Abstract
Aim:
The aim of the present study was to evaluate the efficacy of a bovine derived xenograft Bio-Oss™ and to compare with open flap debridement in human infrabony periodontal defects.
Materials and Methods:
Twelve healthy patients (5 males, 7 females; aged 30-50 years), with no systemic disease with moderate to severe periodontitis were treated. Surgically defects were included only if presence of two or more vertical osseous defects as verified by radiographs with associated probing pocket depth of ≥5.0 mm following non-surgical therapy. Final selection included 24 defects. The defects were randomly assigned treatment with bovine derived xenograft Bio-Oss™ as experimental sites or open flap debridement as control sites. Soft tissue and hard tissue measurements were recorded on the day of surgery and six months post-operatively.
Results:
The results showed significant difference statistically between experimental and control sites in all measurements. Soft tissue measurements for the experimental sites included probing pocket depth reduction of 4.33±0.651 mm and attachment gain of 2.92±0.9003 mm, while the control sites showed a probing pocket depth reduction of 2.92±0.669 mm and a attachment gain of 0.583±0.515 mm. Osseous measurements showed bone fill of 1.936±1.046 mm (54.065±12.642%) for experimental sites and 0.02±0.01 mm (0.534±0.384%) for the control sites. Defect resolution was 50.75% for the experimental sites and 5.45% for the control sites.
Conclusion:
Bio-Oss™ is a bone graft material of considerable promise. However, further long term clinical studies with histological evaluation are warranted.
Keywords: Alveolar bone loss/surgery, Bio-Oss™, bone substitute, bone transplantation, cattle, periodontitis/surgery
INTRODUCTION
Periodontitis, a chronic inflammatory disease of the periodontium results in progressive loss of connective tissue attachment and supporting alveolar bone. In addition to reducing the bone height, it alters the morphologic features of bone leading to an array of osseous defects or deformities. Such periodontal bony defects need to be treated since the pattern of behavior of gingiva is conditioned by the architecture of the underlying supporting alveolar bone. These deformities of the alveolar bone unless corrected may interfere with the eradication of the pockets and make the maintenance difficult, thereby leading to recurrence of the disease.[1]
The ideal goal of periodontal therapy is the reconstruction of the bone and ligamentous attachment that have been destroyed by the disease. Several studies have shown that conventional periodontal therapeutic procedures do not result in regeneration of the supporting tissues, to a predictable degree.[2–5]
This has led to various attempts to reconstruct lost periodontium by using different regenerative methods, one of which is bone grafting procedure. The use of bone grafts to promote periodontal regeneration has been the subject of extensive interest. From the time of Hegedus in 1923[6] and through the studies of Schallhorn in 1970's showed the usefulness of osseous grafts in infrabony defects in order to improve bone regeneration and achieve a greater amount of new connective tissue attachment.[7]
Currently, a bone substitute based upon the inorganic component of bone, a natural derived porous bone mineral of bovine origin called Bio-Oss™ manufactured in Wolhusen and marketed by Geistlich Biomaterials, a Swiss company, has been used in periodontics and oral and maxillofacial surgery. It promises to be biocompatible and supports bone formation. Its morphology, porosity, internal surface, crystalline structure, and chemical composition are reported to be similar to that of human bone. In experimental studies, Bio-Oss™ was found to resorb slowly and integrate in natural remodeling process.[8]
Considering all the above factors the present study has been taken up with the following aims and objectives:
To evaluate Bio-Oss™ a bovine-derived xenograft in the repair of human infrabony vertical defects resulting from moderate to advanced periodontal disease and compare with conventional open flap debridement.
To determine the amount of reduction from baseline osseous defect depth parameters and amount of bone fill subsequent to grafting.
To determine whether the use of this graft would improve the periodontal status of the involved teeth, such as reduction in probing pocket depths, gain in clinical attachment after six months and compare the results obtained with conventional open flap operation without using any graft.
MATERIALS AND METHODS
Bio-Oss™ spongiosa granules size 0.25-1 mm was taken as the implant material. This commercially available xenograft material is a unique, natural, nonantigenic, osteoconductive bone matrix obtained from cattle.
Twelve patients comprising 5 males and 7 females diagnosed to have adult periodontitis being routinely treated in the Department of Periodontology were selected to participate in this study. The age of the subjects ranged from 30 to 50 years.
Patient selection and study design
To be included in the study the subjects had to fulfil the following criteria.
All subjects had to be free of any systemic disease and must not be on any medication
Presence of two or more vertical osseous defects as verified by radiographs with associated probing pocket depths of ≥5.0mm following initial nonsurgical therapy. The defects selected were either one-walled, two-walled, wide three-walled, or combination defects. Narrow three-walled defects were excluded from the study. Final selection included 24 defects
Teeth with furcation involvement and those that were endodontically involved were not included in this study
Teeth subjected to occlusal disharmonies, severe attrition, and facets and excessive mobility were also excluded from the study
Patients must not have had antibiotic therapy in the past 3 months.
A comprehensive medical and dental history was recorded. Patients were then given an explanation of the study purpose and informed consent obtained.
All the patients received initial scaling and root planing with oral hygiene instructions. Approximately 4-6 weeks following the hygiene phase of therapy, patients underwent a re-evaluation examination during which time all the base line scores were recorded.
Clinical parameters
Plaque index (Silness and Loe[9]), Gingival index (Loe and Silness[10]) were recorded at baseline and 6 months for the group of teeth selected for the study.
Probing pocket depth, probing attachment level, gingival recession
Customerized acrylic occlusal stents were prepared on the study casts. A groove was made on the stent by using a fissure bur in an occlusoapical direction at a point where the graft material had to be placed. The grooves helped to provide reproducible alignment of a periodontal probe. The base of the stent served as a reference point to record the soft tissue measurements.[11]
Evaluation of the periodontal soft tissue changes namely probing pocket depth, probing attachment level, and gingival recession were determined by recording the following measurements using Williams periodontal probe.
Reference point (RP) to gingival margin (GM)
Reference point (RP) to cemento-enamel junction (CEJ)
Reference point (RP) to base of the pocket (BOP)
Probing pocket depth was recorded by noting the difference between measurements from the reference point to gingival margin and reference point to base of the pocket.
Probing pocket depth=RP to BOP - RP to GM.
Probing attachment level was calculated by subtracting the distance between reference point to the cemento-enamel junction from the distance between the reference point to base of the pocket.
Probing attachment level=RP to BOP - RP to CEJ.
RP to free gingival margin was measured to determine any change in gingival recession before and after surgery.
Prior to surgery, an intraoral periapical radiograph was taken using an extension cone paralleling device [Figures 1a and 2a]. To standardize radiographic assessment, radiographs were obtained in a constant and reproducible plane using film holders with a template containing impression compound, which was placed in a constant position on a group of teeth and an extension arm that can be precisely attached to both film holder and the X-ray tube.[12] To facilitate recording of precise measurements from the radiographs, a profile projector was used. It consists of a micrometer with which measurements upto 0.01 mm can be made accurately.
Figure 1.
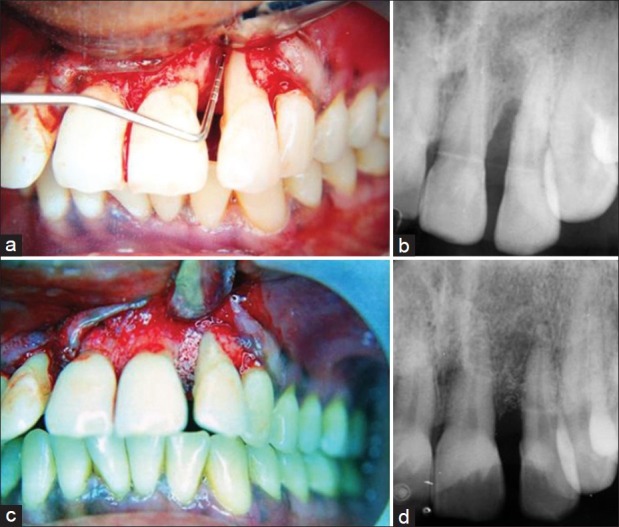
(a) Infrabony defect (mesial to lateral incisor) in the experimental group after debridement; (b) Baseline radiograph showing infrabony defect mesial to lateral incisor; (c) Bio-Oss™ packed into the defect; (d) 6-month post-operative radiograph showing bone fill
Figure 2.
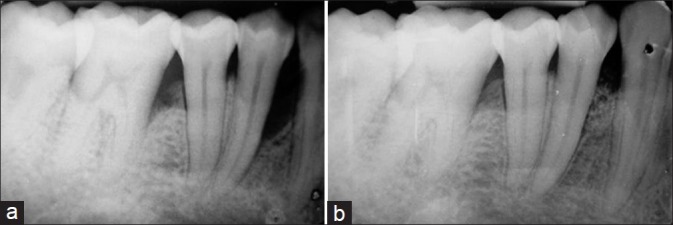
(a) Baseline radiograph showing infrabony defect mesial to first premolar; (b) 6-month post-operative radiograph showing bone fill
Surgical technique, placement of the graft
The defects chosen for the study were randomly assigned either open flap debridement (OFD) or the bovine-derived xenograft (BDX) Bio-Oss™ through the flip of a coin. Upon such determination, the site was either filled with BDX particles or left empty after curettage as a control.
The surgical procedure was performed utilizing local anaesthetics, 2% xylocaine with epinephrine (1:80,000). Intrasulcular incisions with reflection of full thickness flaps were utilized to retain as much soft tissue as possible in order to obtain primary closure. Vertical releasing incisions were not required in any of the patients. Complete debridement of the osseous defect and thorough root planing were done using curettes. The surgical site was repeatedly irrigated with sterile normal saline in order to remove all the debris from the defect. The area was then isolated and dried [Figure 1a].
A non-absorbable black silk suture (4-0 Ethicon Mersilk ™) was placed loosely, left untied in a vertical mattress fashion prior to the filling of the defect. The graft material Bio-Oss™ spongiosa was mixed with a drop of sterile saline as per manufacturer's instructions. The defect was filled to the existing alveolar crest with care being taken not to overfill the defect [Figure 1c]. Primary soft tissue closure was obtained by tying the knots. Care was taken not to displace the graft material while tying the knots. Simple interrupted sutures were utilized mesially and distally wherever necessary. Pressure was applied with a moist gauze for a minimum of 2 minutes post-operatively followed by placement of a periodontal dressing (Coe-Pak™).
The patients were kept under systemic tetracycline therapy of 250 mg qid for 5 days post operatively along with anti-inflammatory analgesics and chlorhexidine (0.2 %) mouth wash.
The periodontal dressing and sutures were removed after 7 days. All the patients were recalled at every one-month interval during which the surgical area was irrigated and further oral hygiene instructions were given. At the 3 month recall, professional prophylaxis was provided.
All the soft and hard tissue measurements taken preoperatively were repeated post-operatively at 6 months to calculate the attachment gain, reduction in probing pocket depth, gingival recession, and amount of bone fill [Figures 1d and 2b].
The following method was employed to determine the radiographic changes before and after the study.[13]
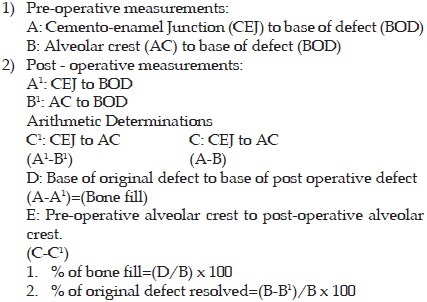
All the data obtained in this study were evaluated statistically by using student's “t” test.
RESULTS
After 24 weeks all clinical parameters were measured and recorded again. All measurements were analyzed statistically using student's “t” test. All patients included in this study participated for the entire study period. All surgical sites showed uneventful healing. No post-operative complications like swelling, hemorrhage or excessive pain was reported by any patient. The graft material was compatible with the surrounding tissues. There were no instances of any reaction of the tissues to the graft material or rejection of the graft in any patient. None of the treated teeth showed any post-surgical mobility. No graft material could be detected outside the defects either at the time of suture removal or subsequent check-up visits.
While there was no distinct variability in the clinical parameters between the experimental and control sites pre-surgically [Table 1 and Figures 3 and 4], a marked improvement was observed in all the parameters in experimental sites at the end of the study period [Table 2 and Figures 5–8].
Table 1.
Comparison of variables between study groups before experiment
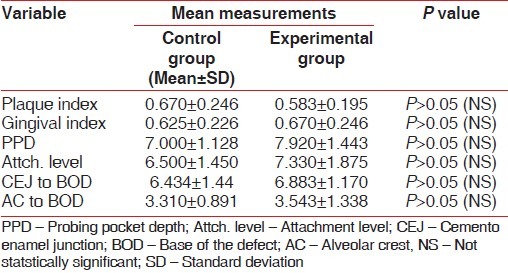
Figure 3.
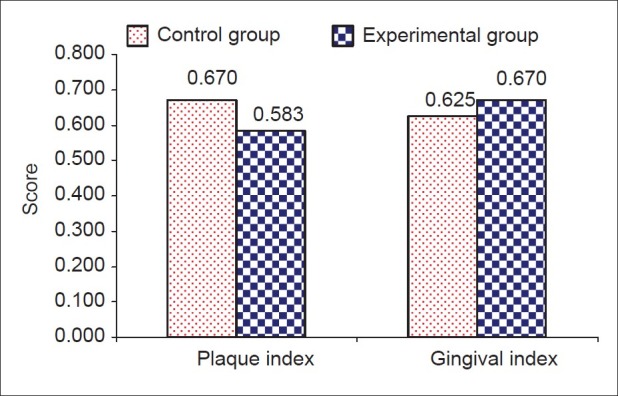
Comparison of variables among study groups before experiment
Figure 4.
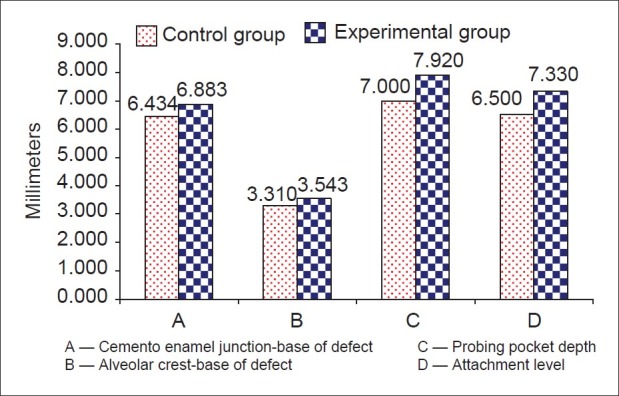
Comparison of variables among study groups before experiment
Table 2.
Comparison of mean changes in variables between control and experimental groups after treatment
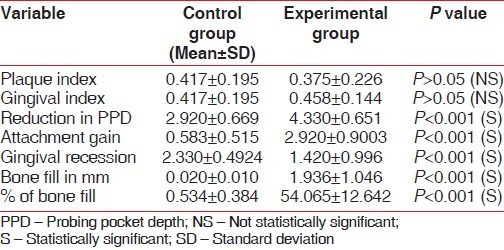
Figure 5.
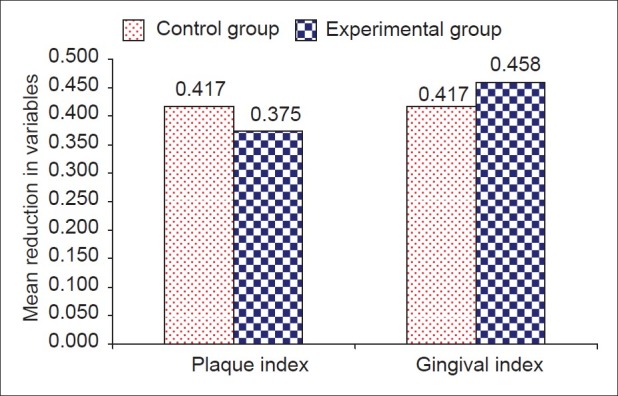
Comparison of reduction in plaque and gingival Index at the end of study period
Figure 8.
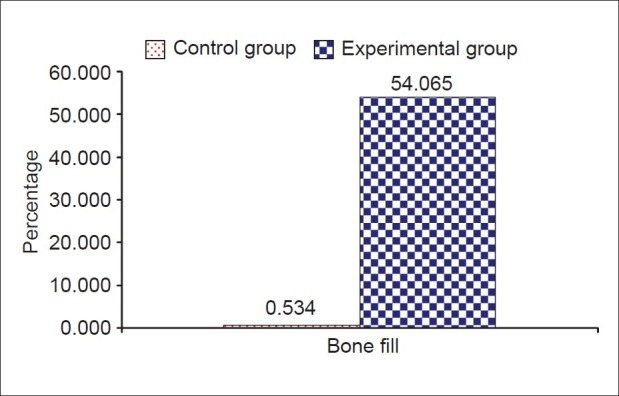
Comparison of mean changes in hard tissue variables (in percentage) between study groups
Figure 6.
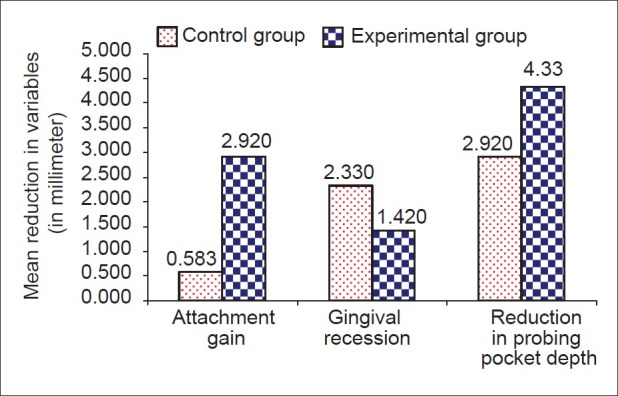
Comparison of mean changes in soft tissue variables between study groups
Figure 7.
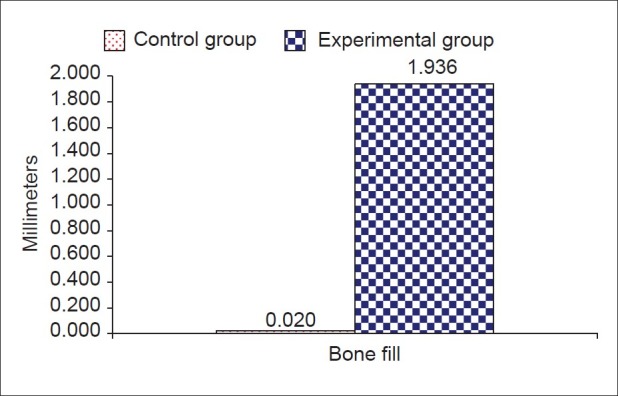
Comparison of mean changes in hard tissue variables (in millimeters) between study groups
DISCUSSION
In the last two decades periodontal therapy has seen significant progress in various aspects. There has been a complete shift from resective pocket eradicative procedures to techniques and methods aimed at regeneration and conservation of the periodontium. Bone being the crux of periodontal disease has obviously received lot of attention. A number of bone grafts have been used in different clinical trials with different degree of success. Upon analysis of the various clinical reports it is seen that most of the bone graft materials such as DFDBA, FDBA, Hydroxyapatite, Ceramics etc., have resulted in significant improvement in the clinical parameters. However, the amount of bone fill, the type of bone tissue that has formed, the exact nature of healing have not been consistent. Many of these bone grafts have been found to be no more than fillers of bone defects without demonstrating any osteogenic or osteoconductive potential.
In the present study, Bio-Oss™ the natural porous bone mineral resulted in significant improvement of all clinical parameters with a mean bone fill of 54.065%. This compares well with the results obtained in other studies using other types of bone grafts.[14–17] This is also comparable to the study of Richardson et al.,[18] where the bone fill was reported to be 55.8% using Bio-Oss™.
Another significant aspect of this study is the negligible amount of bone fill in sites of open flap debridement without the use of graft. This feature was seen despite considerable improvement in other clinical parameters like reduction in probing pocket depth and gain in attachment level. It should be noted here that all the other factors remained the same between experimental and control sites. Hence, this study proves yet again that conventional periodontal therapy does not result in significant bone fill.
Most of the sites treated with simple surgical debridement demonstrated some degree of crestal bone loss (mean - 0.20 mm). Only one site treated with Bio-Oss™ demonstrated crestal bone loss. Four sites showed no difference in the crestal height before and after the study period. Seven sites demonstrated some degree of increase in the height of the alveolar crest (mean 0.10 mm). This compares with the studies of Meffert et al., where hydroxyapatite was used and Schallhorn RG and Hiatt WH[19] where cross-matched iliac allograft of cancellous bone and marrow was used. The reason for crestal bone loss in the solitary case could have been due to the presence of a very wide osseous defect where it would not have been possible to fill up the entire defect with the graft material.
One of the advantages of using any of the popular bone graft material in vogue the minimum recession of the gingiva that is seen post-operatively. In the present study also sites treated with Bio-Oss™ showed significantly less recession than the control sites.
Like in earlier studies[20] Bio-Oss™ exhibited favorable handling properties such as:
Ease of delivery to the site,
Ease of packing the material into the defect,
Ability of the material to demonstrate an adhesion once placed in the defect, even with significant hemorrhage of the wound site, providing a stable graft.
The type of bone defects taken up in this study varied from three-walled defects to one-walled defects, often combined ones. Whereas, in strictly three-walled so called intrabony defects the potential for bone fill is quite predictable, this is not so with combined or bizarre defects. Hence, from the results of this study, it appears that morphologic variations in the osseous defects did not interfere with the efficacy of Bio-Oss™.
The methods employed for evaluating the results of this study were simple. Many other studies advocated reentry procedure at the end of the study period to assess the results. However, re-entry was not considered in this study as the procedure although enables changes in the bone levels to be measured visually, still does not show the actual type of healing that has occurred. Since none of the teeth included in the study required extraction histological study was obviously not taken up.
The results of this study suggested that Bio-Oss™ as a bone graft material is quite predictable when used in infrabony defects in bringing about bone fill and improvement in clinical parameters. However, further investigation with a larger sample on a prolonged post-operative follow-up is required to conclusively establish the efficacy of this material. Further, histological evaluation to determine other changes such as the type of new bone that is formed, any evidence of cementogenesis, regeneration of the attachment apparatus should be taken up in the future studies.
CONCLUSION
This study envisaged to evaluate the efficacy of a bovine derived xenograft Bio-Oss™ and to compare with open flap debridement in human infrabony periodontal defects.
Twelve patients who were diagnosed to have adult periodontitis having at least two angular defects were taken up for the study. Periodontal flap operations were carried out in the selected sites. Twelve sites were filled with Bio-Oss™ constituting the experimental group and the other 12 sites were surgically debrided without graft placement thereby serving as controls. The results showed that significant improvement in all clinical parameters were observed in sites treated with Bio-Oss™ than compared to control sites. This included reduction in probing pocket depth, gain in attachment level and bone fill. In addition sites treated with Bio-Oss™ demonstrated minimum post-surgical gingival recession compared to control sites.
The control sites although exhibited considerable reduction in probing pocket depth and gain of attachment did not however show any appreciable bone fill. Bio-Oss™ was well tolerated by the tissues with no cases showing any exfoliation of the graft, incompatibility or any other complications.
It is concluded that Bio-Oss™ is a bone graft material of considerable promise. However, further long term clinical studies with histological evaluation are warranted.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Schluger S. Osseous resection- A basic principle in periodontal surgery. Oral Surg Oral Med Oral Pathol. 1949;2:316–25. doi: 10.1016/0030-4220(49)90363-1. [DOI] [PubMed] [Google Scholar]
- 2.Bowers G, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part III. J Periodontol. 1989;60:683–93. doi: 10.1902/jop.1989.60.12.683. [DOI] [PubMed] [Google Scholar]
- 3.Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery.II.Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980;7:224–31. doi: 10.1111/j.1600-051x.1980.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 4.Cortellini P, Bowers G. Periodontal regeneration of intrabony defects: An evidence based treatment approach. Int J Periodontol Rest Dent. 1995;15:128–45. [PubMed] [Google Scholar]
- 5.Listgarten MA, Rosenberg MM. Histological study of repair following new attachment procedures in human periodontal lesions. J Periodontol. 1979;50:333–44. doi: 10.1902/jop.1979.50.7.333. [DOI] [PubMed] [Google Scholar]
- 6.Hegedus Z. The rebuilding of the alveolar processes by bone transplantation. Dent Cosmos. 1923;65:736–742. [Google Scholar]
- 7.Schallhorn RG. Present status of osseous grafting procedures. J Periodontol. 1997;48:570–6. doi: 10.1902/jop.1977.48.9.570. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RE, Mullarky RH, Noble B, Comeau RL, Neiders ME. Phenotypic characterization of mononuclear cells following anorganic bovine bone implantation in rats. J Periodontol. 1994;65:1008–15. doi: 10.1902/jop.1994.65.11.1008. [DOI] [PubMed] [Google Scholar]
- 9.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal conditions. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 11.Clark DC, Quee TC, Bergeron MJ, Chan EC, Lautar – Lemay C, Gruchy de K. Reliability of attachment level measurements using the cemento enamel junction and plastic stent. J Periodontol. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 12.Kelly GP, Cain RJ, Knowles JW, Nissle RR, Burgett FG, Shick RA, et al. Radiographs in clinical periodontal trials. J Periodontol. 1975;46:381–6. doi: 10.1902/jop.1975.46.7.381. [DOI] [PubMed] [Google Scholar]
- 13.Meffert RA, Thomas JR, Hamilton KM, Brownstein CN. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J Periodontol. 1985;56:63–73. doi: 10.1902/jop.1985.56.2.63. [DOI] [PubMed] [Google Scholar]
- 14.Mora F, Quhayoun JP. Clinical evaluation of natural and porous hydroxylapatite implants in periodontal bone lesions: Results of a 1-year follow – up. J Clin Periodontol. 1995;22:877–84. doi: 10.1111/j.1600-051x.1995.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 15.Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of FDBA and DFDBA in human periodontal osseous defects. J Periodontol. 1989;60:655–63. doi: 10.1902/jop.1989.60.12.655. [DOI] [PubMed] [Google Scholar]
- 16.Yukna RA. Osseous defect responses to hydroxylapatite grafting versus open flap debridement. J Clin Periodontol. 1989;16:398–402. doi: 10.1111/j.1600-051x.1989.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 17.Yukna RA. HTR Polymer Grafts in human periodontal osseous defects. J Periodontol. 1990;61:633–42. doi: 10.1902/jop.1990.61.10.633. [DOI] [PubMed] [Google Scholar]
- 18.Richardson CR, Mellonig JT, Burnsvold MA, Mc Donnell HT, Cochran DL. Clinical evaluation of Bio-Oss a bovine derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 19.Schallhorn RG, Hiatt WH. Human Allografts of Iliac cancellous Bone and Marrow in periodontal osseous defects. II. Clinical observations. J Periodontol. 1972;43:67–81. doi: 10.1902/jop.1972.43.2.67. [DOI] [PubMed] [Google Scholar]
- 20.Camelo M, Schenk RK, Rasperini G, Nevins M. Clinical, Radiographic and Histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Guide. Int J Periodont Rest Dent. 1998;18:321–31. [PubMed] [Google Scholar]


