Abstract
Background:
Periodontitis is a bacterial disease modified by multiple factors. Interleukin-1 (IL-1) is a key regulator of the host response and a major modulator of extracellular matrix catabolism and bone resorption. It has been reported that variations in IL-1 gene are associated with increased susceptibility to periodontitis. The aims of the study were 1) to analyze the distribution of single nucleotide polymorphism of IL-1 (IL-1A-+4845 and IL-1B-+3954) and 2) to correlate the association of the composite genotype with the severity of chronic periodontitis.
Materials and Methods:
Sixty patients aged above 35 years were selected. Following a periodontal examination, using the clinical parameters plaque index, gingival bleeding index, probing depth, and clinical attachment loss (CAL), the selected subjects were categorized into four groups of differing disease severity based on CAL. Five milliliters of venous blood was drawn. DNA was isolated by phenol chloroform method. Amplification of IL-1A+4845 and IL-1B+3954 was done by polymerase chain reaction (PCR). Detection of genotype was done using restriction fragment length polymorphism using the enzymes FnU4HI for IL-1A and TaqI for IL-1B. The results obtained were analyzed statistically.
Results:
The frequencies of IL-1A-+4845 and IL-1B-+3954were significantly greater in severe periodontitis patients. The distribution of composite genotype (allele 2 of IL-1A+4845and allele 2 of IL-1B+3954) also correlated with the severity of periodontitis. Genotype-positive subjects had a higher mean bleeding index (%) when compared to genotype-negative patients. But no correlation was observed between mean plaque level among genotype-positive and -negative subjects.
Conclusion:
IL-1 gene polymorphism IL-1A+4845, IL-1B+3954 and composite genotype is an indicator of susceptibility to severe periodontitis in adults.
Keywords: Composite genotype, gene polymorphism, interleukin-1, periodontitis, susceptibility
INTRODUCTION
Periodontitis is a chronic inflammatory disease initiated by predominantly gram-negative anaerobes[1] that activate the immune and inflammatory system of the host, tissue destruction being the result of the host response in the process of bacterial evasion.[2] However, the clinical picture of the disease varies highly[3] between individuals, with variations observed in the susceptibility to the disease as well as in the pattern of disease progression.
Studies by Michalowicz et al.[4,5] have shown that a significant part of this inter-individual variation can be attributable to genetic factors. The search for candidate genes to determine gene polymorphism centered on the cytokine network due to their substantiated role in periodontitis. Proinflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) are key mediators of the inflammatory process and IL-1 has been studied extensively as it is a more potent inducer of bone resorption.[6,7] Increased levels of IL-1 have been found in both the gingival crevicular fluid[6] and gingival tissues[8,9] of patients with adult periodontitis. Variation in cytokine levels between individuals has been well documented[10] and may contribute to disease susceptibility. Such differences may be attributed in part to the polymorphic cytokine genes since particular alleles have been associated with increased cytokine levels.
The first study of cytokine gene polymorphism was reported by Kornman[11] who found a significant association between severe adult periodontitis and composite genotype, namely, allele 2 of a single nucleotide polymorphism (SNP) of IL-1A+4845 and IL-1B+3954 located on chromosome 2q13. Following this, several studies have been conducted exploring the role of IL-1 gene polymorphism as a severity factor in periodontitis in various population and ethnic groups.[12,13] The purpose of the present study was to investigate the distribution of IL-1 gene polymorphism in the South Indian population and to correlate the association of composite genotype with severity of periodontitis.
MATERIALS AND METHODS
Sixty patients attending the Department of Periodontics of Tamil Nadu Government Dental College, Chennai, of above 35 years of age and either sex, were screened and selected. Subjects with history of smoking, pregnancy/lactation, diabetes, bleeding disorders, severely compromised immune function, immunosuppressive chemotherapy, requiring antibiotic prophylaxis for dental procedures, on nonsteroidal anti-inflammatory drug for the past 3 years or on antibiotics for the past 6 months were excluded. Selected subjects received a full mouth periodontal examination to assess the extent of plaque using plaque index,[14] gingival inflammation using the gingival bleeding index,[15] pocket probing depth, and clinical attachment level (CAL). Based on the average full mouth CAL, these subjects were categorized into four groups, each comprising 15 subjects, as follows:
Group A (healthy control): Subjects with healthy periodontium
Group B (mild periodontitis): Subjects exhibiting no more than 1–2 mm of CAL
Group C (moderate periodontitis): Subjects exhibiting 3–4 mm of CAL
Group D (severe periodontitis): Subjects exhibiting ≥5 mm of CAL
The study protocol was presented and approved by the ethical committee in the institution.
Five milliliters of venous blood was drawn from the antecubital vein and transferred to a vacutainer containing ethylenediaminetetraacetic acid (EDTA; 3%) and stored at −20°C. Deoxyribonucleic acid (DNA) was extracted by phenol chloroform method and ethanol precipitation.[16] The isolated genomic DNA was suspended in 500 μl of TE buffer and stored at −20°C till further genotyping. Genotyping was carried on for the locus IL-1A+4845 and IL-1B+3954 using polymerase chain reaction (PCR) and restriction fragment length product (RFLP) procedure.
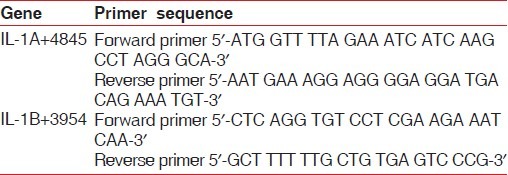
PCR was carried out using a 20-μl reaction mix solution containing 0.2 μl of Taq polymerase (5 U/μl), 2 μl of KCl buffer, 1.6 μl of each primer [Table 1] (0.4 pM), 2 μl of MgCl2 (2.5 mM), 1.6 μl of dNTP (0.2 mM), and 2 μl of DNA on a thermocycler.
Table 1.
Primer sequences of interleukin-1 gene
IL-1A+4845: Cycling was carried as follows: 95°C for 3 min, 35 cycles of 94°C for 1 min, 54°C for 1 min, 72°C for 2 min, and finally one cycle of 72°C for 5 min. 10 μl of amplicon was digested with 1 μl of FNU4H1 enzyme (10 U/μl), yielding two fragments of 124 and 29 bp in subjects homozygous for allele 1 and one fragment of 153 bp in subjects homozygous for allele 2. All the three fragments were present in heterozygous individuals.76 bp served as the restriction site [Figure 1].
Figure 1.
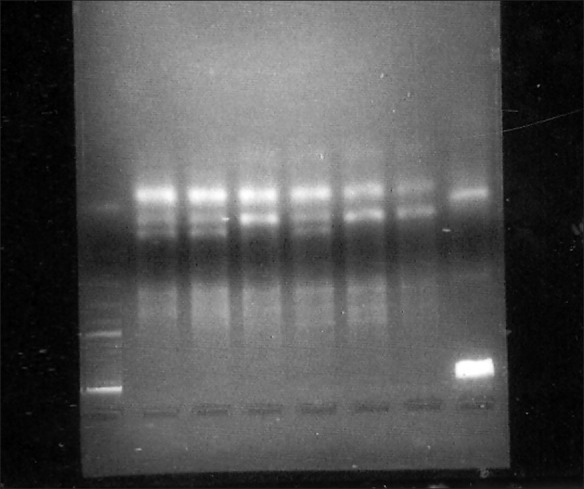
4% agarose gel electrophoresis of RFLP with ethidium bromide staining of IL-1+4845. Lanes 2, 3, 5: homozygous for allele 1 (124 bp); lanes 4, 6, 7: heterozygous for the allele 1 and 2 (124 bp and 153 bp). lane 8-100 bp ladder. Lane numbering proceds from right to left
IL-1B+3954: Cycling was performed as follows: 1 cycle 95°C for 3 min, 35 cycles each at 94°C for 1 min, 67.5°C for 1 min, 74°C for 1 min, and finally 1 cycle 72°C for 8 min. Digestion of 10 μl of amplicon with TaqI enzyme at 65°C yielded two fragments of 85+97 bp (allele 1) and a single 182 bp fragment (allele 2). All three fragments were present in heterozygous individuals. 12 bp served as the restriction control site [Figure 2]. A subject was considered IL-1 genotype positive if at least one allele 2 was present; i.e., homozygous or heterozygous for the less common allele 2.
Figure 2.
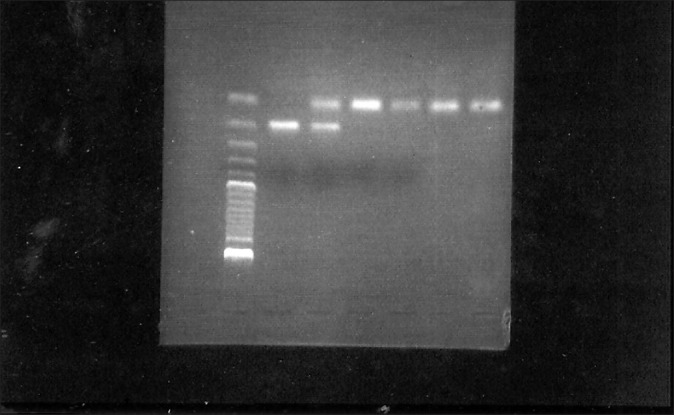
4% agarose gel electrophoresis of RFLP with ethidium bromide staining of IL-1B+3954. Lanes 1–4: homozygous for allele 1 (85+97 bp); lane 6: homozygous for allele 2 (182 bp); lane 5: heterozygous condition (182 bp and 85+97 bp). Lane 7 100 bp ladder. Lane numbering proceeds from right to left
Statistical analysis
Mean and standard deviation of the clinical parameters, namely, plaque index and gingival bleeding index, were estimated for each group. Mean values were compared by Analysis of Variance (ANOVA). Multiple range test by Turkey's Honestly Significant Difference (HSD) was employed to identify the significant groups. Chi-square test with Mantel–Haenszel test of linear association was used to determine the association between severity of periodontitis and IL-1 composite genotype. The mean value of plaque index and gingival bleeding index was calculated among genotype-positive and genotype-negative subjects and their significance was determined using Student's t-test
RESULTS
The clinical parameters, plaque index, and gingival bleeding index were analyzed among the groups to determine their prediction for severe periodontal disease. The mean plaque score of moderate and severe periodontitis subjects (groups C and D, respectively) was significantly higher than that of the healthy and mild periodontitis subjects (groups A and B, respectively) as seen in Table 2, but there was no statistical significant difference between group A and group B or between group C and group D. Table 2 also compares the mean of bleeding index among the different groups. There was a significant increase in the percentage of bleeding sites, progressively from group A (5.9±2.7) to group D (84.73±19.45) (P<0.001).
Table 2.
Correlation of the clinical parameters among different groups

Sixty subjects were evaluated to study the distribution of IL-1 gene polymorphism. Eighteen subjects were positive for IL-1A+4845 genotype (28%), with a high frequency of positive subjects observed among the group D subjects and the least frequency observed among the healthy subjects. This difference achieved statistical significance at 5% level (χ2 =3.99, df=1). Similar trend was noticed for the allele 2 of IL-1B+3954 gene, with the frequency progressively increasing from group A through group B, C, to group D, which was significant at 5% level (χ2 =5.82, df=1). The combined presence of both the IL-1A and IL-1B alleles, i.e., composite genotype, was studied. The frequency was higher in group D (33.33%) when compared with group C (20%), group B (6.75%), and group A (6.7%). This difference brought out a significant correlation between the severe form of periodontitis and the presence of composite genotype (χ2 =4.62, df=1, odds ratio healthy vs. severe=7) [Table 3].
Table 3.
Distribution of genotype among different groups
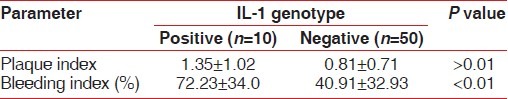
Comparing the mean plaque score among composite genotype positive and negative subjects, it was found that there was no significant correlation (1.35 vs. 0.81) [Table 4]. This was in contrast to the significant association which was observed between the severity of disease and the amount of plaque [Table 2]. But the mean value of percentage of bleeding sites was significantly higher among the genotype-positive subjects (72.23) than the genotype-negative subjects (40.91) as seen in Table 4.
Table 4.
Clinical parameters of IL-1 composite genotypepositive and negative subjects
DISCUSSION
The variability among individuals in the clinical response to the presence of bacterial plaque has been well recognized. No direct cause and effect process is associated with the development and progression of disease, but rather the effects of bacterial accumulation will be modulated by biologic, systemic environmental and behavioral factors. Among the biologic aspects, heredity certainly affects the response of the host. The finding that a specific genotype in the IL-1 gene cluster correlates with severe periodontitis suggests a genetic mechanism by which some individuals may have a more vigorous immuno-inflammatory response leading to more severe periodontitis. Several studies have shown variations in the distribution of composite genotype in association with periodontitis patients from different geographic areas and ethnic groups.[11,13,17–20] Studies examining ethnic population from Europe have reported consistently that approximately 30% of the individuals are genotype positive. Walker et al.[18] reported a genotype-positive prevalence of 15% in an African American population. For a Chinese population,[19] no significant association was found, with only a prevalence of 2.3%. Among the Indian population, where the prevalence of periodontitis is high, studies has shown conflicting results. A prevalence of 11.66% of composite genotype has been found in the Maharashtrian ethnic group, correlating with the severity of periodontitis.[21] In a study evaluating the prevalence of only IL-1B polymorphism among the South Indian subjects, a higher percentage of genotype was observed among cases, but this observation did not reach statistical significance.[22] These findings suggest possible racial variation and ethnic specificity. Till date, no study has been done to analyze both IL-1A and IL-1B polymorphism in the South Indian population. Hence, this study attempted to determine the prevalence of IL-1 gene polymorphism of the composite genotype and also aimed to ascertain the composite genotype as a susceptibility factor to the severe form of periodontitis.
Smoking by itself is a strong risk factor and its role in periodontitis has been well established.[23,24] In the presence of smoking as a risk factor, the predictive value of other variables becomes statistically insignificant.[11] Hence, this study excluded smokers.
Composite genotype, as stated by Kornman et al.,[11] refers to the presence of a specific polymorphism allele 2 at IL-1A-889 and IL-1B+3953 loci. Currently, genetic tests assess for the presence of at least one copy of allele 2 at IL-1A+4845 and at least one copy of allele 2 at IL-1B+3954. The IL-1A+4845 polymorphism is easier to identify than IL-1A-889 polymorphism and is concordant to it. In addition, IL-1B+3953 polymorphism has been renumbered and is now referred as IL-1B+3954.
Studies by Kornman et al.[11] and McDevitt et al.[13] have well documented the association of the composite genotype with the severe form of periodontitis. The findings of the present study are also in concurrence with the previous studies, establishing a positive correlation between composite genotype and periodontitis severity among South Indians. The underlying mechanism for this increased severity has been demonstrated by Pociot et al[25], diGiovine et al.,[26] who established that in response to the same bacterial challenge, genotype-positive individuals produce two to four times more IL-1. Engebretson[27] et al. further established that levels of IL-1 in both gingival crevicular fluid and tissue are about 2.5 times higher in genotype-positive individuals. These findings substantiate that the presence of the polymorphism results in a greater production of IL-1 to a bacterial challenge, thus predisposing to a more severe form of the disease.
The percentage of genotype-positive cases among Caucasians was 29.1% in the study done by Kornman[11] among non-smokers. The present study has yielded a percentage of 16.66%, the prevalence of which is lower when compared to the previous studies,[11,17,20] but higher when compared to the prevalence of 11.66% observed among the Maharashtrian ethnic group. Further larger studies may be needed to corroborate these results.
The individual carriage rates of IL-1A and IL-1B genes have also been analyzed. A significant correlation was observed when the individual polymorphism was compared with the severity of periodontitis. Studies exploring this aspect have not yielded consistent results,[11,12,22,28] probably emphasizing the ethnic variation existing among different groups.
Genotype-positive patients had a higher bleeding index than the negative patients which may be attributed to the hyperresponsiveness of the individual to bacterial plaque, thus predisposing to a severe inflammatory state. Similar association has also been reported by former studies.[21,29] However, the mean plaque index in the study did not vary significantly between genotype-positive and -negative patients, suggesting that though genotype-positive patients may have a lesser mean plaque value, in the presence of the genotype, they are predisposed to a more severe form of the disease. This can be explained by the study done by Socransky and Haffajee[30] who observed that mean counts of specific subgingival species were significantly higher among genotype-positive patients. Prominent among the species detected were Tanerella forsythus, Treponema denticola, and Fusobacterium nucleatum. Thus, the higher levels of red and orange complex rather than the higher quantity of plaque as measured by the plaque index were strongly associated with periodontal disease among the genotype-positive subjects.
In conclusion, the increased frequency of IL-1A+4845 allele 2 and IL-1B+3954 allele 2 genotypes observed in South Indian subjects with advanced chronic periodontitis when compared to that of early and moderate disease brings out a significant positive association between the composite genotype and severity of the disease in this particular population group. However, further studies with a larger sample size are needed to clearly validate these results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000;1994(5):78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 2.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of development, clinical implications and future directions. Periodontol. 2000;1997(14):216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ. Assessment of risk of periodontal disease. Compend suppl. 1994;18:S714–7. [PubMed] [Google Scholar]
- 4.Michalowicz BS. Genetic and heritable risk factors in periodontal disease. J Periodontol. 1994;65:479–89. doi: 10.1902/jop.1994.65.5s.479. [DOI] [PubMed] [Google Scholar]
- 5.Michalowicz BS, Aeppli D, Virag JG, Klump DG, Hinrichs JE, Segal NL, et al. Periodontal findings in adult twins. J Periodontol. 1991;62:293–9. doi: 10.1902/jop.1991.62.5.293. [DOI] [PubMed] [Google Scholar]
- 6.Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison AC. Measurement of interleukin-1α and 1β in gingival crevicular fluid: Implications for the pathogenesis of periodontal disease. J Periodontal Res. 1990;25:156–63. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 7.Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;92:504–9. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 8.Honig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F. Increased Interleukin - 1β (IL - 1β) concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–7. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 9.Stashenko P, Fujiyoshi P, Obernesser MS, Prostak L, Haffajee AD, Socransky SS. Levels of interleukin 1β in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–54. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 10.Molvig J, Baek L, Christensen P, Manogue KR, Vlassara H, Platz P, et al. Endotoxin-stimulated human monocyte secretion of interleukin 1, tumour necrosis factor α, and prostaglandin E2 shows stable interindividual differences. Scand J Immunol. 1988;27:705–16. doi: 10.1111/j.1365-3083.1988.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 11.Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;34:72–7. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 12.Papapanou PN, Neiderud AM, Sandros J, Dahlen G. Interleukin-1 gene polymorphism and periodontal status.A case-control study. J Clin Periodontol. 2001;28:389–96. doi: 10.1034/j.1600-051x.2001.028005389.x. [DOI] [PubMed] [Google Scholar]
- 13.McDevitt MJ, Wang HY, Knobelman C, Newman MG, di Giovine FS, Timms J, et al. Interleukin-1 genetic association with periodontitis in clinical practice. J Periodontol. 2000;71:156–63. doi: 10.1902/jop.2000.71.2.156. [DOI] [PubMed] [Google Scholar]
- 14.Silness P, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 15.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory Manual. In: Nolan C, editor. Cold Spring Harbor. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 1–3. [Google Scholar]
- 17.Caffesse RG, De La Rosa RM, De La Rosa GM, Weltman R. Effect of interleukin-1 gene polymorphism in a periodontally healthy Hispanic population treated with mucogingival surgery. J Clin Periodontol. 2002;29:177–81. doi: 10.1034/j.1600-051x.2002.290213.x. [DOI] [PubMed] [Google Scholar]
- 18.Walker SJ, Van Dyke TE, Rich S, Kornman KS, di Giovine FS, Hart TC. Genetic polymorphisms of the IL-1α and IL-1β genes in African–American LJP patients and an African-American control population. J Periodontol. 2000;71:723–8. doi: 10.1902/jop.2000.71.5.723. [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC, Wu Y, Wang HY, Sorrell J, di Giovine FS, Duff GW. Low prevalence of a periodontitis associated interleukin-1 composite genotype in individuals of Chinese heritage. J Periodontol. 2000;71:164–71. doi: 10.1902/jop.2000.71.2.164. [DOI] [PubMed] [Google Scholar]
- 20.Anusaksathien O, Sukboon A, Sitthiphong P, Teanpaisan R. Distribution of IL-B+3954 and IL-1A-889 genetic variation in a Thai population group. J Periodontol. 2003;74:1796–02. doi: 10.1902/jop.2003.74.12.1796. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian Ethnicity. J Periodontol. 2006;77:1515–21. doi: 10.1902/jop.2006.050427. [DOI] [PubMed] [Google Scholar]
- 22.Kaarthikeyan G, Jayakumar ND, Padmalatha O, Sheeja V, Sankari M, Anandan B. Analysis of the association between interleukin -1β (+3954) gene polymorphism and chronic periodontitis in a sample of the south Indian population. Indian J Dent Res. 2009;20:37–40. doi: 10.4103/0970-9290.49061. [DOI] [PubMed] [Google Scholar]
- 23.Haber J. Smoking is a major risk factor for periodontitis. Curr Opin Periodontol. 1994:12–8. [PubMed] [Google Scholar]
- 24.Schenkein HA, Gunsolley JC, Koertge TE, Schenkein J, Tew JG. Smoking and its effect on early onset periodontitis. J Am Dent Assoc. 1995;126:1107–13. doi: 10.14219/jada.archive.1995.0327. [DOI] [PubMed] [Google Scholar]
- 25.Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1β (IL-1β) gene correlates with IL-1β secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 26.di Giovine F, Camp NJ, Cox A, Chaudhary AG, Sorrell J, Crane A, et al. Detection and population analysis of IL-1 and TNF gene polymorphisms. In: Balkwill F, editor. Cytokine Molecular Biology. Oxford: Oxford University Press; 2000. pp. 21–46. [Google Scholar]
- 27.Engebretson SP, Lamster IB, Herrera Abreu M, Celenti RS, Timms JM, Chaudhary AG, et al. The influence of interleukin gene polymorphism on expression of interleukin-1β and tumor necrosis factor-α in periodontal tissue and gingival crevicular fluid. J Periodontol. 1999;70:567–73. doi: 10.1902/jop.1999.70.6.567. [DOI] [PubMed] [Google Scholar]
- 28.Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM. Interleukin 1β +3953 allele 2: association with disease status in adult periodontitis. J Clin Periodontol. 1998;25:781–5. doi: 10.1111/j.1600-051x.1998.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 29.Lang NP, Tonetti MS, Suter J, Sorrell J, Duff GW, Kornman KS. Effect of interleukin - 1 gene polymorphisms on gingival inflammation assessed by bleeding on probing in a periodontal maintenance population. J Periodontol Res. 2000;35:102–7. doi: 10.1034/j.1600-0765.2000.035002102.x. [DOI] [PubMed] [Google Scholar]
- 30.Socransky SS, Haffajee AD, Smith C, Duff GW. Microbiological parameters associated with IL-1 gene polymorphisms in periodontitis patients. J Clin Periodontol. 2000;27:810–8. doi: 10.1034/j.1600-051x.2000.027011810.x. [DOI] [PubMed] [Google Scholar]


