Abstract
Background/objectives
In order to guide and improve rehabilitation interventions for grip function after spinal cord injury (SCI), it is important to have a detailed understanding of the motor control strategies that the central nervous system uses to control the hand. We examined whether changes in the motor control of the hand after SCI are manifested in the form of changes to muscle synergies. We further sought to determine a correlation between functional ability and the extent of muscle synergy disruption.
Methods
Surface electromyographic (EMG) data were recorded from 8 hand muscles in 10 able-bodied subjects and 6 subjects with SCI as they performed various functional tasks using grip types relevant to activities of daily living. Muscle synergies were extracted using non-negative matrix factorization. Functional performance in each task was quantified using a 5-point clinical scale.
Results
The synergies most commonly observed in able-bodied subjects were co-activation of extensor digitorum communis and extensor indicis proprius, as well as of flexor digitorum superficialis with flexor carpi ulnaris. The proportion of subjects in which particular synergies occurred was significantly different for subjects with SCI compared to able-bodied subjects (P < 0.001). Deviations from the average able-bodied synergies in subject with SCI were found to be poorly correlated (r = −0.04) with functional ability.
Conclusions
Results suggest that the disruptions and re-organizations of neural circuitry after SCI are reflected by the extracted muscle synergies, but the question of how muscle synergies can guide rehabilitation interventions remains open.
Keywords: Spinal cord injuries, Rehabilitation, Hand function, Muscle synergies, Rehabilitation, Electromyography, Motor control, Activities of daily living
Introduction
The restoration of hand function following cervical spinal cord injury (SCI, Table 1) is crucial to the affected individual's independence and quality of life. In several surveys, individuals with tetraplegia have rated improvements in hand function as their top priority.1,2 Rehabilitation after SCI consists in large part of task-directed training in which patients perform many repetitions of movements relevant to activities of daily living (ADLs). In this context, understanding the motor control strategies that the central nervous system (CNS) uses to govern hand movements in able-bodied individuals may be useful in guiding the rehabilitation process after cervical SCI and ensuring that the exercises performed are effective for restoring functional ability.3
Table 1.
List of abbreviations
| Action Research Arm Test | ARAT |
| Activities of daily living | ADL |
| Central nervous system | CNS |
| Electromyography | EMG |
| Extensor carpi radialis | ECR |
| Extensor digitorum communis | EDC |
| Extensor indicis proprius | EIP |
| First dorsal interosseus | DI1 |
| Flexor carpi radialis | FCR |
| Flexor carpi ulnaris | FCU |
| Flexor digitorum superficialis | FDS |
| Graded and redefined assessment of strength, sensibility, and prehension | GRASSP |
| International standards for neurological classification of spinal cord injury | ISNCSCI |
| Spinal cord injury | SCI |
| Thenar eminence muscle group | TEMG |
| Upper extremity motor score | UEMS |
Muscle synergies are a motor control paradigm that has been actively investigated and discussed in recent years.4–7 This framework postulates a modular approach to motor control, where the CNS activates predefined combinations of muscles, rather than explicitly controlling individual muscles. If correct, this hypothesis would have interesting implications in neurorehabilitation: by explicitly retraining muscle synergies that are known to be useful in the able-bodied population, functional performance might be improved across a broad range of tasks. In order for muscle synergies to be applicable to rehabilitation in this way, however, there must be a close relationship between the presence or absence of certain synergies and functional ability.
To date, a small number of studies have investigated the existence of muscle synergies in the hand. Maier and Hepp-Reymond8 investigated the correlation and cross-correlation of muscle pairs during thumb-and-index force target tasks, but did not find consistent synergies across subjects. On the other hand, when precisely controlling index finger posture and force direction, Valero-Cuevas9 demonstrated subject-independent muscle activation patterns that scaled across various levels of fingertip force production. Weiss and Flanders10 applied principal component analysis to electromyographic (EMG) data and used the resulting components to describe 52 different static hand postures. Ajiboye and Weir11 used the American Sign Language alphabet to examine whether muscle synergies could serve as a predictive framework for new hand postures; the synergies identified in this study were generally subject specific. Johnston et al.12 found evidence of synergistic control of extrinsic and intrinsic hand muscles during a two-digit task. These studies generally did not investigate the presence of synergies during the different types of hand movements used in ADLs (with the exception of Ref.10), and did not investigate and compare populations with neurological damage.
In order to gain more insight into the implications of the muscle synergy theory for neurorehabilitation, this study addressed two goals: (1) characterize what muscles synergies (if any) are present in able-bodied individuals while using different types of hand grips relevant to ADLs (pulp-to-pulp pinch, cylindrical grasp, tripod grasp, spherical grasp, and lateral key pinch). (2) Determine whether the presence or absence of these synergies after SCI is correlated with functional abilities.
Methods
Data collection
Subjects
Data were collected from 10 able-bodied subjects and 6 subjects who had previously sustained an SCI at the cervical level and been living with SCI for at least 7 months to 9.5 years. All subjects were male and right handed, with the right hand being dominant both pre- and post-injury in the case of the subjects with SCI. The mean age was 34.9 ± 9.7 years for the able-bodied group and 49.2 ± 17.4 years for the SCI group. The injury characteristics of subjects in the SCI group are given in Table 2, following the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).13 Specifically, the strengths of five key muscle groups are evaluated manually according to a 5-point scale, where 0 corresponds to a completely paralyzed muscle and 5 corresponds to normal strength. The five muscle groups are the elbow flexors, wrist extensors, elbow extensors, finger flexors (distal phalanx of middle finger), and finger abductors (little finger), and correspond to the functional integrity within spinal cord segments C5, C6, C7, C8, and T1, respectively. The sum of the strength scores for these five muscle groups yields the upper extremity motor score (UEMS). The motor level is defined as the most caudal spinal segment for which the corresponding muscles has a strength score of 3 or more, with all the more rostral muscles having normal scores of 5/5. The inclusion criterion for our SCI group was to have partially preserved hand function (defined in this case as being able to flex at least one of the index, middle, ring, or little fingers against gravity), and the exclusion criterion was to have had hand surgery (e.g. tendon transfer).
Table 2.
Injury characteristics of subjects with SCI
| Subject | Time since injury (years) | Right upper extremity motor score | Right motor level |
|---|---|---|---|
| 1 | 4.2 | 20 (4/4/4/4/4) | C4 |
| 2 | 3.9 | 21 (5/5/3/4/4) | C7 |
| 3 | 2 | 25 (5/5/5/5/5) | T1* |
| 4 | 3.5 | 23 (5/5/5/5/3) | T1 |
| 5 | 0.6 | 23 (5/5/5/5/3) | T1 |
| 6 | 9.5 | 23 (5/5/5/4/4) | C8 |
UEMS and motor level for the right arm are computed as per the ISNCSCI.13 Details of the motor scores for each of the five key muscle groups (see text) are provided in parentheses.
*Sensory scores from the thoracic dermatomes are not available, and T1 is therefore an approximation of the motor level, which according to the ISNCSCI would be determined in this case by the most caudal thoracic dermatome with normal sensory function.
Experimental protocol
Each subject was asked to perform the following grip tasks, which involved different muscles or movement strategies and are all adapted from existing hand function assessments (Graded and Redefined Assessment of Strength, Sensibility, and Prehension, GRASSP14 and Action Research Arm Test, ARAT15) to ensure relevance to ADLs:
Gripping and lifting a light block (175 g) 30 cm using the thumb and index finger in a pulp-to-pulp pinch (Fig. 1a).
Gripping and lifting a heavier block (500 g) 30 cm using the thumb and index finger (same grip as in Fig. 1a).
Gripping and lifting a cylinder (500 g) 30 cm using a cylindrical grasp (whole hand, Fig. 1b).
Gripping and lifting a disk (300 g) 30 cm using a tripod grasp (thumb, index and middle finger, Fig. 1c).
Rotating a disk 180° (jar lid) using a spherical grasp (whole hand, Fig. 1d).
Gripping and lifting a baseball (142 g) 30 cm using a spherical grasp (whole hand, Fig. 1e).
Gripping and lifting a marble 30 cm using a pulp-to-pulp pinch (thumb and index finger, Fig. 1f).
Gripping a key, lifting it 30 cm and turning it 90° using a lateral key pinch (thumb and index finger, Fig. 1g). For SCI subjects, picking up a key lying flat on a table can be challenging, so the key was placed in a leaning position or on a small pedestal to make it easier to pick up, if necessary.
Figure 1.

Pictures of the different types of grips used during the data collection. See text for a complete description of each object and grip.
The desired grip postures for each task were demonstrated to the subjects before starting the trials. In each case, the subjects were comfortably seated and started with the hand on the table in front of them, with the forearm pronated and the wrist in a neutral position. An auditory timing signal was provided to mark the beginning of each trial. The subjects reached out, grabbed the object with the designated grip, and moved it to its target position. In all lifting tasks (tasks 1, 2, 3, 4, 6, and 7), the target position was the top of a 30 cm-high shelf placed on a table located at waist level. Once the object was placed on the shelf, subjects let go of the object for 1 second, then took hold of it again, returned it to its initial starting position, and placed their hand in the resting position. This marked the end of the trial. In the rotation tasks (i.e. tasks 5 and 8), subjects rotated the object to its target position but did not let go. After 1 second, the objects were returned to their original position and the hand placed back in its resting posture. Ten trials were performed for each task. The choice of tasks and objects was designed to cover a variety of grip types meaningful to ADLs, ensure that loads reflective of everyday objects were produced (as a result of the lifting), and yet avoid imposing too much variability or difficulty in the reaching component of the movement.
The ability of each subject with SCI to perform the required tasks was assessed using the scale in Table 3. This scale was obtained from the GRASSP, a clinical test developed specifically to assess the hand function of individuals with tetraplegia.14 A total functional score was obtained by adding the scores for all eight tasks, for a maximum score of 40. Note that we did not perform the GRASSP test itself, but simply borrowed its rating scale because it can be easily generalized to various grip types while still providing meaningful information about hand function.
Table 3.
Scale used for the assessment of hand function in the subjects with SCI (from GRASSP14*)
| Score | Description |
|---|---|
| 0 | Task cannot be conducted at all |
| 1 | Task cannot be completed (less than 50% of the task) |
| 2 | Task is not completed (50% or more of the task) |
| 3 | Task is conducted (completed) using tenodesis or an alternative grasp other than the expected grasp |
| 4 | Task is conducted using the expected grasp with difficulty (lack of smooth movement or difficult slow movement) |
| 5 | Task is conducted without difficulties using the expected grasping pattern and unaffected hand function |
*Note that the scale is being used outside of its intended use as part of the GRASSP, and has not been psychometrically validated for use in isolation. Refer to main text for details.
The experimental protocol was approved by the local ethics board of The University of British Columbia (UBC).
EMG recordings
Surface EMG data were recorded from eight muscles: the flexor digitorum superficialis (FDS), the first dorsal interosseus (DI1), the flexor carpi ulnaris (FCU), the flexor carpi radialis (FCR), the extensor carpi radialis (ECR), the extensor digitorum communis (EDC), the extensor indicis proprius (EIP), and the thenar eminence muscle group (TEMG). The placement of the electrodes was based on muscle palpation. Preliminary recordings were performed and electrode placements adjusted as necessary to ensure that each channel accurately reflected its target muscle and that cross-talk was minimized, based on visual inspection of the recordings while subjects were instructed to perform movements designed to contract specific muscles.
EMG signals were recorded using a 16-channel amplifier with bipolar electrodes (BagnoliTM-16 system, Delsys Inc., Boston, MA, USA) at a sampling rate of 2 kHz. The DI1 and TEMG muscles were amplified with a gain of ×1000 and all other muscles with a gain of ×10 000 (DI1 and TEMG produced signals with larger amplitudes, such that a lower gain was necessary to prevent saturation). The amplifier system band-passed filtered the EMG data between 20 and 450 Hz.
Data processing
Identification of muscle synergies
Time-invariant muscle synergies were extracted using a non-negative matrix factorization algorithm, as described in Refs.5,16 These synergies aim to represent fundamental patterns of muscle activity that can be weighted and combined to produce different movements. They are therefore extracted from datasets in which several different movements are represented, in our case the eight tasks described in the previous section.
EMG recordings were first normalized to have unit variance, in order for the synergy extraction process to be based on the pattern rather than magnitude of muscle activation. The recordings were then full-wave rectified and low-pass filtered using a fourth order Butterworth filter with a cut-off frequency of 20 Hz to extract the signal envelope. Zero-lag filtering was produced by using a sequence a forward and reverse digital filtering (Matlab “filtfilt” function). Finally, the signals were integrated over 10 milliseconds bins to reduce the amount of data to process.
For each subject, the resulting time series for all trials of all tasks were concatenated into a matrix M, with eight rows (one per muscle) and N columns (N varied by subject due to variations in trial durations, but always reflected 80 trials, as a result of the 10 trials conducted for each of 8 tasks). In brief, the matrix factorization algorithm uses a multiplicative update rule to decompose M into two matrices W and H, such that M = W × H. Further details about the update rules can be found in Ref.17 W has one row per muscle (i.e. eight rows in our study) and one column per muscle synergy. The number of synergies must be pre-defined. H has one row per synergy, and N columns. Therefore, the columns of W represent the muscle synergies, while the columns of H represent the variations in time of the activation of each synergy.
In order to choose the number of synergies, the factorization process was carried out for each possible number of synergies between one and eight (eight being the number of muscles from which EMG data was recorded). In each case, the mean across all able-bodied subjects of the amount of the variance of M explained by the decomposition W × H was computed (R2 value). The number of synergies chosen was the lowest value needed to explain 85% of the variance. This threshold is high enough to produce synergies that meaningfully reflect underlying activity, while setting the threshold higher could result in a set of relatively trivial single-muscle synergies (see Results).
Note that in a few cases (subject 1, 2 tasks), subjects with SCI were unable to perform the tasks due to insufficient hand function. In those situations, the corresponding sections of M were padded with zeros.
Comparison of muscle synergies in able bodied and SCI groups
Once muscle synergies have been extracted for each subject, two questions need to be addressed. First, how similar are the observed synergies between able-bodied subjects? Second, are there observable differences between the synergies of able-bodied subjects and subjects with SCI? For the purpose of comparing synergies between individuals in a manner that is robust to minor variations, each synergy was normalized to its highest entry and defined by which muscles exceeded a threshold of 0.5 (i.e. half the highest entry). We then computed the percentage of patients in which each synergy occurred, for the able bodied and SCI groups.
Relationship between synergies and function in SCI group
In order to obtain a representative set of able-bodied muscle synergies, the synergies identified for all able-bodied subjects (before thresholding) were pooled, each normalized to its highest entry, and fed into a k-means clustering algorithm. The number of clusters was equal to the number of synergies per subject. The clustering was based on squared Euclidean distance, was repeated 10 times with random initializations, and the solution with the smallest within-cluster sums of point-to-centroid distances was retained. The resulting cluster centroids were taken to represent the “typical” synergies for able-bodied subjects. For each SCI subject, the Frobenius norm of the difference between the 8 × S synergy matrix W (each column normalized to its highest entry) and the 8 × S matrix of cluster centroids was computed, where S is the number of synergies. As the columns of W are not in any particular order, the process was repeated for all possible permutations of the columns of W, and the smallest difference norm retained. This difference measure, denoted D, reflects how similar each SCI subject's synergies are to typical able-bodied synergies.
Finally, the differences computed above were correlated with the functional scores of the subjects (obtained as described in “Experimental protocol”).
Results
Fig. 2 shows the percentage of variance explained as a function of the number of synergies. The number of synergies required to explain 85% of variance is 5, and the remainder of the analysis is therefore conducted using five synergies. Meeting a higher threshold of variance explained would require more synergies (e.g. seven synergies are required to account for 95% of the variance), but this in turn will result in more single-muscle “synergies”, as the number of synergies approaches the number of recording channels in our experiments, which was eight. The choice of five synergies represents a trade-off between identifying meaningful synergies and accounting for more variance.
Figure 2.
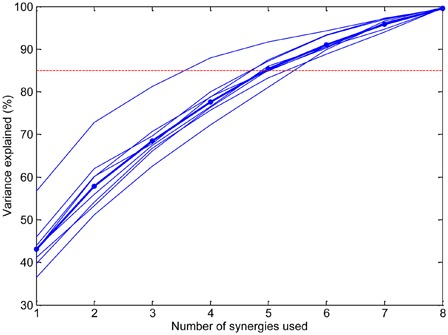
Percentage of signal variance explained as a function of the number of synergies. The dotted line shows the threshold used (85%) for choosing the number of synergies. The thin lines correspond to individual able-bodied subjects and the thick line shows the mean.
Table 4 shows the muscle synergies observed for the able bodied and SCI groups. Among able-bodied subjects, the most commonly observed multiple-muscle synergies were co-activation of EDC and EIP, and of FDS with FCU. These synergies were also found in individuals with SCI, but in a smaller proportion of subjects. In contrast, the co-activation of ECR and FCR was more common among subjects with SCI than among able-bodied subjects. The single-muscle synergies DI1 and TEMG were common in both groups. A χ2 test comparing the proportions of all the observed synergies in the able bodied and SCI groups (i.e. comparing the two columns of Table 4) confirmed that the difference was statistically significant (P < 0.001).
Table 4.
Synergies occurring, for the able-bodied and SCI groups
| Muscles involved in synergy | Frequency of occurrence of each synergy |
|
|---|---|---|
| Able-bodied subjects | Subjects with SCI | |
| EDC, EIP | 7/10 (70%) | 2/6 (33.3%) |
| DI1 | 7/10 (70%) | 4/6 (66.7%) |
| TEMG | 6/10 (60%) | 4/6 (66.7%) |
| FDS, FCU | 6/10 (60%) | 2/6 (33.3%) |
| ECR | 3/10 (30%) | 0 |
| FCR, ECR, EDC | 3/10 (30%) | 0 |
| FCR, ECR | 2/10 (20%) | 4/6 (66.7%) |
| DI1, TEMG | 2/10 (20%) | 1/6 (16.7%) |
| FCR | 2/10 (20%) | 1/6 (16.7%) |
| FCU | 2/10 (20%) | 2/6 (33.3%) |
| FDS | 2/10 (20%) | 2/6 (33.3%) |
| ECR, EDC | 1/10 (10%) | 1/6 (16.7%) |
| EIP | 1/10 (10%) | 0 |
| FCU, FCR, TEMG | 1/10 (10%) | 0 |
| DI1, EDC, TEMG | 1/10 (10%) | 0 |
| FDS, FCR | 1/10 (10%) | 0 |
| ECR, EDC, EIP | 1/10 (10%) | 1/6 (16.7%) |
| FCU, FCR | 1/10 (10%) | 0 |
| FDS, FCU, FCR | 1/10 (10%) | 0 |
| EDC | 0 | 2/6 (33.3%) |
| DI1, FCR, EIP | 0 | 1/6 (16.7%) |
| FDS, FCU, EIP | 0 | 1/6 (16.7%) |
| FDS, FCR, ECR | 0 | 1/6 (16.7%) |
| FDS,EIP, TEMG | 0 | 1/6 (16.7%) |
The results are expressed as a ratio between the number of subjects in which a given synergy was observed and the total number of subjects in that group (able bodied or SCI). The numbers in parentheses express the results as percentages to facilitate comparison between the two groups. For the purposes of comparisons between individuals, the entries in each synergy are normalized to the highest entry and thresholded at 0.5.
Fig. 3 shows the centroids of the able-bodied synergy clusters. These results are consistent with those of Table 4 in that the cluster centroids correspond well to the synergies outlined in the previous paragraph, namely EDC and EIP, FDS and FCU, ECR and FCR, DI1, and TEMG.
Figure 3.
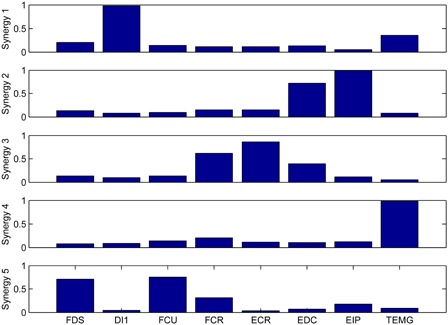
Average synergies in able-bodied subjects. Each synergy is the centroid of a cluster in the pooled set of all able-bodied subject synergies.
Fig. 4 shows each SCI subject's difference measure D and functional score. The series of D values is normalized to its largest entry and the functional scores are normalized to the maximum possible score (40). If the disruption of muscle synergies after SCI was closely linked to functional abilities, the two plots in Fig. 4 would be expected to show a clear inverse relationship (i.e. the more an individual's synergies deviate from the typical able-bodied synergies, the lower that individual's function would be expected to be). However, Fig. 4 shows that the two quantities were poorly correlated. The correlation coefficient was −0.04, and the relationship was not statistically significant (P = 0.94).
Figure 4.
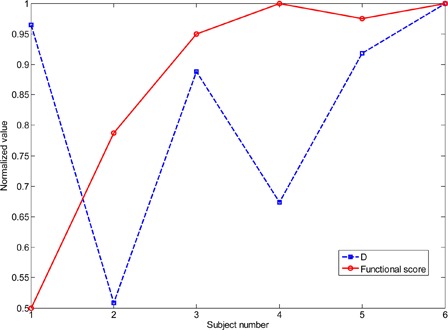
Difference D of each SCI subject's muscle synergies from the average able-bodied synergies show in Fig. 3 (dotted line; see text for details of how D is computed), as well as each SCI subject's functional score (solid line). The series of D values is normalized to its largest entry and the functional scores are normalized to the maximum possible score (40).
Discussion
Several time-invariant synergies were observed to occur consistently in a substantial proportion of able-bodied subjects. In subjects with SCI, similar synergies were observed, but in different proportions. No clear relationship was found between the functional abilities of subjects with SCI and those subjects' deviation from able-bodied synergy patterns.
Synergies observed
The most common multiple-muscle synergies in the able-bodied population were EDC and EIP and FDS and FCU. The first of these is not unexpected, since many tasks involving finger extension tasks can be expected to recruit both EDC and EIP. The FDS and FCU synergy suggests that a wrist flexion was often used to position the hand during a grasping action, though this may be a product of the specific set of tasks employed in this study (see Methods and Fig. 1). The most common synergy in SCI subjects was FCR and ECR, which was also one of the average able-bodied synergies (synergy 3 in Fig. 3). This synergy could be the result of the agonist and antagonist co-contracting, most likely to help stabilize the wrist. The single-muscle synergies DI1 and TEMG were common in both groups, possibly because of the need for independent fine thumb and index finger movements in many dextrous tasks. It is important to note, however, that we were only monitoring a subset of the muscles governing hand movements, and so other unobserved muscles may be involved in these “single-muscle” synergies.
Differences in muscle synergies between individuals with and without SCI would be expected in a relatively trivial sense if some muscles were completely paralyzed and displayed no EMG activity. In our experiments, however, the SCI subjects retained a fairly high level of upper-extremity motor function (Table 2) and most often displayed muscle paresis rather than complete paralysis. During the initial manual muscle testing evaluation of each patient's motor function (Table 2), no scores of 0 were recorded in any of the muscle groups of the ISNCSCI exam. Furthermore, since all EMG signals were normalized to have unit variance, variations in muscle strength due to paresis are not expected to have a significant impact on the identified synergies, except once again in the case of complete paralysis. Synergy differences between the two groups are therefore more likely due to the effects of SCI (adaptation through central plasticity and disruptions in central or peripheral descending pathways), rather than to paralysis.
Relationship between synergies and function
The lack of a relationship between functional ability and deviations from able-bodied synergies could be due to several factors. First, compensatory movement strategies can be used to successfully accomplish tasks using alternate muscle activation patterns. Although we instructed subjects in our experiments to use particular grip types for each task to the best of their abilities, minor variations can nonetheless be expected, particularly given the redundancy of the neuromuscular system. Small compensatory changes in motor control strategies following SCI could lead to the alteration of motor synergies without having a very noticeable impact on function. In other words, differences in synergies between able-bodied subjects and subjects with SCI may be due to a different set of synergies having been learned or recruited over the time since injury, with the alternate synergies being sufficiently effective to maintain functional ability. Second, minor variations in functional ability might not have been captured by the scale used (Table 3). We chose to evaluate the function using a clinical scale because the motivation of this work was to explore the implications of the muscle synergy framework for neurorehabilitation, but it is possible that different measures of hand function might have shown a closer relationship to synergy differences. Finally, it is important to recall that although there was a certain amount of consistency in the muscle synergies of able-bodied subjects, there was also variability: the most common synergy occurred in only 7 out of 10 subjects (Table 4). Therefore, since some able-bodied subjects showed unimpaired function, but did not display the typical synergy patterns exemplified by Fig. 3, it is not surprising that some subjects with SCI also displayed high levels of function without those synergies.
Limitations
A limitation of this study is the use of surface EMG. The electrodes for FCR and ECR were relatively close to one another and, to a lesser extent, those for EDC and EIP were close to one another as well. Although electrode placement was adjusted to minimize cross-talk at the beginning of each experiment, we cannot exclude the possibility that some synergies might have been influenced by residual cross-talk. Cross-talk effects cannot account, however, for the differences observed between subjects with and without SCI, suggesting that the identified muscle synergies are genuine rather than an artifact of recording technique. Surface EMG also does not capture the behavior of deeper muscles (e.g. flexor digitorum profundus), so we cannot assess the contributions of those muscles to the synergies. Nevertheless, our data are sufficient to establish a change in synergies after SCI, which was our objective.
Another limitation is the small number of subjects with SCI, but the correlation between the quantities shown in Fig. 4 is still sufficiently low to provide confidence about the robustness of our results. The nature of our experiments restricted our SCI subject population to individuals having sustained cervical-level injuries yet retaining enough hand function to perform at least some of the tasks. Because of these requirements most of our subjects were highly functional compared to many individuals with tetraplegia, but even in this specific population muscle synergies were found to differ from those of able-bodied individuals.
The robust identification of muscle synergies requires a sufficient amount of variability in the movements being performed. Our focus on tasks relevant to ADLs may have restricted this variability, which may account for the lack of clear plateau in Fig. 2. However, because our objective was to relate the muscle synergy framework to neurorehabilitation of the hand, our choice of tasks was judged to be an acceptable compromise between variability and practical relevance, and proved sufficient to identify reasonably consistent synergies in a group of able-bodied subjects.
A final limitation to our study is that data on sensory impairment were not collected. Although we can expect that some degree of sensory impairment was present (most likely partial), the probable consequence of the missing tactile and proprioceptive information would be to alter the magnitude of the grip forces. This phenomenon has been observed in other pathologies involving sensory loss and relates to increasing the “safety margin” of the grip force (e.g. Ref.18–20). However, our analysis normalizes the EMG recordings to have unit variance, making it insensitive to magnitudes of activation. Furthermore, the tasks used did not involve any rapid or unexpected changes in load forces, and visual information was available at all times, making it likely that the control of grip for these tasks relied primarily on feed-forward mechanisms rather than on sensory feedback.21 We therefore do not expect sensory loss to have been one of the central mechanisms underlying our results.
Future work
The disruption of the muscle synergies measured by surface EMG could be due either to central disruption of the synergies themselves, or to changes in the recruitment of the synergies (i.e. their weighting, which could be altered by disruptions to the descending pathways). Although the latter alternative seems more likely in SCI, given that the brain is generally unaffected, our study was designed to identify the presence of disruptions, rather than elucidate the underlying mechanism. Future work examining how synergies change within a short time-frame after SCI, rather than chronically as studied here, may help to shed more light on the nature of the synergy adjustments: if the synergy disruptions are due to central mechanisms, then they could be expected to be smaller right after injury, before any significant central reorganization has occurred. Characterizing the time-course of synergy alterations after SCI may also provide more insights into how this type of analysis can inform the nature and timing of the rehabilitation process. A key consideration before applying this methodology to larger samples and at different time points is the need to involve individuals with a range of functional abilities, including those with insufficient hand function to perform the tasks used in the present study. To this end, tasks should be defined that focus on more proximal upper-limb function, making it possible to adapt the methodology to the level of injury of the subject.
The existence of postural synergies in hand function has been described by a number of investigators,10,22,23 but the link with the results described here is complex: hand postures are governed by biomechanical coupling as well as muscle activity, and furthermore the redundant nature of the system allows a particular posture to be produced by different patterns of muscle activation. Future work could focus on how postural rather than muscular synergies are affected by SCI, and whether these disruptions have more implications for rehabilitation interventions.
Conclusion
The goal of this study was to see whether there is a clear link between the presence of certain muscle synergies and hand function after SCI. No such link was found between functional ability and the amount of deviation from the average able-bodied synergies. The results presented here are therefore not sufficient to demonstrate how the muscle synergy framework can be used to guide new rehabilitation interventions, for the subject population examined. We did find moderate inter-subject consistency in the muscle synergies that underlie hand grips used in ADLs. Similar synergies occurred after SCI, but with different frequencies, suggesting that the disruptions and re-organizations occasioned by the injury are measurably reflected in the motor control patterns of the hand. Further study is required to understand how these re-organizations may occur after SCI.
Acknowledgements
This paper is based on the award-winning research abstract presented at the 5th National Spinal Cord Injury Conference, Toronto, Ontario, Canada 2012. This work was supported by the Sensorimotor Computation Major Thematic grant from the Peter Wall Institute for Advanced Studies. Additional support was provided by the Canada Research Chairs Program, the Human Frontier Science Program, NSERC, and CFI. The authors thank Dr Andrea d'Avella for his helpful comments on the manuscript, as well as Philippa Taylor for help with subject recruitment.
References
- 1.Snoek GJ, Ijzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004;42(9):526–32 [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21(10):1371–83 [DOI] [PubMed] [Google Scholar]
- 3.Backus D. Exploring the potential for neural recovery after incomplete tetraplegia through nonsurgical interventions. PMR 2010;(12 Suppl 2):S279–85 [DOI] [PubMed] [Google Scholar]
- 4.d'Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behaviour. Nat Neurosci 2003;6(3):300–8 [DOI] [PubMed] [Google Scholar]
- 5.Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviour. J Neurosci 2005;25(27):6419–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bizzi E, Cheung VC, d'Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev 2008;57(1):125–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overduin SA, d'Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci 2008;28(4):880–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res 1995;103(1):123–36 [DOI] [PubMed] [Google Scholar]
- 9.Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol 2000;83(3):1469–79 [DOI] [PubMed] [Google Scholar]
- 10.Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol 2004;92(1):523–35 [DOI] [PubMed] [Google Scholar]
- 11.Ajiboye AB, Weir RF. Muscle synergies as a predictive framework for the EMG patterns of new hand postures. J Neural Eng 2009;6(3):036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston JA, Bobich LR, Santello M. Coordination of intrinsic and extrinsic hand muscle activity as a function of wrist joint angle during two-digit grasping. Neurosci Lett 2010;474(2):104–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Spinal Injury Association International standards for neurological classification of spinal cord injury, revised 2011. J Spinal Cord Med 2011;34(6):535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalsi-Ryan S, Curt A, Fehlings MG, Verrier MC. Assessment of the hand in tetraplegia using the graded redefined assessment of strength, sensibility and prehension (GRASSP): impairment versus function. Top Spinal Cord Inj Rehabil 2009;14(4):34–46 [Google Scholar]
- 15.Carroll D. A quantitative test of upper extremity function. J Chronic Dis 1965;18(5):479–91 [DOI] [PubMed] [Google Scholar]
- 16.Cheung VC, d'Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviour. J Neurophysiol 2009;101(3):1235–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DD, Seung HS. Algorithms for non-negative matrix factorization. In: Leen TK, Dietterich TG, Tresp V, (eds.) Advances in neural information processing systems. Vol. 13 Cambridge, MA: MIT Press, 2001. p. 556–62 [Google Scholar]
- 18.Muller F, Dichgans J. Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Exp Brain Res 1994;101(3):485–92 [DOI] [PubMed] [Google Scholar]
- 19.Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol 2003;114(5):915–29 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Johnston JA, Ross MA, Smith AA, Coakley BJ, Gleason EA, et al. Effects of carpal tunnel syndrome on adaptation of multi-digit forces to object weight for whole-hand manipulation. PLoS ONE 2011;6(11):e27715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 2009;10(5):345–59 [DOI] [PubMed] [Google Scholar]
- 22.Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. J Neurosci 1998;18(23):10105–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latash ML, Friedman J, Kim SW, Feldman AG, Zatsiorsky VM. Prehension synergies and control with referent hand configurations. Exp Brain Res 2010;202(1):213–29 [DOI] [PMC free article] [PubMed] [Google Scholar]


