Abstract
Component and coalesced health risks of the cardiometabolic syndrome (CMS) are commonly reported in persons with spinal cord injuries (SCIs). These CMS hazards are also co-morbid with physical deconditioning and elevated pro-atherogenic inflammatory cytokines, both of which are common after SCI and worsen the prognosis for all-cause cardiovascular disease. This article describes a systematic procedure for individualized CMS risk assessment after SCI, and emphasizes evidence-based and intuition-centered countermeasures to disease. A unified approach will propose therapeutic lifestyle intervention as a routine plan for aggressive primary prevention in this risk-susceptible population. Customization of dietary and exercise plans then follow, identifying shortfalls in diet and activity patterns, and ways in which these healthy lifestyles can be more substantially embraced by both stakeholders with SCI and their health care providers. In cases where lifestyle intervention utilizing diet and exercise is unsuccessful in countering risks, available pharmacotherapies and a preferred therapeutic agent are proposed according to authoritative standards. The over-arching purpose of the monograph is to create an operational framework in which existing evidence-based approaches or heuristic modeling becomes best practice. In this way persons with SCI can lead more active and healthy lives.
Keywords: Drug therapy, Exercise therapy, Metabolic cardiovascular syndrome, Nutrition therapy, Spinal cord injuries, Atherogenesis, Tetraplegia, Paraplegia
Introduction
The health hazards imposed by all-cause cardiovascular disease (CVD) and co-morbid endocrine disorders in persons with spinal cord injuries (SCIs) have been the subject of considerable clinical concern and research attention.1–4 Although known by various names, the descriptor “cardiometabolic syndrome” (CMS) has been adopted to represent a complex array of these risks, which by evidence-based diagnosis encompass the five component hazards of central obesity, hypertriglyceridemia, low plasma high-density lipoprotein cholesterol (HDL-C), hypertension, and fasting hyperglycemia (Table 1).5,6 Left untreated, these disorders incite atherosclerotic plaque formation and premature CVD, and in the process pose a health threat considered so menacing that clustering of three or more CMS components confers the same health risk as a clinical finding of extant coronary artery disease.7
Table 1.
American Heart Association/NCEP Guideline (Updated) for Diagnosis of Cardiometabolic Syndrome7
| Risk | Criterion |
|---|---|
| Waist circumference | Men >40 inches (102 cm) |
| Women >35 inches (88 cm) | |
| Fasting triglycerides | ≥150 mg/dl (1.7 mmol/l) |
| HDL-C | Men <40 mg/dl (1.03 mmol/l) |
| Women <50 mg/dl (1.29 mmol/l) | |
| Blood pressure | ≥130/85 mmHg, or use of medication for hypertension |
| Fasting glucose | ≥100 mg/dl (5.6 mmol/l), or use of medication for hyperglycemia |
Convincing evidence now supports the threat to persons with SCI for an accelerated trajectory of CMS.4,8,9 Component risks of central obesity,10–12 impaired fasting glucose and frank diabetes mellitus,13–15 and both fasting dyslipidemia1,16,17 and exaggerated postprandial lipemia18,19 underpin this threat, and are observed earlier in the lifespan of those with SCI than in the general population. Although the described risk profile might appear disheartening for those sustaining SCI, it thus far fails to include the disease-accelerating risks of sedentary lifestyle and physical deconditioning commonly reported after SCI,9,20,21 and neglects to consider emerging reports of exaggerated post-injury inflammatory cytokines now thought to be instigators of pro-atherogenic activity.22–24 Although the latter risks are not formally included among evidence-based CMS component hazards, their disease-promoting threats are so extensively characterized and firmly entrenched among key CVD risks that they warrant attention as legitimate therapeutic targets in persons with SCI.
Although findings of component risks and risk clustering after SCI might otherwise paint a bleak long-term heath picture, lifestyle modification, and medical management of patients with CMS continue to improve as risk-assessment tools become more precise25 and treatment interventions more effectively targeted.26 These tools and treatments are typically tested for population-specific efficacy, thus satisfying the contemporary focus on evidence-based treatments as trustworthy standards that drive health care assessment, intervention, and services. It is also prudent to rely on general population guidelines and best practical suppositions in cases where population-specific assessment is lacking, especially when lack of aggressive primary prevention can allay profound health risks and functional decline. The latter “heuristic” approaches are based on our intuitive judgments, experience, and general discovery,27 and effectively serve where exhaustive testing of tools and treatments has yet to be undertaken. That expressed, the current monograph will examine both evidence-based and heuristic approaches to customized management of CMS in persons with SCI. When possible the discussion will be grounded in the evidence-based disease guidelines of the American Heart Association/National Cholesterol Education Project Adult Treatment Panel III,28 established disease prevention approaches adopting therapeutic lifestyle interventions (TLIs) of diet and exercise, and a last resort adoption of medication as a disease countermeasure when TLI has failed to achieve health goals. A paradigm for management of CMS risk components occurring after SCI is shown in Fig. 1.
Figure 1.
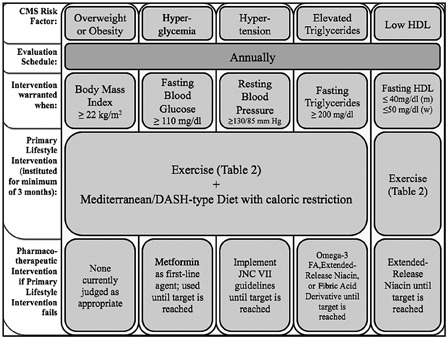
A paradigm for management of CMS risk components occurring after SCI.
Need for dietary therapy
The CMS in humans is broadly influenced by dietary habits and nutritional status. Both may be significantly altered after SCI due to changes in the metabolic milieu (e.g. loss of metabolically active tissue), physical barriers (e.g. access to food shopping and grocery store shelving), environment (e.g. institutional food), functional challenges (e.g. difficulties encountered in preparing food), and social factors (e.g. food provided as comfort by family/friends).29 As persons with SCI already live in an “obesogenic environment”30 before sustaining injuries, this reality and other factors combine to thereafter make healthy nutrition habits all the more challenging. In order to provide recommendations that address the problematic metabolic milieu and associated diseases, dietary intakes and resulting nutritional status of persons with SCI must be identified. Key nutritional factors affecting the metabolic milieu include overall caloric intake and macronutrient composition, the latter focusing on risks imposed by dietary carbohydrate and fat intake. In addition, cholesterol overconsumption can have a major impact on lipid profiles, and some micronutrients and imprudent alcohol intake may similarly constitute dietary health risks.
Dietary intake
Total caloric intake
Data reported since 2008 indicate that men with SCI consume an average of 500–600 fewer kilocalories than the ∼2600 kcal standard for men in the general population,31,32 whereas caloric intake for women with SCI is about the same or slightly (∼100 kcal) lower than the typical intake of ∼1800 kcal.31,33–35 Previous studies also report a broader range of caloric restriction for men (∼300–900 kcal) for men than women (∼300 kcal).36–39 Whether these deficits are sufficient to offset gender-dependent reductions of caloric expenditure is unknown due to a lack of information on total caloric expenditure changes following SCI. Nonetheless, data comparing resting energy expenditure and average daily caloric ingestion indicate a surplus intake of ∼300–500 kcal per day.34,38,39 Although this excess intake may appear insignificant, even a small yet sustained caloric surplus will eventually lead to weight gain, unfavorable lipid profiles, reduced glycemic control, disease, and increased mortality. No differences in caloric or macronutrient intake have been identified for level or completeness of injury, or acute vs. chronic SCI.31,33,34,40 This observation contrasts earlier studies that reported low caloric intakes for persons with tetraplegia, but not paraplegia.41,42 More precise data on total caloric expenditure in relation to total energy expenditure are needed to make specific dietary recommendations for persons with SCI, and better matching of caloric intake and expenditure should represent a primary goal of dietary therapy.
Fat and cholesterol intakes
Despite a lower intake of kilocalories, most (not all)43 studies report that persons with SCI consume levels of fat that approach or exceed the recommended level.31,32,34–38,40,44 Unlike overall caloric intake, percent macronutrient intake appears similar in men and women.31,33,40 Saturated fat intakes are also at the high end of, or beyond the recommended limit (<10% of total calories,31,32,36,37,40 although several studies reported a decline with duration of injury.44,45 High fat intake is commonly associated with weight gain, and particularly high saturated fat intakes appear to negatively affect metabolic profiles and chronic disease outcomes.46,47 There is also evidence for a direct relationship between high fat intake and serum triglycerides (TGs) in persons with SCI.44 Unlike total and saturated fat intakes, persons with SCI tend to be below the recommended limit for cholesterol intake (<300 mg).32,36,37,40,44
Carbohydrate intake
Carbohydrate intake after SCI appears to fall within recommended ranges of 45–55% total calories according to recent studies.31,33–35,40 However, most persons with SCI are below recommended intakes of fruit and vegetable servings, and have excess intake of simple carbohydrates. Both are robustly associated with unfavorable metabolic profiles.36,40,48 Given these data, it is not surprising that fiber intakes are low34,36,48 even among women.31,33,37,40 This is of particular relevance to persons with SCI as fiber consumption has been related to levels of the cardioprotective high-density lipoprotein (HDL) cholesterol.49–52 However, high-fiber diets (20–30 g/day) may stimulate undesirable changes in bowel function that differ from the non-disabled population, which may render high fiber diets impractical for persons with SCI.33,53,54 Future studies will need to determine the best ratio and sources for simple and complex carbohydrates that satisfy specific nutrient needs after SCI.
Micronutrients and alcohol
Despite having intake of multivitamin and mineral supplements that typically rival those of the general population, low intakes of several micronutrients have been reported for a significant proportion (i.e. >10%) of the SCI population. These are summarized in Table 2, and include vitamins A, B5, C, D, E, and K, biotin, calcium, chloride, chromium, copper, folate, iodine, molybdenum, magnesium, niacin, potassium, riboflavin, thiamin, and zinc.31,33,34,36,42,48,57 Occasional differences based on gender, injury level, or injury completeness have been reported, although detailed information on this dietary attribute remains scarce.31,33,37 Micronutrient status may also be suboptimal in the acute phase of recovery from injury, but improve over time.55 Almost all of the above listed micronutrients have been linked to glycemic and/or lipid profiles,58–71 but a detailed exploration of these deficiencies and their association with CMS risks of dysglycemia and dyslipidemia exceeds the scope of this monograph. In addition, only a few of these deficiencies have consistently been reported to be prevalent in persons with SCI over the last 10 years. The strongest evidence for deficiencies in a substantial part of the SCI population is available for vitamin B5, C, D, E, and biotin.31,33,34,36,56
Table 2.
List of micronutrients with at least one study reporting deviations from recommended intake
| Micronutrient | Study | Sample | Intake status | RDA* (18–50 years) |
|
|---|---|---|---|---|---|
| Female | Male | ||||
| Vitamin A | Peiffer et al.41 | 17 male, 1 female (18–80 years) | Average above recommendation | 700 µg | 900 µg |
| Laven et al.55 | 46 male, 5 female (18–60 years) | 62% deficient | |||
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Male average intake below recommendation | |||
| Moussavi et al.56 | 79 male, 31 female (22–82 years) | 16% below reference range for serum levels | |||
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 92% of males and 57% of females below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average above recommendation | |||
| Vitamin B5 (pantothenic acid) | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Vitamin B6 | Levine et al.37 | 24 male, 9 female (10–50+ years) | Average above recommendation** | 1.3 mg | 1.3 mg |
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 24% of males below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average above recommendation | |||
| Vitamin C | Peiffer et al.41 | 17 male, 1 female (18–80 years) | Average above recommendation | 75 mg | 90 mg |
| Barboriak42 | 37 male (average 33 years) | Average above recommendation | |||
| Laven et al.55 | 46 male, 5 female (18–60 years) | 25% deficient in plasma values (2–4wk post-injury only) | |||
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average above recommendation | |||
| Moussavi et al.56 | 79 male, 31 female (22–82 years) | 37% below reference range for serum levels | |||
| Tomey et al.36 | 95 male (20–59 years) | 25% below recommendation | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average above recommendation | |||
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 52% of males, 14% of females below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation for males | |||
| Vitamin D | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | 15 µg | 15 µg |
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Vitamin E | Levine et al.37 | 24 male, 9 female (10–50+ years) | Average below recommendation | 15 µg | 15 µg |
| Moussavi et al.56 | 79 male, 31 female (22–82 years) | “Significant proportion” below serum reference range (average within range) | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Vitamin K | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average recommended intake | 90 µg | 120 µg |
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average above recommendation | |||
| Biotin | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average above recommendation | |||
| Calcium | Peiffer et al.41 | 17 male, 1 female (18–80 years) | Average at or above recommendation | 1000 (mg) | 1000 (mg) |
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average below recommendation | |||
| Tomey et al.36 | 95 male (20–59 years) | Average below recommendation (43% below “safety level”) | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average at recommendation | |||
| Chloride | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Chromium | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Copper | Laven et al.55 | 46 male, 5 female (18–60 years) | 11% of plasma levels below recommendation | 900 µg | 900 µg |
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average above recommendation** | |||
| Folate | Laven et al.55 | 46 male, 5 female (18–60 years) | Average of plasma levels above recommendation | 400 µg | 400 µg |
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average below recommendation | |||
| Tomey et al.36 | 95 male (20–59 years) | 33% below recommendation | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation (males only) | |||
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 75–79% below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Iodine | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Iron | Peiffer et al.41 | 17 male, 1 female (18–80 years) | Average above recommendation | 18 mg | 8 mg |
| Tomey et al.36 | 95 male (20–59 years) | “Nearly everyone” met recommendation | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average above recommendation | |||
| Walters et al.33 | 63 male, 14 female (19–70+ years) | Average above recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Molybdenum | Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | N/A | |
| Magnesium | Levine et al.37 | 24 male, 9 female (10–50+ years) | Average below recommendation | 310–320 mg | 400–420 mg |
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 89% of males, 71% of females below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation** | |||
| Potassium | Barboriak42 | 37 male (average 33 years) | Average below recommendation | 4700 mg | 4700 mg |
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average below recommendation | |||
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Average below recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation | |||
| Thiamin | Peiffer et al.41 | 17 male, 1 female (18–80 years) | Average above recommendation | 1.1 mg | 1.3 mg |
| Barboriak42 | 37 male (average 33 years) | Average above recommendation | |||
| Laven et al.55 | 46 male, 5 female (18–60 years) | 24% below average | |||
| Levine et al.37 | 24 male, 9 female (10–50+ years) | Average above recommendation | |||
| Walters et al.33 | 63 male, 14 female (19–70+ years) | 22% of males, 14% of females deficient | |||
| Zinc | Levine et al.37 | 24 male, 9 female (10–50+ years) | Male average below, females** above recommendation | 8 mg | 11 mg |
| Groah et al.27,31 | 61 male, 12 female (20–73 years) | Male average below***, females above recommendation | |||
| Perret et al.34 | 18 male, 6 female (22–48 years) | Average below recommendation for males | |||
*RDA (Recommended Daily Intake) Dietary Guidelines for Americans 2010.
**Original article reports it as below recommendation but recommendation is different from USDA 2010.
***Original article reports it as within recommendation but recommendation is different from USDA 2010.
Another dietary component that may have significant impact on the metabolic milieu is alcohol. Low-to-moderate levels of daily alcohol intake (≤ 0–25 g/day) have been associated with decreased coronary heart disease, largely due to positive effects on cholesterol profiles, particularly elevated HDL.72–74 Although higher for men than for women, and for people with paraplegia compared with tetraplegia, overall mean alcohol consumption was reported to be low (<10 mg/day) among persons with SCI.31 However, there are several limitations of self-report data (as used in most studies on alcohol intake) such as recall bias and underreporting.75,76 Despite the low reported average, intake heavy drinking and alcohol abuse are more prevalent in the SCI than the general population.75,76 Although a detailed evaluation of alcohol abuse goes beyond the scope of this manuscript, clinicians should be aware of SCI-related risks associated with alcohol abuse such as pain, acceptance of injury, life satisfaction, and depression75,76 For individuals who were already at-risk drinkers prior to injury, it has been suggested that the early period after SCI may present a “window of opportunity” for effecting a change in alcohol abuse.77
Nutritional recommendations
Dietary patterns resembling those of persons with SCI, i.e. relying on high levels of fat and simple sugars, are often associated with dyslipidemia and poor glycemic control. Dietary recommendations to address these risks are usually in line with those adopted for the general population, and have been reviewed elsewhere.29,78 Specific recommendations represented by two contemporary dietary strategies, the Mediterranean-style and dietary approaches to stop hypertension diets, are summarized in Fig. 2. Unfortunately, data examining effects of specific dietary interventions in persons with SCI are very limited. In patients with chronic SCI, dietary counseling that adopted recommendations of the American Heart Association resulted in positive changes in lipid profiles,79 although changes were limited in scope and of questionable value in altering disease trends. This intervention employed general nutrition advice such as eating an adequate, varied diet and reducing fat intake (particularly saturated fats and cholesterol). At the 16-month follow-up, reductions in Total Cholesterol (TC), low-density lipoprotein (LDL), and TG were observed, but no change in HDL. By contrast, a weight-management intervention consisting of diet, exercise, and behavior modification classes elicited several favorable changes in 16 overweight or obese persons with SCI.80 These include reduced caloric, saturated fat, and cholesterol intake as well as increased fiber intake and weight loss with preservation of lean tissue mass. However, no significant changes in lipid profiles were observed, except a decrease in HDL. The latter finding is unusual as we and others have reported positive changes in lipid profiles with exercise alone, particularly on HDL levels (see exercise section), although a reduction of HDL is not uncommon under conditions of excessive caloric restriction. Other “holistic” interventions showed also no changes in lipid profiles.81,82 Future investigation will need to clarify whether there is a consistently negligible or adverse negative effect on lipid profile with these types of interventions and if and why they seemingly differ from exercise only interventions.
Figure 2.
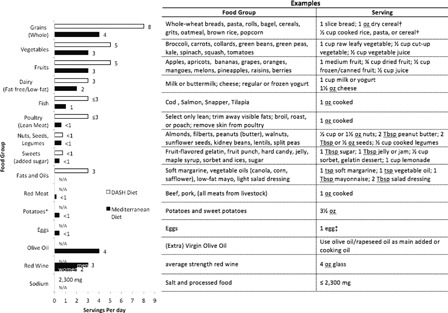
Dietary recommendations based on the Mediterranean and DASH diets. Adapted from http://www.nhlbi.nih.gov/health/health-topics/topics/dash/followdash.html and http://www.patient.co.uk/health/Mediterranean-Diet-Summary-and-Chart.htm. Note: The Mediterranean-style diets are sometimes considered high in fat content and calories for sedentary populations, although the fats are generally monounsaturated and not the more atherogenic saturated fats. The DASH diet is usually prescribed for hypertension management to a greater degree than fat loss, although high intake of fruits and vegetables in the diet may favor the same weight reduction goals. DASH, Dietary Approaches to Stop Hypertension. *Potatoes and eggs are included in the vegetable and lean meat groups, respectively, for the DASH diet. †Serving sizes vary between ½ cup and 1¼ cups, depending on cereal type. Check the product's Nutrition Facts label. ‡Eggs are high in cholesterol, therefore limit egg yolk intake to ≤4 per week.
Beyond dietary modification alone recent interest has focused on dietary interventions supplementing with omega-3 fatty acids (FAs).29,83,84 Six months of omega-3 FA administration in a cohort of 19 men with SCI resulted in a 10-fold increase in plasma omega-3 FA values, but did not affect lipid profile.85 However, the 2.25 g/day of omega-3 FA provided in this study may have been below the effective supplement dose range, which has been suggested to be 3–5 g/day.86 Given the wide variety of proposed beneficial effects of omega-3 FA including lowering of TG, hypertension, and atherosclerotic plaque formation,29,87,88 future investigations with more applicable dosing appear warranted.
In summary, despite low caloric intake compared with the general population, persons with SCI may still experience a caloric surplus, and select foods containing both high levels of fat and saturated fat. When combined with excessive levels of simple carbohydrate and low fiber intake these dietary choices disfavor a healthy metabolic profile. Randomized controlled trials are needed to determine whether diet interventions can successfully modify the undesirable metabolic milieu reported in persons with SCI, and whether these diets can ameliorate component hazards of the CMS.
Exercise/physical activity to enhance metabolic health
Physical activity levels among persons with SCI
Physical activity, exercise, and/or, sport participation rates are low among persons with SCI. Among 985 wheelchair-dependent persons with SCI in the United Kingdom injured at least one year, ∼63% reported participating in little (∼10%) to no (∼53%) weekly sports activity.89 Ginis et al.90 corroborated this low rate of physical inactivity. Among 695 non-ambulatory community-dwelling Canadians with SCI, 50% reported no leisure time physical activity (LTPA) in the preceding 3 days.91 Among the 50% who engaged in LTPA, median activity time was 33 minutes per day. Similar SCI-specific information is not available for the United States. However, 2009–2010 data from a population-based survey of community dwelling persons in the United States (National Health Interview Survey) indicated 50% of persons with a disability report no LTPA in the preceding month.92 Collectively, these data confirm the widespread belief that physical inactivity is highly prevalent among persons with SCI.
Physical activity and metabolic health in SCI
Although physical inactivity is rampant among persons with SCI, a growing body of scientific evidence suggests a beneficial impact of physical activity on metabolic health, primarily on fasting lipids. However, little-to-no research has addressed whether exercise/physical activity can reduce fat mass and/or attenuate systemic inflammation among persons with SCI. We have limited our summary of the possible benefits of physical activity to studies examining upper extremity exercise. Although the metabolic benefits of lower extremity functional electrical stimulation and body weight supported treadmill training have been evaluated, these therapies are cost prohibitive, less widely available than voluntary arm exercise, and are not widely available for use by persons with SCI.
Physical activity to improve dyslipidemia and glucose/insulin homeostasis
Two studies have demonstrated that upper extremity based, moderate (or greater) intensity exercise performed for 30–45 minutes three times weekly for 8–12 weeks effectively improves fasting lipids in persons with SCI.93,94 An early study by Hooker et al.95 reported improved fasting lipids after 8 weeks of moderate intensity wheelchair ergometer propulsion performed three times weekly for 20 minutes daily. Activity/rest interval type programs may also be effective in lipid and glycemic management, as de Groot et al.96 demonstrated that 8 weeks of three times weekly moderate intensity “interval” training improved fasting lipids and improved insulin sensitivity. The “intervals” consisted of three minutes of moderate intensity arm exercise followed by 2 minutes of rest, repeated for 1 hour, for 36 accumulated minutes of activity, with the goal of maintaining an intensity at 70–80% of heart rate reserve. An interval-based approach may be an ideal approach to enable use of high-intensity exercise among deconditioned persons with SCI.
Observational studies widely support the findings of intervention-focused investigations. Cross-sectional studies report more favorable lipid profiles (i.e. higher HDL, lower TG, and reduced LDL) among persons with tetraplegia and paraplegia who are habitually highly active or fit relative to their peers.23,97,98 Buchholz et al.99 reported a lower prevalence of insulin resistance among active than inactive persons with SCI. Observational data from the Dutch longitudinal study suggest a favorable relationship between aerobic power, muscle strength, and HDL, TG, LDL/HDL ratio, and TC/HDL ratio across rehabilitation and the first year of discharge from post-injury rehabilitation.100
SCI/disability-specific physical activity recommendations
Although an existing body of evidence strongly supports health benefits of physical activity for persons with SCI, it remains insufficient to provide a unified recommendation for this specific disability population.101 We thus defer to authoritative recommendations developed for all persons with a disability (Table 3). Perhaps surprising, the weekly aerobic physical activity and resistance training recommendations to improve health are the same for adults with and without disabilities. The U.S. Department of Health and Human services states, “When adults with disabilities are not able to meet the Guidelines, they should engage in regular physical activity according to their abilities and should avoid inactivity. Adults with disabilities should consult their health-care provider about the amounts and types of physical activity that are appropriate for their abilities.” The World Health Organization states, “These recommendations can be valid for adults with disabilities. However, adjustments for each individual based on their exercise capacity and specific health risks or limitations may be needed.” At this time, the Canadian Society for Exercise Physiology does not provide recommendations for persons with disabilities. However, recommendations to improve fitness in persons with SCI have been developed by SCI Action Canada, a research-medical-community collaborative effort to advance physical activity participation among Canadians living with a SCI. Those who developed these recommendations noted that there was insufficient evidence to unequivocally conclude that the minimum recommendation to improve fitness were also sufficient to reduce CVD risk.102
Table 3.
U.S. Department of Health and Human Services and the World Health Organization recommended levels of physical activity for adults aged 18–64 years
| Aerobic activity |
|||
|---|---|---|---|
| Total weekly duration | At least 150 minutes (2 hours, 30 minutes) | At least 75 minutes (1 hour, 15 minutes) | |
| Duration per session | Variable, but at least 10 minutes | Variable, but at least 10 minutes | |
| How many days each week? | Most days of the week | Most days of the week | |
| Intensity each session | Moderate | Vigorous | |
| Activities that feel somewhat hard, but you can keep doing them for a while without getting tired* | Activities that make you feel like you are working really hard, almost at your maximum, and you cannot do these activities for very long without getting tired* | ||
| Type of activity | Any activity which achieves the above | Any activity which achieves the above | |
| AND | |||
| Resistance Training Activity | |||
| Number of days per week | At least 2 days a week | ||
| Number of exercises each day | All major muscle groups | ||
| If upper and lower body, then 8–10 exercises† | |||
| If just upper body, then 4–5 exercises‡, with a focus on shoulder depressors and scapular stabilizers§ | |||
| For each exercise | At least 1 set of 8–10 repetitions, using enough weight so that you can barely, but safely finish the final few repetitions | ||
*Intensity description from SCIAction physical activity guidelines for adults with spinal cord injury (http://www.sciactioncanada.ca/guidelines/).
†Total number of upper and lower body exercises from American College of Sports Medicine/American Heart Association physical activity recommendations.99
‡Total number of upper body exercises represents our suggestion and is not drawn from an authoritative source.
§Recommendation to focus on shoulder depressors and scapular stabilizers from the Consortium for Spinal Cord Medicine Clinical Practice Guideline for Preservation of Upper Limb Function Following Spinal Cord Injury.100
To improve global health, the U.S. Department of Health and Human Services103 and the Canadian Society for Exercise Physiology,104 recommend adults with disabilities in the 18- to 64-year age range accumulate at least 150 weekly minutes of moderate intensity aerobic physical activity, with bouts of activity spaced across the week. Alternatively, adults can accrue 75 weekly minutes of vigorous intensity aerobic physical activity, or an equivalent combination of moderate and vigorous activity. Any “bout” of moderate or vigorous physical activity lasting 10 minutes or more is counted toward this weekly total. Each of these authoritative bodies also recommends resistance training performed at least twice a week, in addition to the aerobic physical activity goals. To achieve important fitness benefits, SCI Action Canada recommends that persons with SCI should engage in at least 20 minutes of twice-weekly moderate-to-vigorous intensity aerobic physical activity, and engage in strength training another two times a week.102 Importantly, these groups consistently stress that some activity is better than none, even if weekly goals are not met or the person's abilities are limited; activity beyond the minimum confers additional health benefits; and that the greatest health benefits occur in those who transition from an entirely sedentary lifestyle to performing any physical activity whatsoever.
Historically, exercise and physical activity recommendations to improve health have focused on the moderate-to-vigorous end of the intensity spectrum as an exercise goal. However, recent recommendations encourage “avoiding” inactivity in addition to achieving the target amounts of weekly moderate-to-vigorous aerobic physical activity. The “avoiding” inactivity recommendation is based on the goal to limit extended periods of stationary, motionless, e.g. “sedentary”, behavior by engaging in very light to light-intensity, non-exercise, physical activity throughout the day. This recommendation confers meaningful health benefits to all persons regardless of current fitness status or total daily moderate or vigorous physical activity. This paradigm shift provides a powerful opportunity to enhance the health of the SCI community. The barriers to limiting stationary, non-movement behavior such as watching television, sitting at a desk, or surfing the internet, and alternatively engaging in very light to light activity including moving around the house or performing basic daily activities, are much lower than the barriers to engaging in moderate-to-vigorous physical activity several times a week. We direct interested readers to the recent perspective of Manns et al.105 for an in-depth discussion of this paradigm shift, and the implications for persons with a disability.
Need for medical clearance and/or supervised physical activity
Health risks associated with physical activity are outweighed by benefits for most persons with SCI.106 A recent evidence-based consensus report defined the needs for both medical clearance and supervised exercise in clinical populations, including SCI.106 For persons with SCI, they recommend that the following groups seek medical clearance before becoming more physically active: (1) persons less than 6 months post-SCI; (2) persons with established autonomic dysreflexia; (3) persons who experience resting or exertional hypotension; and (4) persons with recurrent or recent (within the previous 6 months) musculoskeletal injury that is worsened by physical activity.106 Once medically cleared, those who are less than 6 months post-SCI should exercise in a supervised environment with trained exercise personnel.106 In cases where medical clearance has been obtained, the authors provide no additional guideline for need for supervised physical activity in cases of autonomic dysreflexia or symptomatic hypotension. Individuals with recent or recurrent musculoskeletal injuries that may be worsened by physical activity should exercise under supervision of appropriately trained exercise professionals once medical clearance is obtained. Finally, persons who are unaccustomed to vigorous exercise should be supervised by specially trained personnel when initiating a vigorous exercise program.106 The consensus document cautions that this recommendation should not be interpreted as a restriction on participation in wheelchair sports by persons with chronic SCI. Instead, those with SCI need only satisfy the previously identified clearance issues before undertaking sports participation, and then follow-up as needed with a qualified exercise professional. Additionally, the authors of this opinion are moot in describing how long supervision is indicated once the level of conditioning has improved and adjustment to the demands of vigorous exercise has occurred.
Customizing a physical activity prescription
Once an individual with SCI has been cleared for physical activity, the next step is to generate a customized physical activity prescription. The goal of customization is to help the person with SCI develop a new, sustainable pathway to becoming physically active. The targeted physical activity “behavior” is constant across all persons with SCI, i.e. avoid inactivity by engaging in as much light/very light activity as possible; accumulate 150 minutes of moderate intensity activity weekly; and resistance train twice weekly. The programs will be customized by the pathway each person will take to increase their physical activity levels, and consider barriers they must overcome. Customization of a physical activity prescription requires engaging the individual to jointly develop a behavior change plan that empowers them to engage in the appropriate amount of physical activity. Discussion of behavior change theory, practice, and applications to improve physical activity among persons with SCI is beyond the scope of this study. However, we refer readers to the work of Arbour-Nicitopoulos, Ginis, and Latimer, who demonstrated that action-coping planning increased LTPA among persons with SCI when compared with action planning alone.107 Action planning requires an individual to declare a priori the type, intensity, duration, days/time, and locations of the physical activity, e.g. I will propel my wheelchair (type) at a moderate intensity (intensity) for 30 minutes (duration) Monday through Friday (days) after work (time) at the local high school track (location). Action coping planning includes the action planning plus a priori identification of potential barriers and their solution, e.g. if rain disrupts my plan, I will propel at the local indoor mall instead of the track.
Pharmacotherapeutic approaches to management of cardiometabolic risks
In most cases, lifestyle therapies incorporating decreased caloric intake and increased daily caloric expenditure effectively serve as first-line treatments for CMS. However, these approaches may have questionable effectiveness for persons with SCI, as loss of body fat may require unattainable or unreasonable levels of caloric restriction,9 and as basal and exercise-induced caloric expenditures are decreased in patients with tetraplegia because of diminished active muscle mass and adrenergic dysfunction accompanying injury above the level of spinal sympathetic outflow.108 Severe caloric restriction may also lower HDL,79,109 making the effort counterproductive. For those in whom the first-line approaches of diet and exercise fail to modify CMS component risks, evidence-based guidelines and current practice standards recommend pharmacotherapy.6,110
Weight-loss drugs
With the October 2010 withdrawal of sibutramine from the European and North American markets, orlistat (Brand names Alli and Xenical, Brentford, Middlesex, UK) became the sole drug currently approved for reduction of body mass. However, the mechanism of drug action that decreases caloric uptake by inhibiting intestinal lipase activity sometimes results in steatorrhea and socially discomforting incontinence.111 No evidence suggests that the drug is suitable for use by those with a neurogenic bowel, or would be appropriate for use by persons with SCI.
Dysglycemia
Candidate drugs to treat dysglycemia after SCI have been reviewed by Goldberg.112 Six classes of oral medication are currently approved to treat elevated blood glucose in people with type 2 diabetes: metformin (Glucophage, Princeton, NJ; Fortamet, Atlanta, GA and others), sulfonylureas (Amaryl, Bridgewater, NJ; Glucotrol, New York, NY and others), thiazolidinediones (Avandia, Brentford, Middlesex, UK and Actos, Deerfield, IL), meglitinides (Starlix, East Hanover, NJ and Prandin, Princeton, NJ), Dipeptidyl peptidase-4 inhibitors (Januvia, Whitehouse Station, NJ, USA and Onglyza, Princeton, NJ), and Glucagon-like peptide-1 receptor agonists (Byetta, San Diego, CA and Victoza, Princeton, NJ). Most of the described drugs lower hemoglobin A1c levels – a surrogate for chronic glycemic control – by 0.5–2.0%, which depending on pre-treatment A1c levels above the 6.5–7% clinical target for those with diabetes mellitus may require more than one agent. Recent evidence-based guidelines, including a consensus algorithm for initiation and adjustment of therapy, identified metformin as a preferred first-line agent for lowering glycemia,113 as it is less prone to cause hypoglycemia and water retention than other drugs, and is available in a generic formulation that favors cost-effectiveness.114 Use of other glycemic-lowering therapies such as sulfonylureas and meglitinides are more prone to hypoglycemia, and the alpha-glucosidase inhibitors commonly cause GI discomfort from gas, both of which make them less appealing for therapy in those with SCI. Similarly unattractive are the thiazolidinediones, which both have black box warnings for congestive heart disease, and are associated with weight gain. None of these agents have been subjected to the rigors of randomized controlled trial in persons with SCI, although no evidence suggests that either benefits or adverse effects would differ from those reported in the non-disabled population.
Dyslipidemia
Five classes of agents are currently used to treat lipid disorders occurring in the general population: hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors (“Statins”), niacin (as an extended-release (ER) formulation), fibric acid derivatives (i.e. fibrates), bile–acid sequestrates, and cholesterol-uptake blockers. Their expected effects are outlined in Table 4. Another treatment option involves daily high-dose (3.8 g) omega-3 FA for persons primarily having elevated fasting triglyceride levels and an atherogenic lipoprotein phenotype.115 Goldberg112 has described suggested drug choices and nuances for medication selection in persons with SCI, and Dyson-Hudson and Nash have reviewed testing methods and systematic approaches to Adult Treatment Panel116 III-based treatment decision-making for their need.117
Table 4.
Candidate drugs for treating dyslipidemia and expected effects on key elements of the lipid profile
| Drug class | Candidate drugs | TG %Δ | LDL-C %Δ | HDL-C %Δ |
|---|---|---|---|---|
| HMG-CoA reductase inhibitors: “Statins” | • Atorvastatin (Lipitor, New York, NY) | ↓ 10–30 | ↓ 25–55 | ↑ 5–15 |
| • Lovastatin (Mevacor, Whitehouse Station, NJ, USA) | ||||
| • Pravastatin (Pravacol, Princeton, NJ) | ||||
| • Rasuvastatin (Crestor, Wilmington, DE) | ||||
| • Simvastatin (Zocor, Whitehouse Station, NJ, USA) | ||||
| Cholesterol uptake blocker | • Ezetimibe (Zetia, Whitehouse Station, NJ, USA) | ↓ 5–15 | ↓ 15–20 | – |
| Bile–acid sequestrates | • Cholestyramine (Questran, Spring Valley, NY) | ↑↓ 10–20 | ↑↓ 20–20 | – |
| • Colesevelam (Welchol, Parsippany, NJ) | ||||
| • Colestipol (Colestid, New York, NY) | ||||
| Niacin extended release | • Niaspan, Abbott Park, IL, U.S.A. | ↓ 10–30 | ↓ 5–25 | ↑ 10–35 |
| Fibrates | ↓ 30–50 | ↓0–15 | ↑5–20 | |
| • Tricor (Fenofibrate, Abbott Park, IL) | ||||
| • Lopid (Gemfibrozil, New York, NY) | ||||
| Omega-3 fat* | ↓30–50 | ↓5–15 | ↓5–10 |
*3.8 g daily dose.
Need for intervention on an atherogenic lipid profile can be determined by NCEP ATP III guidelines, which base treatment on whether LDL measured in fasting blood plasma exceeds a criterion target computed from an array of CVD risk factors and predictions.6 In general, an intermediate CVD risk stratification may be used to define need for treatment, which would include individuals having Framingham scores of 1%–20% in the 10-year event risk category, and whose LDL-C levels are >130 mg/dl, or >100 mg/dl in the presence of risk factors including age, hypertension, and/or cigarette smoking or high-sensitivity C-reactive protein >3 µg/l. In the general population, individuals with this profile would likely receive a statin as a first-line drug, although several shortcomings should be considered for use of these agents in persons with SCI. The most widely reported of these limitations involves myopathy,118 a term that describes a spectrum of muscle-related adverse events of myalgia, myositis, rhabdomyolysis, and asymptomatic increase in concentration of creatine kinase enzyme. In most cases these adverse events will be related to simple discomfort and not the more severe nephrotoxic effects of liberated myocyte components.119 Simple discomfort can often be controlled through less aggressive first dosing and, if needed, more conservative dose escalation.120 As myalgia may be related to depletion of coenzyme Q10 (CoQ10), dietary supplementation with CoQ10 has been suggested as a countermeasure to myalgia, although without a fully confirmed evidence of benefit.121 That said, even simple myalgic effects might be compelling in persons with SCI who use upper limb function to sustain routine daily activities, and may require more diligent monitoring to accompany pre-treatment and annual checks for hepatotoxicity. To date, a randomized controlled trial has not been conducted in persons with SCI that examines safety, tolerance, and effectiveness of statin monotherapy.
An alternative to statin drugs for dyslipidemia management involves nicotinic acid (niacin) in ER formulation. Niacin is an older, inexpensive broad-spectrum drug that decreases concentrations of all atherogenic plasma lipids/lipoproteins and is the most effective agent for increasing HDL-C levels.122 In crystalline (i.e. intermediate-release) form, the drug provokes a robust cutaneous flushing, thus compromising patient tolerance when therapeutically dosed.123 However, an ER formulation of niacin (Niaspan, Abbott Park, Illinois, U.S.A.)29 administered with a prostaglandin antagonist (i.e. 325 mg Acetylsalicylic Acid; Aspirin) and gradual dose escalation reduces this discomfort.124 The therapeutic response to Niacin directly remedies the CMS component risk of low HDL, and thus addresses low HDL as the most common lipid disorder sustained by persons with SCI.1,45,125
Unlike other candidate drugs for treating SCI-associated dyslipidemia, ER niacin has been subjected to RCT in persons with SCI. Nash et al.126 performed a randomized controlled trial enrolling persons with SCI have low plasma levels of HDL. Forty-eight weeks of treatment on a dose-escalation schedule showed significant increased fasting HDL-C levels by 24.5%, accompanied by dose-dependent decreases in the global risk predictor ratios of TC/HDL and LDL/HDL, LDL levels, and TC levels (Fig. 3). No evidence of sustained hepatotoxicity or hyperglycemia was observed. Treatment-emergent withdrawals (12.9%) accompanied flushing (n = 1), hypotension/pre-syncope (n = 1), and diarrhea (n = 2), although event rates were lower than those reported for the same agent when treating non-disabled individuals. Although ER niacin use requires diligence in dose escalation, pre-treatment with aspirin to suppress the flush, and abstention from spicy foods, alcohol, and hot showers in the immediate pre-treatment period, its use as a monotherapy is safe, tolerated, and effective for most persons with chronic tetraplegia, and we expect also with paraplegia.
Figure 3.
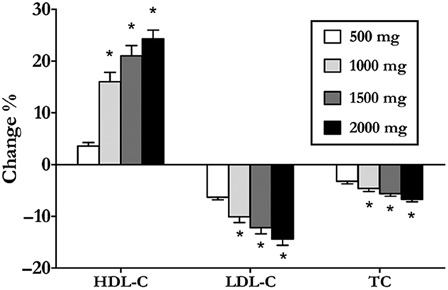
Dose-dependent responses of subjects with chronic tetraplegia to extended-release niacin monotherapy *p < 0.05, change % different from all other dosing levels.126
Conclusions
A disconcerting number of people with SCI develop component risks for CMS as they age with their disability. These risks coalesce to comprise a frank diagnosis of the disorder in an alarming number of these individuals. Evaluation and diagnosis of the CMS now fall within the framework of an evidence-based clinical pathway that effectively assesses risk and defines uniform approaches to both individual risk containment and overall disease management. Lifestyle intervention with exercise and diet remains the cornerstone of effective treatment, and where intervention on CMS once embraced a “one-size-fits-all” plan, expanded research has provided population-specific and patient-centric approaches to both lifestyle and medical management. Exercise programs now offer greater varieties of engaging exercise that enhance activity, life-satisfaction, and health, although multi-dimensional barriers to more substantial participation still need to be surmounted. Customized programs of optimal dietary control must still be refined, although recognition of the imprudent dietary habits in this population and characterization of deficiencies represents an important first step in CMS risk containment. Behavioral approaches enhance compliance and benefit derived from both diet and exercise interventions, and may be necessary to assure that persons with SCI profit from their efforts. Evidence suggests that multi-therapy strategies will be needed to control the more challenging of component risks, such as gain in body mass, which has far reaching implications for maintenance of daily function as well as health. In cases where lifestyle approaches prove inadequate for risk management, pharmacologic control is now available through a population tested drug that is inexpensive and widely available. These customized tools will likely foster a more effective health-centered culture for stakeholders with SCI and their health professionals alike.
Acknowledgement
This article is the basis for Dr Nash's keynote presentation at the 5th National SCI Conference held in October 2012 in Toronto, Ontario, Canada. This research was supported by grants from the National Institute for Disability and Rehabilitation Research (NIDRR), US Department of Education (#H133A011115, #H133G080150), the NIDRR RRTC on Secondary Conditions in SCI (#H133B090002), and the US Department of Defense (DOD) Spinal Cord Injury Research Program (SC090095).
References
- 1.Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil 2007;88(6):751–7 [DOI] [PubMed] [Google Scholar]
- 2.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76 [DOI] [PubMed] [Google Scholar]
- 3.Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk factors in persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med 2010;42(3):272–8 [DOI] [PubMed] [Google Scholar]
- 4.Groah SL, Nash MS, Ward EA, Libin A, Mendez AJ, Burns P, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulmonary Rehabil Prevent. 2011;31(2):73–80 [DOI] [PubMed] [Google Scholar]
- 5.Govindarajan G, Whaley-Connell A, Mugo M, Stump C, Sowers JR. The cardiometabolic syndrome as a cardiovascular risk factor. Am J Med Sci 2005;330(6):311–8 [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106(25):3143–421 [PubMed] [Google Scholar]
- 7.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bombelli M, Mancia G. Metabolic syndrome and cardiometabolic risk: an update. Blood Press 2009;18(1–2):7–16 [DOI] [PubMed] [Google Scholar]
- 8.Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 2000;11(1):109–40 [PubMed] [Google Scholar]
- 9.Cowan RE, Nash MS. Cardiovascular disease, SCI and exercise: unique risks and focused countermeasures. Disabil Rehabil 2010;32(26):2228–36 [DOI] [PubMed] [Google Scholar]
- 10.Ashraf SG, David RG., Jr Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil 2007;12(4):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005;43(9):513–8 [DOI] [PubMed] [Google Scholar]
- 12.Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 13.Duckworth WC, Solomon SS, Jallepalli P, Heckemeyer C, Finnern J, Powers A. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes 1980;29(11):906–10 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 15.Lee MY, Myers J, Hayes A, Madan S, Froelicher VF, Perkash I, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med 2005;28(1):20–5 [DOI] [PubMed] [Google Scholar]
- 16.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77 [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007;86(2):142–52 [DOI] [PubMed] [Google Scholar]
- 18.Nash MS, DeGroot J, Martinez-Arizala A, Mendez AJ. Evidence for an exaggerated postprandial lipemia in chronic paraplegia. J Spinal Cord Med 2005;28(4):320–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmons RR, Garber CE, Cirnigliaro CM, Moyer JM, Kirshblum SC, Galea MD, et al. The influence of visceral fat on the postprandial lipemic response in men with paraplegia. J Am Coll Nutr 2010;29(5):476–81 [DOI] [PubMed] [Google Scholar]
- 20.Nash MS. Central nervous system: spinal cord injury. In: Frontera WR, Dawson DM, Slovik Dm E, (eds.) Exercise in rehabilitation medicine. Champaign, IL: Human Kinetic Publishers; 2006. p. 191–205 [Google Scholar]
- 21.Nash MS. Cardiovascular fitness after spinal cord injuries. In: Lin V, (ed.) Spinal cord medicine. New York, NY: Demos Medical Publications; 2002. p. 637–46 [Google Scholar]
- 22.Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86(2):312–7 [DOI] [PubMed] [Google Scholar]
- 23.Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005;86(6):1176–81 [DOI] [PubMed] [Google Scholar]
- 24.Liang H, Mojtahedi MC, Chen D, Braunschweig CL. Elevated c-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil 2008;89(1):36–41 [DOI] [PubMed] [Google Scholar]
- 25.Vasudevan AR, Ballantyne CM. Cardiometabolic risk assessment: an approach to the prevention of cardiovascular disease and diabetes mellitus. Clin Cornerstone 2005;7(2–3):7–16 [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg HN, Stalenhoef AF. The metabolic syndrome: targeting dyslipidaemia to reduce coronary risk. J Cardiovasc Risk 2003;10(2):121–8 [DOI] [PubMed] [Google Scholar]
- 27.Groah SL, Libin A, Lauderdale M, Kroll T, DeJong G, Hsieh J. Beyond the evidence-based practice paradigm to achieve best practice in rehabilitation medicine: a clinical review. PMR 2009;1(10):941–50 [DOI] [PubMed] [Google Scholar]
- 28.McKenney JM. Update on the National Cholesterol Education Program Adult Treatment Panel III guidelines: getting to goal. Pharmacotherapy 2003;23(9 Pt 2):26S–33S [DOI] [PubMed] [Google Scholar]
- 29.Feasel S, Groah S The impact of diet on cardiovascular disease risk in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil 2009;14(3):56–68 [Google Scholar]
- 30.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health 2006;126(6):262–7 [DOI] [PubMed] [Google Scholar]
- 31.Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med 2009;32(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary guidelines for Americans. 7th ed Washington, DC: Government Printing Office; 2010. p. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters JL, Buchholz AC, Martin Ginis KA. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord 2009;47(4):318–22 [DOI] [PubMed] [Google Scholar]
- 34.Perret C, Stoffel-Kurt N. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med 2011;34(6):569–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr 2008;87(3):600–7 [DOI] [PubMed] [Google Scholar]
- 36.Tomey KM, Chen DM, Wang X, Braunschweig CL. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehabil 2005;86(4):664–71 [DOI] [PubMed] [Google Scholar]
- 37.Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia 1992;30(12):880–9 [DOI] [PubMed] [Google Scholar]
- 38.Aquilani R, Boschi F, Contardi A, Pistarini C, Achilli MP, Fizzotti G, et al. Energy expenditure and nutritional adequacy of rehabilitation paraplegics with asymptomatic bacteriuria and pressure sores. Spinal Cord 2001;39(8):437–41 [DOI] [PubMed] [Google Scholar]
- 39.Lee BY, Agarwal N, Corcoran L, Thoden WR, Del Guercio LR. Assessment of nutritional and metabolic status of paraplegics. J Rehabil Res Dev 1985;22(3):11–7 [DOI] [PubMed] [Google Scholar]
- 40.Sabour H, Javidan AN, Vafa MR, Shidfar F, Nazari M, Saberi H, et al. Calorie and macronutrients intake in people with spinal cord injuries: an analysis by sex and injury-related variables. Nutrition 2012;28(2):143–7 [DOI] [PubMed] [Google Scholar]
- 41.Peiffer SC, Blust P, Leyson JF. Nutritional assessment of the spinal cord injured patient. J Am Diet Assoc 1981;78(5):501–5 [PubMed] [Google Scholar]
- 42.Barboriak JJ, Rooney CB, El Ghatit AZ, Spuda K, Anderson AJ. Nutrition in spinal cord injury patients. J Am Paraplegia Soc 1983;6(2):32–6 [DOI] [PubMed] [Google Scholar]
- 43.Aghaeishahsavari M, Noroozianavval M, Veisi P, Parizad R, Samadikhah J. Cardiovascular disease risk factors in patients with confirmed cardiovascular disease. Saudi Med J 2006;27(9):1358–61 [PubMed] [Google Scholar]
- 44.Moussavi RM, Ribas-Cardus F, Rintala DH, Rodriguez GP. Dietary and serum lipids in individuals with spinal cord injury living in the community. J Rehabil Res Dev 2001;38(2):225–33 [PubMed] [Google Scholar]
- 45.Zlotolow SP, Levy E, Bauman WA. The serum lipoprotein profile in veterans with paraplegia: the relationship to nutritional factors and body mass index. J Am Paraplegia Soc 1992;15(3):158–62 [DOI] [PubMed] [Google Scholar]
- 46.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2012;5:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr 2000;84(4):417–27 [DOI] [PubMed] [Google Scholar]
- 48.Knight KH, Buchholz AC, Martin Ginis KA, Goy RE. Leisure-time physical activity and diet quality are not associated in people with chronic spinal cord injury. Spinal Cord 2011;49(3):381–5 [DOI] [PubMed] [Google Scholar]
- 49.Battista P, Di Primio R, Di Luzio A, Nubile G, Di Tano G. Correlations between dietetic fiber and serum levels of total cholesterol and HDL-cholesterol. Boll Soc Ital Biol Sper 1983;59(1):83–6 [PubMed] [Google Scholar]
- 50.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69(1):30–42 [DOI] [PubMed] [Google Scholar]
- 51.Mietus-Snyder ML, Shigenaga MK, Suh JH, Shenvi SV, Lal A, McHugh T, et al. A nutrient-dense, high-fiber, fruit-based supplement bar increases HDL cholesterol, particularly large HDL, lowers homocysteine, and raises glutathione in a 2-wk trial. FASEB J 2012;26(8):3515–27 Epub 2012 May 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reyna-Villasmil N, Bermudez-Pirela V, Mengual-Moreno E, Arias N, Cano-Ponce C, Leal-Gonzalez E, et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am J Ther 2007;14(2):203–12 [DOI] [PubMed] [Google Scholar]
- 53.Cameron KJ, Nyulasi IB, Collier GR, Brown DJ. Assessment of the effect of increased dietary fibre intake on bowel function in patients with spinal cord injury. Spinal Cord 1996;34(5):277–83 [DOI] [PubMed] [Google Scholar]
- 54.Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord 2010;48(10):718–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laven GT, Huang CT, DeVivo MJ, Stover SL, Kuhlemeier KV, Fine PR. Nutritional status during the acute stage of spinal cord injury. Arch Phys Med Rehabil 1989;70(4):277–82 [PubMed] [Google Scholar]
- 56.Moussavi RM, Garza HM, Eisele SG, Rodriguez G, Rintala DH. Serum levels of vitamins A, C, and E in persons with chronic spinal cord injury living in the community. Arch Phys Med Rehabil 2003;84(7):1061–7 [DOI] [PubMed] [Google Scholar]
- 57.Berger D. Dietary management of the paraplegic patient. Can Serv Med J 1954;10(1):65–70 [PubMed] [Google Scholar]
- 58.Khandare AL, Shankar NH, Kalyanasundaram S, Rao GS. Effect of calcium deficiency induced by fluoride intoxication on lipid metabolism in rabbits. Fluoride 2007;40(3):184–9 [Google Scholar]
- 59.Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res 2010;134(2):119–29 [DOI] [PubMed] [Google Scholar]
- 60.Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 2012;97(1):279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fattoretti P, Bertoni-Freddari C, Casoli T, Di Stefano G, Solazzi M, Giorgetti B. Decreased expression of glucose transport protein (Glut3) in aging and vitamin E deficiency. Ann N Y Acad Sci 2002;973:293–6 [DOI] [PubMed] [Google Scholar]
- 62.Frikke-Schmidt H, Lykkesfeldt J. Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol 2009;104(6):419–33 [DOI] [PubMed] [Google Scholar]
- 63.Grungreiff K, Reinhold D. Liver cirrhosis and ‘liver’ diabetes mellitus are linked by zinc deficiency. Med Hypotheses 2005;64(2):316–7 [DOI] [PubMed] [Google Scholar]
- 64.Kaya A, Altiner A, Ozpinar A. Effect of copper deficiency on blood lipid profile and haematological parameters in broilers. J Vet Med A Physiol Pathol Clin Med 2006;53(8):399–404 [DOI] [PubMed] [Google Scholar]
- 65.Larrieta E, Vega-Monroy ML, Vital P, Aguilera A, German MS, Hafidi ME, et al. Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis. J Nutr Biochem 2012;23(4):392–99 [DOI] [PubMed] [Google Scholar]
- 66.Matthews KA, Rhoten WB, Driscoll HK, Chertow BS. Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr 2004;134(8):1958–63 [DOI] [PubMed] [Google Scholar]
- 67.McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr Med 2008;7(2):48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr 2007;26(2):121–32 [DOI] [PubMed] [Google Scholar]
- 69.Reiterer G, MacDonald R, Browning JD, Morrow J, Matveev SV, Daugherty A, et al. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J Nutr 2005;135(9):2114–8 [DOI] [PubMed] [Google Scholar]
- 70.Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism. 1980;29(6):498–502 [DOI] [PubMed] [Google Scholar]
- 71.Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 2008;31(11):2092–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation 1992;85(3):910–15 [DOI] [PubMed] [Google Scholar]
- 73.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction 2000;95(10):1505–23 [DOI] [PubMed] [Google Scholar]
- 74.Wakabayashi I. Increased body mass index modifies associations between alcohol intake and blood cholesterol profile. Eur J Clin Invest 2012;42(2):179–85 [DOI] [PubMed] [Google Scholar]
- 75.Saunders LL, Krause JS. Psychological factors affecting alcohol use after spinal cord injury. Spinal Cord 2011;49(5):637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tate DG, Forchheimer MB, Krause JS, Meade MA, Bombardier CH. Patterns of alcohol and substance use and abuse in persons with spinal cord injury: risk factors and correlates. Arch Phys Med Rehabil 2004;85(11):1837–47 [DOI] [PubMed] [Google Scholar]
- 77.Bombardier CH, Rimmele CT. Alcohol use and readiness to change after spinal cord injury. Arch Phys Med Rehabil 1998;79(9):1110–15 [DOI] [PubMed] [Google Scholar]
- 78.Phillips E, Gater DR. A practical Approach for the nutritional management of obesity in spinal cord injury. Top Spinal Cord Inj Rehabil 2007;12(4):64–75 [Google Scholar]
- 79.Szlachcic Y, Adkins RH, Adal T, Yee F, Bauman W, Waters RL. The effect of dietary intervention on lipid profiles in individuals with spinal cord injury. J Spinal Cord Med 2001;24(1):26–9 [DOI] [PubMed] [Google Scholar]
- 80.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44(2):82–91 [DOI] [PubMed] [Google Scholar]
- 81.Liusuwan RA, Widman LM, Abresch RT, Styne DM, McDonald CM. Body composition and resting energy expenditure in patients aged 11 to 21 years with spinal cord dysfunction compared to controls: comparisons and relationships among the groups. J Spinal Cord Med 2007;30Suppl. 1:105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zemper ED, Tate DG, Roller S, Forchheimer M, Chiodo A, Nelson VS, et al. Assessment of a holistic wellness program for persons with spinal cord injury. Am J Phys Med Rehabil 2003;82(12):957–68 Quiz 969–71 [DOI] [PubMed] [Google Scholar]
- 83.Emon ST, Irban AG, Bozkurt SU, Akakin D, Konya D, Ozgen S. Effects of parenteral nutritional support with fish-oil emulsion on spinal cord recovery in rats with traumatic spinal cord injury. Turk Neurosurg 2011;21(2):197–202 [DOI] [PubMed] [Google Scholar]
- 84.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, et al. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain 2007;130(Pt 11):3004–19 [DOI] [PubMed] [Google Scholar]
- 85.Javierre C, Vidal J, Segura R, Medina J, Garrido E. Continual supplementation with n-3 fatty acids does not modify plasma lipid profile in spinal cord injury patients. Spinal Cord 2005;43(9):527–30 [DOI] [PubMed] [Google Scholar]
- 86.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106(21):2747–57 [DOI] [PubMed] [Google Scholar]
- 87.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997;655 Suppl.:1645S–54S [DOI] [PubMed] [Google Scholar]
- 88.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 2003;23(2):151–2 [DOI] [PubMed] [Google Scholar]
- 89.Tasiemski T, Bergstrom E, Savic G, Gardner BP. Sports, recreation and employment following spinal cord injury–a pilot study. Spinal Cord 2000;38(3):173–84 [DOI] [PubMed] [Google Scholar]
- 90.Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, Buchholz AC, Bray SR, Craven BC, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part II: activity types, intensities, and durations. Arch Phys Med Rehabil 2010;91(5):729–33 [DOI] [PubMed] [Google Scholar]
- 91.Ginis KA, Latimer AE, Arbour-Nicitopoulos KP, Buchholz AC, Bray SR, Craven BC, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part I: demographic and injury-related correlates. Arch Phys Med Rehabil 2010;91(5):722–8 [DOI] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention. National Center for Health Statistics. Health Indicators Warehouse. 2009–2010. Available from: http://healthindicators.gov/Indicators/Leisure-time-physical-activity-none-percent_1313/National_0/Profile/Data . [Google Scholar]
- 93.El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 2005;43(5):299–305 [DOI] [PubMed] [Google Scholar]
- 94.Nash MS, Jacobs PL, Mendez AJ, Goldberg RB. Circuit resistance training improves the atherogenic lipid profile in persons with chronic paraplegia. J Spinal Cord Med 2001;24(1):2–9 [DOI] [PubMed] [Google Scholar]
- 95.Hooker SP, Wells CL. Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc 1989;21(1):18–22 [DOI] [PubMed] [Google Scholar]
- 96.de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord 2003;41(12):673–9 [DOI] [PubMed] [Google Scholar]
- 97.Dallmeijer AJ, Hopman MT, van der Woude LH. Lipid, lipoprotein, and apolipoprotein profiles in active and sedentary men with tetraplegia. Arch Phys Med Rehabil 1997;78(11):1173–6 [DOI] [PubMed] [Google Scholar]
- 98.Janssen TW, van Oers CA, van Kamp GJ, TenVoorde BJ, van der Woude LH, Hollander AP. Coronary heart disease risk indicators, aerobic power, and physical activity in men with spinal cord injuries. Arch Phys Med Rehabil 1997;78(7):697–705 [DOI] [PubMed] [Google Scholar]
- 99.Buchholz AC, Martin Ginis KA, Bray SR, Craven BC, Hicks AL, Hayes KC, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab 2009;34(4):640–7 [DOI] [PubMed] [Google Scholar]
- 100.de Groot S, Dallmeijer AJ, Post MW, Angenot EL, van den Berg-Emons RJ, van der Woude LH. Prospective analysis of lipid profiles in persons with a spinal cord injury during and 1 year after inpatient rehabilitation. Arch Phys Med Rehabil 2008;89(3):531–7 [DOI] [PubMed] [Google Scholar]
- 101.Carlson KF, Wilt TJ, Taylor BC, Goldish GD, Niewoehner CB, Shamliyan TA, et al. Effect of exercise on disorders of carbohydrate and lipid metabolism in adults with traumatic spinal cord injury: systematic review of the evidence. J Spinal Cord Med 2009;32(4):361–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ginis KA, Hicks AL, Latimer AE, Warburton DE, Bourne C, Ditor DS, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011;49(11):1088–96 [DOI] [PubMed] [Google Scholar]
- 103.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. Centers for Disease Control and Prevention, National Center for Health Statistics. 2008. Available from: www.hhs.gov . [Google Scholar]
- 104.Canadian Society for Exercise Physiology, Canadian Physical Activity Guidelines, 2012 Scientific Statements. Available from: www.csep.ca/guidelines. [Google Scholar]
- 105.Manns PJ, Dunstan DW, Owen N, Healy GN. Addressing the nonexercise part of the activity continuum: a more realistic and achievable approach to activity programming for adults with mobility disability? Phys Ther 2012;92(4):614–25 [DOI] [PubMed] [Google Scholar]
- 106.Warburton DE, Gledhill N, Jamnik VK, Bredin SS, McKenzie DC, Stone J, et al. Evidence-based risk assessment and recommendations for physical activity clearance: Consensus Document 2011 (1) (1) This paper is one of a selection of papers published in this Special Issue, entitled Evidence-based risk assessment and recommendations for physical activity clearance, and has undergone the Journal's usual peer review process. Appl Physiol Nutr Metab 2011;36Suppl. 1:S266–98 [DOI] [PubMed] [Google Scholar]
- 107.Arbour-Nicitopoulos KP, Ginis KA. Universal accessibility of ‘accessible’ fitness and recreational facilities for persons with mobility disabilities. Adapt Phys Activ Q 2011;28(1):1–15 [DOI] [PubMed] [Google Scholar]
- 108.Nash MS. Exercise as a health-promoting activity following spinal cord injury. J Neurol Phys Ther 2005;29(2):87–103, 106 [DOI] [PubMed] [Google Scholar]
- 109.Weltman A, Matter S, Stamford BA. Caloric restriction and/or mild exercise: effects on serum lipids and body composition. Am J Clin Nutri 1980;33(5):1002–9 [DOI] [PubMed] [Google Scholar]
- 110.Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J 2001;22(7):554–72 [DOI] [PubMed] [Google Scholar]
- 111.Kopelman P, Groot Gde H, Rissanen A, Rossner S, Toubro S, Palmer R, et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity 2010;18(1):108–15 [DOI] [PubMed] [Google Scholar]
- 112.Goldberg R. Guideline-driven Intervention on SCI-Associated dyslipididemia, metabolic syndrome, and glucose intolerance using pharmacological agents. Top Spinal Cord Inj Rehabil 2009;14(3):46–57 [Google Scholar]
- 113.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52(1):17–30 [DOI] [PubMed] [Google Scholar]
- 114.Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147(6):386–99 [DOI] [PubMed] [Google Scholar]
- 115.Goldberg RB, Sabharwal AK. Fish oil in the treatment of dyslipidemia. Curr Opin Endocrinol Diabetes Obes 2008;15(2):167–74 [DOI] [PubMed] [Google Scholar]
- 116.Khachatryan V, Sirunyan AM, Tumasyan A, Adam W, Bergauer T, Dragicevic M, et al. Search for quark compositeness with the dijet centrality ratio in pp collisions at radicals = 7 TeV. Phys Rev Lett 2010;105(2):022002 Epub 2010 Jul 6 [DOI] [PubMed] [Google Scholar]
- 117.Dyson-Hudson T, Nash MS. Guideline-driven assessment of cardiovascular disease and related risks after spinal cord injury. Top Spinal Cord Inj Rehabil 2009;14:32–45 [Google Scholar]
- 118.Farmer JA. Statins and myotoxicity. Curr Atheroscler Rep 2003;5(2):96–100 [DOI] [PubMed] [Google Scholar]
- 119.Klopstock T. Drug-induced myopathies. Curr Opin Neurol 2008;21(5):590–5 [DOI] [PubMed] [Google Scholar]
- 120.Nash MS, Goldberg RB. Higher starting doses of atorvastatin may reduce LDL-cholesterol levels more than lower doses. Commentary. Evid Based Cardiovasc Med 2005;9(2):98–101 [DOI] [PubMed] [Google Scholar]
- 121.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol 2007;49(23):2231–7 [DOI] [PubMed] [Google Scholar]
- 122.Goldberg AC. Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study. Am J Cardiol 1998;82(12A):35U–8U [DOI] [PubMed] [Google Scholar]
- 123.Knopp RH, Ginsberg J, Albers JJ, Hoff C, Ogilvie JT, Warnick GR, et al. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin. Metabolism 1985;34(7):642–50 [DOI] [PubMed] [Google Scholar]
- 124.Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol 1998;82(12A):29U–34U [DOI] [PubMed] [Google Scholar]
- 125.Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil 1986;67(7):445–50 [PubMed] [Google Scholar]
- 126.Nash MS, Lewis JE, Dyson-Hudson TA, Szlachcic Y, Yee F, Mendez AJ, et al. Safety, tolerance, and efficacy of extended-release niacin monotherapy for treating dyslipidemia risks in persons with chronic tetraplegia: a randomized multicenter controlled trial. Arch Phys Med Rehabil 2011;92(3):399–410 [DOI] [PubMed] [Google Scholar]


