10.1379/1466-1268(2003)008<0348:hspria>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gastpar R, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asea A, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 86.Moroi Y, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci U S A. 2000;97:3485–90. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lehner T, et al. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur J Immunol. 2000;30:594–603. doi: 10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 88.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 89.Singh-Jasuja H, et al. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–5. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 90.Kuppner MC, et al. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602–9. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 91.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–85. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 93.Doody AD, et al. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–92. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Subjeck J, Yang G, Repasky E, Wang XY. Generation of anti-tumor immunity using mammalian heat shock protein 70 DNA vaccines for cancer immunotherapy. Vaccine. 2006;24:5360–70. doi: 10.1016/j.vaccine.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 95.Gong J, et al. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol. 2010;184:488–96. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bausinger H, et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–13. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 97.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–9. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 98.Cwiklinska H, et al. Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int Immunol. 2003;15:241–9. doi: 10.1093/intimm/dxg022. [DOI] [PubMed] [Google Scholar]
- 99.Mycko MP, et al. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the HLA class II. J Immunol. 2004;172:202–13. doi: 10.4049/jimmunol.172.1.202. [DOI] [PubMed] [Google Scholar]
- 100.Lund BT, et al. Association of MBP peptides with Hsp70 in normal appearing human white matter. J Neurol Sci. 2006;249:122–34. doi: 10.1016/j.jns.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 101.Galazka G, et al. Brain-derived heat shock protein 70-peptide complexes induce NK cell-dependent tolerance to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:1588–99. doi: 10.4049/jimmunol.176.3.1588. [DOI] [PubMed] [Google Scholar]
- 102.Galazka G, et al. EAE tolerance induction with Hsp70-peptide complexes depends on H60 and NKG2D activity. J Immunol. 2007;179:4503–12. doi: 10.4049/jimmunol.179.7.4503. [DOI] [PubMed] [Google Scholar]
- 103.Mycko MP, et al. A heat shock protein gene (Hsp70.1) is critically involved in the generation of the immune response to myelin antigen. Eur J Immunol. 2008;38:1999–2013. doi: 10.1002/eji.200737661. [DOI] [PubMed] [Google Scholar]
- 104.Battistini L, et al. Gamma delta T cell receptor analysis supports a role for HSP 70 selection of lymphocytes in multiple sclerosis lesions. Mol Med. 1995;1:554–62. [PMC free article] [PubMed] [Google Scholar]
- 105.Freedman MS, Ruijs TC, Selin LK, Antel JP. Peripheral blood gamma-delta T cells lyse fresh human brain-derived oligodendrocytes. Ann Neurol. 1991;30:794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- 106.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 107.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–71. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cwiklinska H, Mycko MP, Szymanska B, Matysiak M, Selmaj KW. Aberrant stress-induced Hsp70 expression in immune cells in multiple sclerosis. J Neurosci Res. 2010;88:3102–10. doi: 10.1002/jnr.22476. [DOI] [PubMed] [Google Scholar]
- 110.Routsias JG, Tzioufas AG. The role of chaperone proteins in autoimmunity. Ann N Y Acad Sci. 2006;1088:52–64. doi: 10.1196/annals.1366.029. [DOI] [PubMed] [Google Scholar]
- 111.Lamb JR, et al. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1:191–6. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- 112.Munk ME, et al. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989;143:2844–9. [PubMed] [Google Scholar]
- 113.Abulafia-Lapid R, et al. T cell proliferative responses of type 1 diabetes patients and healthy individuals to human hsp60 and its peptides. J Autoimmun. 1999;12:121–9. doi: 10.1006/jaut.1998.0262. [DOI] [PubMed] [Google Scholar]
- 114.Perschinka H, et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–5. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 115.Res PC, et al. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988;2:478–80. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 116.Salvetti M, et al. T-lymphocyte reactivity to the recombinant mycobacterial 65- and 70-kDa heat shock proteins in multiple sclerosis. J Autoimmun. 1992;5:691–702. doi: 10.1016/0896-8411(92)90186-t. [DOI] [PubMed] [Google Scholar]
- 117.Salvetti M, et al. The immune response to mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J Neuroimmunol. 1996;65:143–53. doi: 10.1016/0165-5728(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 118.Stinissen P, et al. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J Immunol. 1995;154:4883–94. [PubMed] [Google Scholar]
- 119.Chiba S, et al. Autoantibodies against HSP70 family proteins were detected in the cerebrospinal fluid from patients with multiple sclerosis. J Neurol Sci. 2006;241:39–43. doi: 10.1016/j.jns.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Jorgensen C, Gedon E, Jaquet C, Sany J. Gastric administration of recombinant 65 kDa heat shock protein delays the severity of type II collagen induced arthritis in mice. J Rheumatol. 1998;25:763–7. [PubMed] [Google Scholar]
- 121.Wendling U, et al. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–7. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- 122.Chandawarkar RY, Wagh MS, Kovalchin JT, Srivastava P. Immune modulation with high-dose heat-shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis. Int Immunol. 2004;16:615–24. doi: 10.1093/intimm/dxh063. [DOI] [PubMed] [Google Scholar]
- 123.Birnbaum G, et al. Heat shock proteins and experimental autoimmune encephalomyelitis. II: environmental infection and extra-neuraxial inflammation alter the course of chronic relapsing encephalomyelitis. J Neuroimmunol. 1998;90:149–61. doi: 10.1016/s0165-5728(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 124.Raz I, et al. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 125.Wieten L, et al. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS One. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 127.Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–9. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 129.Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- 130.Pockley AG, et al. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–7. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- 131.Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–8. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- 132.Birnbaum G, Kotilinek L. Heat shock or stress proteins and their role as autoantigens in multiple sclerosis. Ann N Y Acad Sci. 1997;835:157–67. doi: 10.1111/j.1749-6632.1997.tb48627.x. [DOI] [PubMed] [Google Scholar]
- 133.Bustamante MF, et al. Implication of the Toll-like receptor 4 pathway in the response to interferon-beta in multiple sclerosis. Ann Neurol. 2011;70:634–45. doi: 10.1002/ana.22511. [DOI] [PubMed] [Google Scholar]
- 134.Yokota S, Chiba S, Furuyama H, Fujii N. Cerebrospinal fluids containing anti-HSP70 autoantibodies from multiple sclerosis patients augment HSP70-induced proinflammatory cytokine production in monocytic cells. J Neuroimmunol. 2010;218:129–33. doi: 10.1016/j.jneuroim.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 135.Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–51. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 136.Favatier F, Bornman L, Hightower LE, Gunther E, Polla BS. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–55. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985;5:330–41. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramachandran S, Bell RB. Heat shock protein 70 gene polymorphisms and multiple sclerosis. Tissue Antigens. 1995;46:140–1. doi: 10.1111/j.1399-0039.1995.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 139.Cascino I, et al. HSP70–1 promoter region polymorphism tested in three autoimmune diseases. Immunogenetics. 1994;39:291–3. doi: 10.1007/BF00188795. [DOI] [PubMed] [Google Scholar]
- 140.Niino M, et al. Heat shock protein 70 gene polymorphism in Japanese patients with multiple sclerosis. Tissue Antigens. 2001;58:93–6. doi: 10.1034/j.1399-0039.2001.580205.x. [DOI] [PubMed] [Google Scholar]
- 141.Satoh J, et al. Microarray analysis identifies an aberrant expression of apoptosis and DNA damage-regulatory genes in multiple sclerosis. Neurobiol Dis. 2005;18:537–50. doi: 10.1016/j.nbd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 142.Mandel M, Gurevich M, Pauzner R, Kaminski N, Achiron A. Autoimmunity gene expression portrait: specific signature that intersects or differentiates between multiple sclerosis and systemic lupus erythematosus. Clin Exp Immunol. 2004;138:164–70. doi: 10.1111/j.1365-2249.2004.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bomprezzi R, et al. Gene expression profile in multiple sclerosis patients and healthy controls: identifying pathways relevant to disease. Hum Mol Genet. 2003;12:2191–9. doi: 10.1093/hmg/ddg221. [DOI] [PubMed] [Google Scholar]
- 144.Cudkowicz ME, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve. 2008;38:837–44. doi: 10.1002/mus.21059. [DOI] [PubMed] [Google Scholar]
- 145.Traynor BJ, et al. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–7. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 146.Benn SC, Brown RH., Jr Putting the heat on ALS. Nat Med. 2004;10:345–7. doi: 10.1038/nm0404-345. [DOI] [PubMed] [Google Scholar]
- 147.Kieran D, et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–5. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 148.Dello Russo C, et al. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem. 2006;99:1351–62. doi: 10.1111/j.1471-4159.2006.04221.x. [DOI] [PubMed] [Google Scholar]
- 149.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–5. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 150.Ohtsuka K, Kawashima D, Gu Y, Saito K. Inducers and co-inducers of molecular chaperones. Int J Hyperthermia. 2005;21:703–11. doi: 10.1080/02656730500384248. [DOI] [PubMed] [Google Scholar]
- 151.Eriksen JL, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–9. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fu YF, et al. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide derivative, prevents experimental autoimmune encephalomyelitis via inhibiting T cell activation. J Neuroimmunol. 2006;175:142–51. doi: 10.1016/j.jneuroim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Mei Y, Feng D, Xu L. Triptolide modulates T-cell inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:2441–9. doi: 10.1002/jnr.21683. [DOI] [PubMed] [Google Scholar]
- 154.Kizelsztein P, Komarnytsky S, Raskin I. Oral administration of triptolide ameliorates the clinical signs of experimental autoimmune encephalomyelitis (EAE) by induction of HSP70 and stabilization of NF-kappaB/IkappaBalpha transcriptional complex. J Neuroimmunol. 2009;217:28–37. doi: 10.1016/j.jneuroim.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 155.Liu Q, et al. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004;319:980–6. doi: 10.1016/j.bbrc.2004.04.201. [DOI] [PubMed] [Google Scholar]
- 156.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–22. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 157.Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11:377–83. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]