Abstract
Background:
Several synthetic alloplastic materials have been used in the past as an implant in infrabony defects with a goal to reconstruct the lost part of attachment apparatus via new osseous tissue formation. The present study was undertaken to evaluate and compare clinico-radiographically, the effect of bioactive glass (BG), hydroxyapatite (HA), and BG-HA composite bone graft particles in the treatment of human infra-bony periodontal defects.
Materials and Methods:
Indigenous synthetic HA, BG, and BG-HA composite bone graft materials were developed in the laboratory. Twenty eight infrabony periodontal defects were equally distributed (i.e., seven defects) into four groups. The defects were treated separately with three types of graft materials and non-grafted manner (open flap debridement alone, control) to evaluate both the soft and hard tissue responses after six months of surgery. Evaluation was done by studying different parameters such as plaque index, gingival index, relative attachment level, probing pocket depth, and radiographic bone fill in Intra Oral Peri-Apical radiograph.
Results:
The healing of defects was uneventful and free of any biological complications. The gain in relative attachment level, reduction of probing pocket depth, and bone fill was statistically significant in all four groups. BG and BG-HA synthetic bone graft implanted sites showed significant bone fill (P<0.05) than hydroxyapatite and unimplanted control sites.
Conclusion:
The performance of BG and its composite was better compared to HA and open flap debridement alone for the reconstruction of infrabony defects. The BG-HA composite particles may effectively be used as an alternative bone graft material for infrabony defects.
Keywords: Alloplastic materials, BG-HA composite grafts in infrabony defects, bone defect fill, infrabony defects, open flap debridement, reconstruction
INTRODUCTION
Regeneration of the lost periodontium as a consequence of periodontal diseases yet unachieved by surgery and/or any other means to its previously existing normal physiologic level is one of the main goals of periodontal therapy.[1] Conventional periodontal treatments, such as scaling and root planing, are highly effective at repairing disease-related defects and halting the progression of periodontitis but do little to promote regeneration of the lost periodontium. Regenerative periodontal surgery using both “Tissue Engineering” and “In situ tissue regeneration” is modified continuously to modulate regenerative processes, though they may have variable success rate and efficacy in human jaw bones.[2] All the currently available bone graft materials neither “cure – all” nor cure up to 100% of any periodontal defect. Ceramics, collagen, and polymers are the recently used alloplastic materials;[3] they can be either bioactive or bioinert. Bioactive ceramics include hydroxyapatite (HA), fluorapatite, bioactive glass (BG), and tricalcium phosphate. Tricalcium phosphate (TCP) has unpredictable high rate of bioresorption and incapable to induce periodontal regeneration.[4] Hydroxyapatite is the most extensively researched material in periodontal defects. Synthetic HA is a biocompatible, nontoxic, slowly resorbing, osteoconductive, osteophillic material and has close structural and chemical resemblance to bone mineral, but not identical.[4] Bioactive glass can develop a chemical bond with living hard tissues through the development of a surface layer of carbonated hydroxyapatite.[5] When BG is exposed to tissue fluid, it is covered by silica rich gel and top of which calcium phosphate rich layer is formed that promotes absorption and concentration of osteoblast cells to form an extra cellular matrix and mineralization.[6] Kim et al.[7] reported that BG guides and promotes osteogenesis and allows rapid bone formation. Fetner et al.[8] had studied comparison of TCP, HA, BG with an unimplanted control and found that bioactive glass (Perioglas) produced significant bone and cementum repairs. On the other hand, Ong et al.[9] reported no significant effect of BG in periodontal defects.
Therefore, the present study was aimed to evaluate and compare clinically and radiographically the effect of HA, BG, and BG-HA composite bone graft particles in the treatment of human infrabony periodontal defects.
MATERIALS AND METHODS
This study was carried out in the Department of Periodontics, Dr. R. Ahmed Dental College, Kolkata, in association with the Central Glass and Ceramic Research Institute, Kolkata. Indigenous HA particles and bioactive glass particles [SiO2 = 43-44 wt. %, Na2 B4 O7.10H2O=6-7 wt. %, Na2CO3 =11-12 wt. %CaCO3 =29-30 wt. %, (NH4)2HPO4 =8-9 wt. %, TiO2 =1-2 wt. %] that were developed (by co-precipitation technique and conventional glass melting technique) and characterized # earlier both in vitro and in vivo[10] were used for the present study. BG-HA composite graft was developed by milling the scaffold made up of 50% BG and 50% HA powder at 820°C.
Twenty two systemically healthy patients (12 male and 10 female), aged from 30 to 56 years (mean age, 43 years) were selected for this study. Radiographic examination revealed 28 three walled infrabony defects, and out of the 22 patients, 4 patients demonstrated multiple angular periodontal defects [Figure 1f], and were treated by open flap debridement (OFD) alone in one defect and OFD with identical synthetic bone graft (HA or BG or BG-HA) in the others. None of the patients received two or more grafts. The informed consent was obtained from all the patients and the study was approved by the institutional ethical committee of Dr. R. Ahmed Dental College, Kolkata. The infrabony defect sites were divided equally into four groups (seven in each group) - Group I (Open Flap Debridement [OFD] was done alone), Group II (OFD with HA), Group III (OFD with BG), and Group IV (OFD with BG [50%] – HA [50%] composite bone graft filling sites). Patients selected for the study fulfilled the following inclusion criteria; at least one clinical evidence of an infrabony defect, radiographic evidence of bone loss, systemically healthy patients, no history of long term antibiotics or other medications usage, and no history of invasive periodontal therapy for the past six months at the recipient site. Acrylic stents with one prepared vertical groove were used as fixed reference position (i.e. junction of vertical groove and lower border of the stent) and for proper alignment of graduated periodontal probe University of North Carolina (UNC-15)** in subsequent measurements.
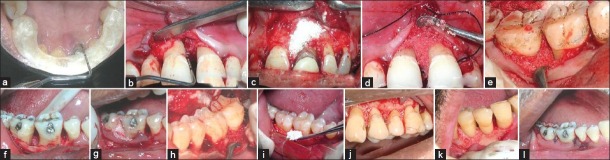
Figure 1.
(a) Recording of relative attachment gain using acrylic stent, (b,f) infrabony defects after curettage and debridement; infrabony defects implanted with (c,i) bioactive glass, (d,j,k) hydroxyapatite and (e,g,h) BG-HA composite
The following clinical parameters were recorded to the nearest millimeter with the help of a UNC-15 probe for each surgical site during surgery (baseline) and six months post-operatively. All the measurements as well as clinical procedures were conducted by a single examiner to exclude inter-examiner variation.
Soft tissue parameters were evaluated by plaque index (PI),[11] gingival index (GI),[12] probing pocket depth (PPD), and relative attachment level (RAL). RAL is the distance from a fixed reference point to base of the periodontal pocket [Figure 1a].
Hard tissue parameters were evaluated by depth of the defect (DOD) and radiographic bone fill as seen in Intra Oral Peri-Apical (IOPA) radiograph. DOD is the distance from the fixed reference point to the deepest part of the defect that was measured at baseline by bone sounding and via surgical entry and after six months post-operatively through bone sounding only. The radiographic bone fill was assessed comparing six months post-operative radiographs with the baseline radiographs which were taken prior to the surgery.
Each patient was prepared for surgery with oral hygiene instructions, scaling and root planing at least three months prior to surgery, adjunctive chemical plaque control, and occlusal adjustment was performed, whenever necessary. Patients were re-evaluated to assess clinical parameters and plaque control four weeks after initial therapy.
After extra and intra oral disinfection and local anesthesia (2% Lignocaine) application, crevicular incisions were given and the flaps were elevated. Depth of the defect from the fixed reference point (apical end of the stent) to the deepest point of the defect was measured for baseline reference. After thorough debridement and root planning [Figure 1b], an adequate quantity of the sterile synthetic bone graft particles were mixed with a few drops of saline in a sterile dappen dish. The graft recipient site was selected randomly. Before the placement of the graft, a 3-0 non resorbable silk suture was passed through the buccal and lingual papillae and the suture was left loose to prevent removal of the graft particles by the passage of the needle as well as the suture material [Figure 1d]. After placement of the graft [Figure 1c–k], silk sutures were used to approximate the lingual and buccal flaps using interrupted sutures [Figure 1l] and conventional periodontal dressing was given. In seven defect sites, no synthetic bone graft particles were implanted (Group I). Asepsis and gentle tissue handling, which are important considerations for regenerative procedures, were strictly followed in each surgery.
Patients were prescribed 0.2% Chlorhexidine twice daily for two weeks, oral antimicrobials (500 mg amoxicillin thrice daily × 7 days), analgesic (400 mg ibuprofen thrice daily × 3 days), antacids (Ranitidine 150mg twice daily × 3 days), and routine post surgical instructions were explained. Patients were recalled after one week for suture removal and six months after surgery. At each of the recall visits oral hygiene was checked and oral hygiene instructions were reinforced.
Statistical analysis
Statistical analysis was employed to compare the study results using a software program£ . For each outcome measurement, at least 95% confidence limit was estimated for change of all observations from baseline to six months post-operatively. Inter-group comparisons of changes in soft tissue and hard tissue parameters from baseline to six months post surgery were made between Group I (unimplanted control), Group II (HA), Group III (BG), and Group IV (BG-HA composite) by independent sample ‘t’ test and within the respective groups themselves (intra-group) through a paired student ‘t’ test in case of periodontal defects.
RESULTS AND OBSERVATIONS
Healing was uneventful in all the groups. In the intra-group comparison (paired student ‘t’ test), changes in soft tissue and hard tissue parameters of periodontal defects from baseline to six months post surgery for Group I (unimplanted control), Group II (HA), Group III (BG), and Group IV (BG-HA composite) are presented in Table 1, and the inter-group comparison (independent sample ‘t’ test) are presented in Table 2. No sites in Group I, Group II, Group III, and Group IV displayed 100% vertical defect resolution. Mean plaque index scores for all the four groups were 1 at baseline, one week and 6 months after surgery (no statistically significant differences-P>0.05). Mean gingival index scores at baseline and after six months of surgery for all the four groups were shown in Table 1 (ranged from 0 to 1). Paired t-value of gingival index (GI) scores at baseline, 1 week and 6 months after surgery in the four groups showed no statistically significant differences (P>0.05). The PPD values for the grafted sites ranged from 6 to 10 mm and in the control sites ranged from 6 to 8 mm at baseline. At six months, probing pocket depth values for the HA sites ranged from 4 to 6 mm, 2 to 4 mm for the bioactive glass group, 3 to 4 mm for HA-BG composite bone graft group, and 3 to 5 mm in the control group. The mean of PPD values for all the groups at baseline and six months after surgery were presented in Table 1. All the groups resulted in significant reductions in PPD (P<0.01). When comparison was done between the four groups bioactive glass group showed statistically significant reductions in PPD at 5% level compared to Gr-I and Gr-II [Table 2]. The RAL values at baseline ranged from 10 mm to 14 mm for HA group, 11 to 16 mm for bioactive glass group, 11 to 15 mm for HA-BG composite group and 10 to 12 mm in control group. At six months, RAL values for the HA sites ranged from 8 to 9 mm and 7 to 9 mm for all the remaining groups. The mean of RAL values for all the groups at baseline and 6 months after surgery were presented in Table 1. All the four groups resulted in significant gain in relative attachment level (P<0.01). When comparison was done between the four groups, HA-BG composite group showed statistically significant gain in RAL at 5% level compared to control group only [Table 2]. The Depth of Defect (DOD) values at baseline ranged from 11 to 15 mm for HA, 12 to 16 mm for bioactive glass group, 12 to 16 mm for HA-BG composite group, and 11 to 14 mm in control group. At six months, DOD values for the HA sites ranged from 9 to 11 mm, 8 to 10 mm for the bioactive glass group, 8 to 12 mm for HA-BG composite group, and 9 to 10 mm in the control group. The mean of DOD values for all the groups at baseline and 6 months after surgery were presented in Table 1. All the four groups showed statistically significant (P<0.01) amount of defect fill from baseline to six months post operatively. The bioactive glass and HA-BG composite group resulted in a greater defect fill as compared to the HA and control group [Table 2], but inter group comparison between the HA-BG composite and Bioactive glass group showed no statistically significant difference in defect fill [Table 2].
Table 1.
Intra-group comparisons of mean and SD of soft tissue and hard tissue parameters (mm)
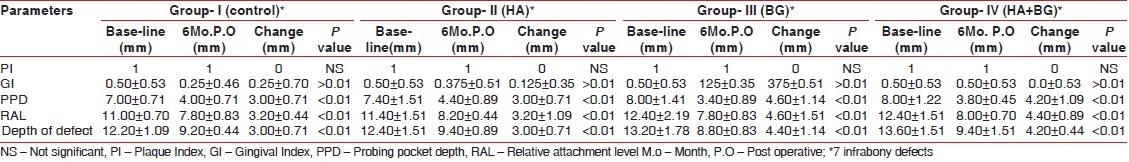
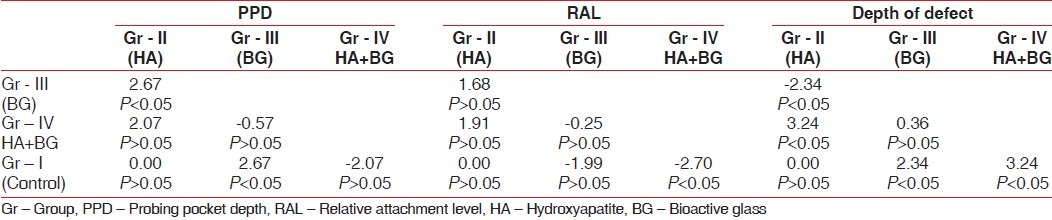
Table 2.
Inter group comparisons (Independent sample t-test) of different parameters studied
In Figure 2, the representative IOPA X-ray image of the healing site of Group I, Group II, Group III, and Group IV, A1, A2, A3, and A4 show pre-operative and B1, B2, B3, and B4 show six months post operative pictures of the respective groups of healing of infrabony periodontal defects. In the post operative pictures, B3 (Group III) and B4 (Group IV) showed increased angular defect fill compared to B1 (Group I) and B2 (Group II) in respect of their pre-operative view (A3, A4 and A1, A2).
Figure 2.
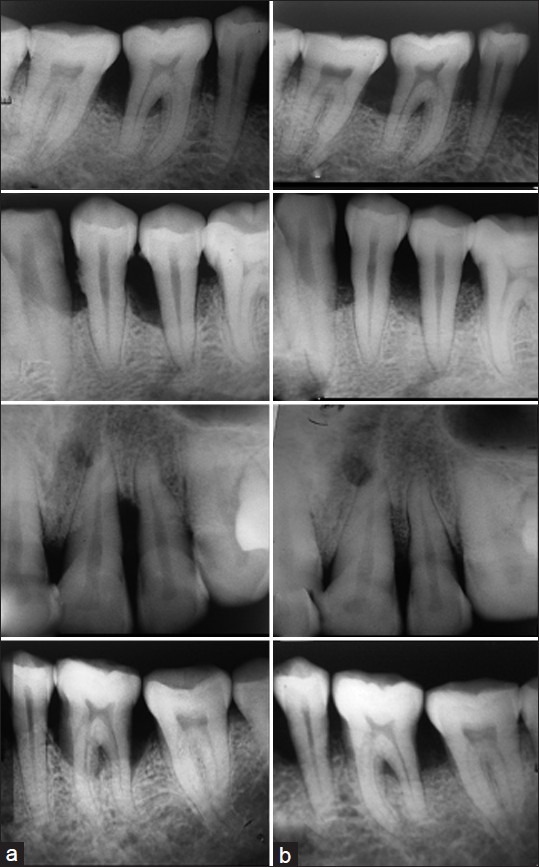
Pre-operative (a) and six months post-operative (b) view of Group I, Group II, Group III, and Group IV (periodontal defects), respectively
DISCUSSION
A multitude of new materials have been used for promoting periodontal regeneration in infrabony defects where bioactive glass (BG) has gained momentum in the recent years.
Long-term success with regenerative procedures has been directly correlated with plaque control.[1] Statistically insignificant differences (P>0.01) in PI scores throughout the study period in the four groups [Table 1] suggesting that minimum amount of plaque did not interfere with regenerative process of any particular group and all the patients maintained their oral hygiene properly throughout the study periods,[9] though insignificant deterioration in plaque scores might be found sometimes.[13]
Similarly, statistically insignificant differences (P>0.01) in GI scores in all the four groups [Table 1] suggesting that all the synthetic graft materials used were biocompatible, non toxic, didn′t elicit any inflammatory and/or foreign body responses to the tissues, and no apparent retardation of normal bone mineralization process that claimed to be primary requisites for graft materials.[14]
Intra-group comparisons of changes in PPD, RAL, and Depth of the Defect [Table 1] from baseline to six months post surgery for all four groups showed statistically significant improvement (P<0.01) suggesting that both OFD and OFD plus synthetic bone graft particles were effective in the treatment of infra bony periodontal defects.[15]
In inter-group comparisons [Table 2], there was significant improvement (P<0.05) in the probing pocket depth in Group-III (BG) as compared to the Group-I (control) and Group-II (HA) suggesting that BG composition (43-44 Wt% SiO2 ) used in this study was below 52% by weight of SiO2 that could form a bond with soft tissue collagen.[16] It acted as a biologic membrane retarding epithelial downgrowth[8] and appeared to facilitates growth and repair of soft connective tissues. But statistically insignificant differences of the changes in probing pocket depth between the Group-III and Group-IV (BG-HA composite) suggesting that soft tissue bonding property of BG might have some role in Group-IV (BG-HA composite) sites.
The inter-group comparison between mean gain in RAL in the HA, BG, and control sites were found [Table 2] to be statistically insignificant suggesting that all three treatment modalities were effective in the treatment of infra bony periodontal defects with a greater trend toward improvement in the BG sites, as also observed by Zamet et al.[17] But statistically significant gain (P<0.05) in RAL in the Group-IV (porous BG-HA composite) as compared to Group-I sites (control) suggesting that strong chemical bonding of hydroxy carbonate apatite layer (HCA) to collagen created the firm attachment to the host tissue. However, the gain in attachment could be due to new attachment, healing by long junctional epithelium or change in soft tissues post-operatively.
Although surgical entry is considered to be the most accurate method to assess depth of the defect but it was evaluated through bone sounding because, trauma to regenerated tissue, unavoidable bone loss with each exposure, patient's discomfort, ethical concerns for re-entry procedure were the limitations of open bone measurements.[18]
In the present study, no difference was observed with the bone level measurement recorded through bone sounding and via surgical entry during surgery (at baseline) indicating that bone sounding alone was adequate to assess bone defect fill causing minimal trauma to the regenerating tissue.[19]
The mean defect fill in the four groups at the end of six months [Table 1] falls within the same range or slightly more of the study of Laurell et al.[20] DOD demonstrated greater defect fill with Group-III and Group-IV as compared to the Group-II (HA) and Group-I (control), but inter group comparison between the Group-III and Group-IV showed no statistically significant differences [Table 2] in defect fill. It suggested that osteostimulatory property of BG might have some role in Group-IV and in improving defect fill noted in this study.[8,17,21]
In Group-I (Control) sites [Figure 2, B1], relatively slow radiographic bone fill was seen in IOPA radiograph which might be due to early recolonization of cells from the gingival soft tissues to the defect sites and resulted in filling of defects with radiolucent soft connective tissue.[22]
In Group-II (HA) sites [Figure 2, B2], apparently slow bone formation which might be due to partial resorption followed by encapsulation of HA particles with radiolucent soft connective tissue.[23] It was also suggested that HA did not appear to stimulate rapid bone growth in the defect but acted simply as an osteoconductive agent.[4] There is no silicon contained in the HA, consequently an incubation period for the HA implant is necessary to accumulate biological hydroxyapatite on the implant, which would delay the deposition process of biological hydroxyapatite on the surface of the implant.[24]
In Group-III (BG, [Figure 2, B3]) and Group-IV (BG-HA, [Figure 2, B4]) sites, greater radiographic bone fill in IOPA radiograph might be due to osteostimulatory property of bioactive glass. A greater amount of new bone growth was also observed in the bone defects implanted with bioactive glass when compared to Group-I ([Figure 2], B1 -control) and Group-II ([Figure 2], B2 -HA) suggesting that silica rich layer provide multiple nucleation sites for hydroxy-carbonate-apatite precipitation and crystallization. Schepers et al.[24] also observed similar finding in beagle dogs. Soluble silicon is a potent mitogen for osteoblast progenitor cells. Due to gene activation, they produce several growth factors (IGF-II, TGF-β) and cytokines that modulate osteoblast cell cycle. It resulted in increased osteoblast cell proliferation and differentiation and enhanced synthesis and release of Type-1 collagen, alkaline phosphatase and extra cellular matrix (osteocalcin) from these cells;[25] thus, allows rapid bone formation.[7]
It is known that mineralization of bone involves a concentration enhancement of silicon at the mineralization front. Biologically active SiO2 and apatite species was also responsible for inhibiting the proliferation of fibroblasts at bioactive implant interface.[26]
An added advantage of BG-HA composite bone graft materials was assumed that HA particles with in the composite bone graft materials acted as a scaffold, encircling which osteoid could deposited that resulted in early increase in strength of newly forming bone.
SUMMARY AND CONCLUSION
In view of the present finding, both OFD and OFD plus synthetic bone graft particles are effective in the reconstruction of infra bony periodontal defects, but considering the hard tissue measurements it is concluded that BG and BG-HA composite synthetic bone graft particles lead to better results compared to other groups (Group-I and Group-II). Equally important was the fact that the newly developed indigenous BG and BG-HA composite particles did not cause any extra biological complications and therefore may safely be used as good alternative bone graft materials in humans.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
# Central Glass and Ceramic Research Institute, Kolkata, India.
** Hu-Friedy Inc, Chicago.
£ SPSS version 11, SPSS Inc., Chicago.
REFERENCES
- 1.Rosenberg E, DipDent H, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998;42:467–88. [PubMed] [Google Scholar]
- 2.Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GW. Healing and repair of osseous defects. Dent Clin North Am. 1991;35:469–77. [PubMed] [Google Scholar]
- 4.Jarcho M. Biomaterial aspects of calcium phosphates. Dent Clin North Am. 1986;30:25–47. [PubMed] [Google Scholar]
- 5.Hench LL. Bioceramics: From Concept to Clinic. J Am Ceram Soc. 1991;74:1487–510. [Google Scholar]
- 6.Anderegg CR, Alexander DC, Freidman MA. Bioactive glass particulate in the treatment of molar furcation invasions. J Periodontol. 1999;70:384–7. doi: 10.1902/jop.1999.70.4.384. [DOI] [PubMed] [Google Scholar]
- 7.Kim CK, Kim HY, Chai JK. Effect of a calcium sulfate implant with calcium sulfate barrier on periodontal healing in 3-wall infrabony defects in dogs. J Periodontol. 1998;69:982–8. doi: 10.1902/jop.1998.69.9.982. [DOI] [PubMed] [Google Scholar]
- 8.Fetner AE, Hartigan MS, Low SB. periodontal repair using Perio Glass in non-human primates: Clinical and histologic observations. Compendium. 1994;15:932–5. [PubMed] [Google Scholar]
- 9.Ong MA, Robert ME, Maria IK, Lamont N, Gerald N, Glickman GN, et al. Evaluation of a bioactive glass alloplast in treating periodontal intrabony defects. J Periodontol. 1998;69:1346–54. doi: 10.1902/jop.1998.69.12.1346. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Nandi SK, Kundu B, Datta S, De DK, Roy SK, et al. Interfacial response of hydroxyapatite and tri-calcium phosphate prepared by a novel aqueous combustion method: A comparison with bioglass in vivo implanted in goat. J Biomed Mater Res. 2008;86B:217–27. doi: 10.1002/jbm.b.31009. [DOI] [PubMed] [Google Scholar]
- 11.Silness P, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 12.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 13.Krejci CB, Bissada NF, Farah C, Greenwell H. Clinical evaluation of porous and nonporous hydroxyapatite in the treatment of human periodontal bony defects. J Periodontol. 1987;58:521–8. doi: 10.1902/jop.1987.58.8.521. [DOI] [PubMed] [Google Scholar]
- 14.Shetty V, Han TJ. Alloplastic Materials in reconstructive periodontal surgery. Dent Clin North Am. 1991;35(3):521–30. [PubMed] [Google Scholar]
- 15.Humagain M, Nayek DG, Uppoor AS. A clinical evaluation of bioactive glass particulate in the treatment of mandibular class II furcation defects. Braz J Oral Sci. 2007;6(23):1450–6. [Google Scholar]
- 16.Wilson J, Noletti D. Bonding of Soft Tissues to Bioglass®. In: Yamamuro T, Hench LL, Wilson J, editors. Handbook of Bioactive Ceramics: Bioactive Glasses and Glass- Ceramics. Boca Raton Florida: CRC Press; 1990. p. 1. [Google Scholar]
- 17.Zamet JS, Darbar UR, Griffiths GS, Bulman JS, Bragger U, Burgin W, et al. Particulate bioglass as a grafting material in the treatment of periodontal intrabony defects. J Clin Periodontol. 1997;24:410–8. doi: 10.1111/j.1600-051x.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, Suh JJ, Choi SH, Moon IS, Cho KS, Kim CK, et al. Effects of pretreatment clinical parameters on bioactive glass implantation in intrabony periodontal defects. J Periodontol. 2001;72:730–40. doi: 10.1902/jop.2001.72.6.730. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, Yi SW, Choi SH, Kim CK. Bone Probing Measurement as a Reliable Evaluation of the Bone Level in Periodontal Defects. J Periodontol. 2000;71:729–35. doi: 10.1902/jop.2000.71.5.729. [DOI] [PubMed] [Google Scholar]
- 20.Laurell L, Gottlow J, Zybutz M, Persson R. Treatment of intrabony defects by different surgical procedures: A literature review. J Periodontol. 1998;69:303–13. doi: 10.1902/jop.1998.69.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Oonishi H, Hench LL, Wilson J, Sugihara F, Tsuji E, Matsuura M, et al. Quantitative comparison of bone growth behavior in granules of Bioglass®, A-W glass-ceramic, and hydroxyapatite. J Biomed Mater Res. 2000;51:37–46. doi: 10.1002/(sici)1097-4636(200007)51:1<37::aid-jbm6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 23.Boyne PJ, Shapton BA. The response of surgical periodontal defects to implantation with a hydroxyapatite ceramic. Transactions of 4th Annual Meeting of Society of Biomaterials, 10th International Biomaterials Symposium. 1978:115. [Google Scholar]
- 24.Schepers E, De Clercq M, Ducheyne P, Kempeneers R. Bioactive glass particulate material as a filler for bone lesions. J Oral Rehabil. 1991;8:439–52. doi: 10.1111/j.1365-2842.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 25.Xynox LD, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and Induce Insulin-like Growth Factor II mRNA Expression and Protein Synthesis. Biochem Biophys Res Comm. 2000;276:461–5. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 26.Hench LL, West JK. Biological applications of bioactive glasses. Life Chem Rep. 1996;13:187–241. [Google Scholar]