Abstract
Objective
To evaluate the effects of functional electrical stimulation (FES)-assisted walking on body composition, compared to a non-FES exercise program in individuals with a spinal cord injury (SCI).
Design
Parallel-group randomized controlled trial.
Methods
Individuals with chronic (≥18 months) incomplete SCI (level C2 to T12, AIS C or D) were recruited and randomized to FES-assisted walking (intervention), or aerobic and resistance training (control) sessions thrice-weekly for 16 weeks. Whole body and leg lean mass and whole body fat mass, measured with dual-energy X-ray absorptiometry, and lower-limb muscle cross-sectional area (CSA) and fat CSA, measured with peripheral computed tomography were assessed at baseline, 4 months, and 12 months. Intention-to-treat analyses using repeated measures general linear models were used to assess between-group differences.
Results
Thirty-four individuals were randomized (17 per group); 27 remained at 12 months. There were no significant main effects of FES-assisted walking on body composition variables in intention-to-treat analyses with group means. There was a significant group-by-time interaction for muscle area from baseline to 12 months (P = 0.04). Intention-to-treat analysis of muscle area change scores between baseline and 12 months revealed a significant difference between groups (mean (SD) muscle area change score 212 (517) mms for FES, −136 (268) mms for control, P = 0.026). There were 13 side effects or adverse events deemed related to study participation (7 intervention, 5 control); most were resolved with modifications to the protocol. One fainting episode resulted in a hospital visit and study withdrawal.
Conclusions
Thrice-weekly FES-assisted walking exercise over 4 months did not result in a change in body composition in individuals with chronic, motor incomplete C2 to T12 SCI (AIS classification C and D). However, longer-term follow-up revealed that it might maintain muscle area.
Keywords: Spinal cord injuries, Paraplegia, Tetraplegia, Electrical stimulation, Muscle, Body composition, Exercise, Rehabilitation, Physical, Neuroprostheses, Ambulation
Introduction
After spinal cord injury (SCI), there is a rapid and dramatic loss of muscle mass in the lower limbs, which may predispose individuals with SCI to impaired glucose control, pressure sores, and an increased risk of fracture. Reductions in lower-limb muscle mass or cross-sectional area (CSA) can be 12–25% in only the first 24 weeks post-injury.1,2 Fat mass in individuals with chronic SCI is increased relative to individuals without SCI, which may also contribute to an increased risk of chronic disease (e.g. diabetes, cardiovascular disease).2–4 Muscle atrophy occurs to a greater extent in complete versus incomplete SCI, although average decrements in muscle CSA of ∼25% or more have been observed when individuals with chronic, incomplete SCI were compared to controls without SCI.2–5
In individuals with SCI, functional electrical stimulation (FES) can be used: (i) to produce isometric muscle contractions for the purpose of muscle strengthening6–8; (ii) produce contractions against resistance during cycling or leg extensions6,7,9–20; (iii) as a permanent orthotic device to facilitate gait21,22 or grasping function,42 i.e. neuroprostheses for walking and grasping; and (iv) for promoting neuroplasticity and retraining voluntary grasping and walking functions,41–44 i.e. FES therapies. Despite variability in the type, intensity, duration, and frequency across the FES interventions studied to date, an improvement in muscle mass or area is a consistently observed outcome.6,14,17,19,23–25
Preliminary research suggests that combining FES with gait training may be a means to improve functional mobility.26–28 Previous applications of FES-assisted walking involved neuroprostheses, which are intended as permanent tools for assisting patients during walking.29–31 Many walking neuroprostheses stimulate the flexor withdrawal reflex via the common peroneal nerve, which produces awkward gait and can habituate over time.27 Advances in FES technologies allow FES to be applied directly to motor neurons of several muscle groups in a coordinated manner to enhance voluntary function in individuals with SCI (i.e. FES therapy), instead of substituting it, and avoiding the need for stimulation of reflex arcs.32 FES therapy is applied as a short-term therapeutic intervention with the goal of improving voluntary function, and stimulating reorganization and retraining of intact parts of the central nervous system, so that dependence on the stimulation is reduced. Therefore, in theory, individuals with incomplete SCI could practice FES walking and achieve functional improvements in walking function that would be retained after cessation of therapy. The improved muscle function and mobility should also translate to a reduction in complications associated with immobility, such as muscle atrophy, pressure sores, or bone loss. Whether FES walking can result in increases in muscle size similar to other FES-based exercise interventions, or whether muscle size is maintained or increased after cessation of training is not known. Our team conducted a randomized controlled trial with the primary aim of testing the hypothesis that the application of our FES-assisted walking protocol thrice weekly for 16 weeks could improve functional mobility in individuals with chronic motor incomplete SCI, and that it would remain improved 8 months after cessation of training. Secondary objectives of the trial were to determine if our FES-assisted walking intervention could reduce several secondary complications in individuals with chronic SCI. The current report will address one of the secondary objectives, namely does our FES-assisted walking protocol increase muscle size or reduce body fat in individuals with chronic incomplete SCI, and what is the longer-term effect of 4 months of FES once the intervention is ceased?
Methods
Study design and sample
The design was a parallel group randomized controlled trial conducted at a large SCI rehabilitation hospital. The clinicaltrials.gov registration number is NCT00201968. The study received approval from the research ethics board, and recruitment began in March 2005. The last participant completed follow-up in December 2010. All recruitment, outcome assessment, and intervention and control group activities were conducted at our centre, with the exception of computed tomography scans, which were done at another centre. Participants were individuals with chronic (>18 months), traumatic, incomplete SCI (American Spinal Injury Association Impairment Scale (AIS) C or D) from C2 to T12. Exclusion criteria were: contraindications for FES (cardiac pacemakers, skin lesions at electrode sites, or denervation of targeted muscles); a lower-extremity grade IV pressure ulcer, or a grade II or III pressure ulcers at sites that would be exposed to the harness; uncontrolled hypertension; symptomatic orthostatic hypotension when standing for 15 minutes; and unstable autonomic dysreflexia requiring medication. A letter was sent to potential participants’ family physician to inform them of their participation, with a request to provide medical clearance regarding the safety of their participation.
Randomization
Upon completion of baseline assessments, participants were randomly assigned to the intervention or control group in a 1:1 allocation ratio. The randomization sequence was generated using randperm.m function in Matlab (The MathWorks, Natwick, MA). Envelopes were prepared by a research assistant not involved in enrolling participants; each envelope contained a unique reference number. Each participant selected an unmarked, sealed envelope from a box. This reference number corresponded to another sealed envelope in a separate location that indicated group allocation for that participant.
Intervention and control group activities
Due to the nature of the treatment, it was not possible to blind the participants, so there was no placebo group. The patient's physician was blind to group allocation unless a serious adverse event occurred. Both groups received the same level of attention and were engaged in a form of physical activity for 45 minutes per session, 3 days per week, for 4 months (48 sessions total). An active control was chosen so that any intervention effects were attributed to the FES walking intervention rather than an improved fitness level. Adherence was determined by counting the number of sessions completed. Individuals who missed sessions were permitted to make them up.
Intervention group
Individuals assigned to the intervention group received FES stimulation while ambulating on a body weight support treadmill and harness system (Loko 70, Woodway USA Inc., Foster Ct, Waukesha, WI, USA). The harness was worn at all times for safety. Walking speed was chosen by the person supervising the session, with input by the participant. When necessary, manual assistance was applied by up to three assistants to the participant's lower extremities and lower back to facilitate normal gait. Whether body weight support used was patient-dependent, and varied over time, the amount of weight support used was just enough to assist the participant to achieve standing without knees buckling. The amount of body-weight support and manual assistance was progressively decreased over time with the goal of achieving no support or assistance.
FES therapy was delivered using two Compex Motion, transcutaneous electric stimulators (Compex SA, Switzerland), which used surface self-adhesive stimulation electrodes. Two stimulators worked independently, each stimulating one leg only (i.e. four electrodes from one stimulator were applied to one leg only) and were not synchronized. Instead, each stimulator behaved as an independent system controlling the gait sequence of the designated leg. The stimulators were manually triggered using a push button. The therapist activated the push button shortly after heal-off but before the toe-off phase of the gait cycle. The stimulation sequence was developed such that following the push button activation the entire stimulation sequence was delivered to the targeted muscles in an open-loop control manner. For the majority of participants the push buttons were triggered by the therapist or an assistant. In cases where a participant had good balance control and did not need to hold the handrails, they were given the option to control the stimulation when they initiated steps.
The electrodes were placed on the subject's skin at the motor points above the nerves corresponding to the muscles targeted with FES. Muscles stimulated were quadriceps (electrode size 5 × 10 cm), hamstrings (electrode size 5 × 10 cm), tibialis anterior (electrode size 2.5 × 2.5 cm) and gastrocnemius (electrode size 2.5 × 2.5 cm). The stimulation pulses used were balanced, biphasic, asymmetric, and current-regulated. Pulse-width modulation was used to regulate temporal activity of the muscles and the pulse amplitude was used to regulate muscle contraction strength. Pulse amplitudes were in the range from 8 to 125 mA (they were subject- and muscle-specific), and pulse durations were in the range of 0 to 300 µs. The pulse frequencies were from 20 to 50 Hz. A case example of the parameters: maximum pulse duration 300 µs, pulse frequency was 40 Hz, and maximum pulse amplitudes for the stimulated muscles (left leg) were: quadriceps – 40 mA, hamstrings – 46 mA, tibialis anterior – 56 mA, and gastrocnemius – 30 mA. During stimulation, the targeted muscles were stimulated bilaterally and in a physiologically correct sequence that mimicked the muscle activation sequence observed during ambulation in individuals without paralysis.
Control group
Individuals assigned to the control group participated in an individually tailored exercise program consisting of 20–25 minutes of resistance training (using hand weights, cables, and uppertone) and 20–25 minutes of aerobic training (arm cycling, leg cycling, and walking in parallel bars or on treadmill), supervised by trained kinesiologists. Two to three sets of a resistance training exercise were performed at 12–15 repetition maximum resistance for all major muscle groups that were capable of voluntary activity. The intensity was progressively increased according to tolerance. Aerobic exercise was performed at a moderate (3–5 on the Modified Borg Rating of Perceived Exertion Scale).
Outcome measures
The registered trial listed the primary outcomes as the following health complications: spasticity, muscle atrophy, and bone loss (osteoporosis): no primary outcome was identified among these complications. The current report outlines the effects of the intervention on muscle atrophy (total body fat-free mass, leg lean mass, and calf muscle CSA) and fat mass (total body fat mass and calf fat CSA). Outcomes were assessed at baseline, after 4 months of intervention/control activities, and at 12-month follow-up (8 months after intervention/control activities had ceased). Other trial outcomes (i.e. effects on indices of bone strength, spasticity, etc.) will be reported elsewhere. All technicians performing scans or analysis were blind to group allocation. Side effects of intervention/control group activities and adverse events were a secondary outcome. Participants were monitored for side effects during and in between training sessions, and were instructed to report any event to the trial coordinator. Each event was reviewed by a physician adjudicator that was not a member of the research team to determine if it was related to intervention/control activities.
Total body lean mass and fat mass, and leg lean mass (in kilograms) were evaluated using whole body dual-energy X-ray absorptiometry (DXA, Hologic QDR 4500A, software version 11.1:3, Waltham, MA, USA). Whole body scans were analysed according to the manufacturer's instructions. Measurements of muscle CSA and fat CSA were obtained at the point of largest calf cross section (66% of the tibia length, measuring upward from the distal end) using a StraTec XCT-2000 peripheral quantitative computed tomography (pQCT) scanner (STRATEC Biomedical AG, Birkenfeld, Germany). Scans were analysed with the manufacturer's software (XCT version 5.50). Contour mode 1, peel mode 2, and −100/40 mg/cm3 threshold were used in CALCBD mode to separate pixels containing muscle, bone, or skin from pixels containing adipose tissue, so that total fat CSA (mm2) could be determined. Contour mode 1 and 710 mg/cm3 threshold were used in CORTBD mode to determine the pixels belonging to bone, and finally contour mode 4 and −100/2000 mg/cm3 were used in CORTBD mode to determine the pixels belonging to skin. The bone and skin areas were then subtracted from the area containing muscle, bone, and skin to obtain total muscle CSA (mm2).
Statistical analyses
The trial reporting was done in accordance with the CONSORT criteria, and participant flow through the study was depicted using a CONSORT flow diagram (http://www.consort-statement.org/). Descriptive statistics were used to characterize participant demographic and medical history information, and all outcomes; mean (standard deviation, SD) was used for continuous variables and count (percent, %) was used for categorical variables. Sample size was determined using the outcome that was expected to demonstrate the smallest effect size for the registered trial, namely tibia cortical bone mineral density (BMD) (not reported here). In a report on FES-assisted cycling, Eser et al. reported a standard deviation of 0.03 to 0.06 g/cm3 for the tibial cortical BMD in individuals with SCI, and the estimated clinically meaningful effect was 0.6% per month (21), corresponding to a sample size of 13 per group assuming that alpha is 0.05. Our target was 17 participants per group to account for attrition. Between-group differences, differences over time, and the time by group interaction for comparisons baseline to 4 months and baseline to 12 months were analysed in an intention-to-treat analysis using a repeated measures general linear model. The distribution was tested using Mauchly's test of sphericity. If the distribution violated the assumption of sphericity the Greenhouse–Geisser correction was used. The last available observation was carried forward in the case of missing data. Participants whose data were missing at baseline (i.e. because scans could not be obtained) were excluded from the analysis for that particular variable. A per-protocol analysis was also performed. An alpha of 0.05 (two-tailed) was used for all tests. Bonferroni corrections were performed in the case of multiple comparisons so that the overall level of alpha was 0.05. SPSS version 19 (Armonk, NY, USA) was used for the analyses.
Results
Of the 34 individuals who entered the study, 27 (16 intervention, 11 control) returned for the final assessment (Fig. 1). Demographic, medical history, and impairment characteristics of participants in the intervention and control groups are presented in Table 1. There were no significant differences between groups at baseline for any of the body composition variables (Table 2). On average, fewer sessions were completed by control group participants (34.1 sessions, or 71%) than intervention group participants (42.1, or 88%). Two individuals in the control group were excluded from pQCT analyses of muscle and fat area because of lower-limb oedema or spasticity that impeded scan acquisition, and one additional person had missing data at follow-up for this reason but their data were treated as missing (last observation carried forward).
Figure 1.
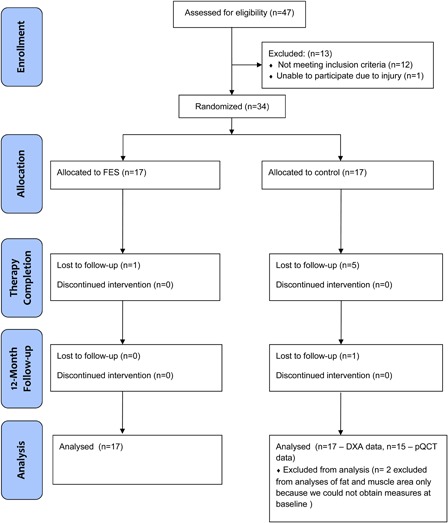
CONSORT flow diagram.
Table 1.
Participant characteristics at the start of the study
| Variable | FES (n = 17) | Control (n = 17) | Combined (n = 34) |
|---|---|---|---|
| Age (years): mean (SD) | 56.6 (14.0) | 54.1 (16.5) | 55.3 (15.1) |
| Gender: no. of males (%) | 14 (82.4) | 12 (70.6) | 26 (76.5) |
| UEMS: mean (SD) | 38.3 (7.4) | 37.5 (13.8) | 37.9 (11.0) |
| LEMS: mean (SD) | 30.4 (8.2) | 27.9 (9.8) | 29.2 (9.0) |
| Duration of injury (years): mean (SD) | 8.75 (9.7) | 10.3 (11.1) | 9.5 (10.3) |
| Tetra AIS C-D: n (%) | 14 (82.4) | 12 (70.6) | 26 (76.5) |
| Height: mean (SD) | 174.3 (7.9) | 173.6 (9.2) | 174.0 (8.4) |
| Weight: mean (SD) | 81.3 (13.1) | 90.7 (39.0) | 86.0 (26.0) |
| No. of training sessions completed: mean (SD) | 42.1 (10.7) | 34.1 (17.6) | 38.1 (14.9) |
UEMS, Upper Extremity Motor Score; LEMS, Lower Extremity Motor Score.
Table 2.
Body composition outcomes used in intention to treat analyses, reported as mean (SD), for intervention and control groups (n = 17 in each group with last observation carried forward in cases of missing data) at baseline, after 4 months of intervention/control activities and at 12-month follow-up
| Baseline |
4 months |
12 months |
||||
|---|---|---|---|---|---|---|
| FES | Control | FES | Control | FES | Control | |
| Calf muscle CSA (mm2) | 7017 (1019) | 6763* (1642) | 7234 (1110) | 6707* (1629) | 7229 (959) | 6627** (1635) |
| Calf fat CSA (mm2) | 2917 (1506) | 2784* (1719) | 2824 (1517) | 2809* (1712) | 2914 (1507) | 2751** (1666) |
| Total body fat mass (kg) | 25.4 (9.5) | 23.2 (10.8) | 24.3 (9.5) | 23.0 (10.7) | 25.2 (9.0) | 23.3 (11.1) |
| Leg lean mass (kg) | 14.9 (5.7) | 16.3 (3.5) | 16.2 (4.8) | 16.6 (3.9) | 16.1 (4.8) | 16.4 (4.3) |
*Two pQCT scans were not performed at this site due to spasms or oedema, so n is 2 less than noted.
**Three pQCT scans were not performed at this site due to spasms or oedema, so n is 3 less than noted.
In intention-to-treat analyses, FES-assisted walking had no significant effect on lower-limb muscle CSA; there was no significant change over time and there were no differences between the groups (Table 2). The interaction was significant (P = 0.04); the control group appears to be losing muscle area over time, whereas the FES group appears to be gaining muscle area over time (Fig. 2). However, neither the between-group differences nor the within-group changes over time were statistically significant when group means were compared for either time point. Intention-to-treat analyses using change scores revealed a significant effect of FES on muscle area after 12 months (mean (SD) change score 212 (517) mms for FES, −136 (268) mms for control, P = 0.026), but not after 4 months (mean (SD) change score 289 (465) mms for FES, −525 (1811) mms for control, P = 0.083). Per-protocol analyses were generally consistent with intention-to-treat analyses. In per-protocol analyses using group means, the group-by-time interaction was significant after 4-month follow-up (P = 0.049) and after 12-month follow-up (0.046) but there was no significant between-group difference after 4-month (P = 0.193), or 12-month (P = 0.168) follow-up. If change scores were used, there was a significant main effect of FES on muscle area at 12 months (P = 0.046). Individual data for muscle area are presented in Fig. 3.
Figure 2.
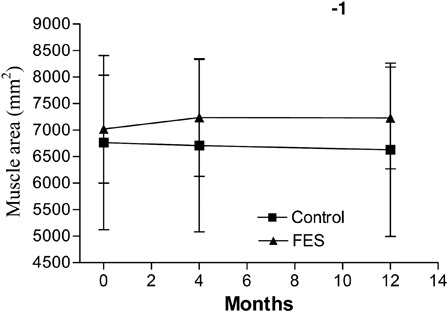
Mean muscle area (mm2) at baseline, and at 4- and 12-month follow-ups, with standard deviations noted using error bars. Missing data were replaced using last observation carried forward; therefore, n = 17 for FES and n = 15 for control groups.
Figure 3.
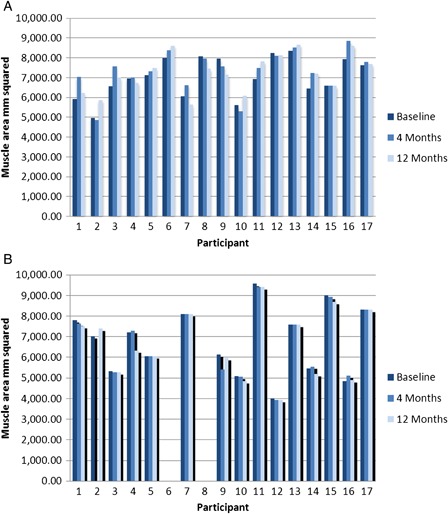
Individual participant data for muscle area (mm2) at baseline, 4-month follow-up and 12-month follow-up for those with available data at baseline, missing data at 4 months and 12 months is replaced using last observation carried forward: (A) FES group (n = 17); (B) Control group (n = 15).
There was no effect of FES-assisted walking on fat CSA in the lower limb; there was no significant change over time, there was no difference between the groups and the interaction was not significant. For total body lean mass, leg lean mass and total body fat mass the changes over time were not significant in intention-to-treat analyses. The difference between the groups was not significant, nor was the group by time interaction in intention-to-treat analyses or per-protocol analyses.
Adverse events and side effects are summarized in Table 3. One individual in the control group experienced fainting/loss of consciousness on his first visit that was deemed due to exercise participation; he was sent to the emergency room. For his safety, he was subsequently sent for a stress test and the attending physician recommended that he be withdrawn from the study. Four additional events occurring in the control group were deemed to be related to exercise participation but did not result in withdrawals: pectoral muscle strain; swollen knees; left elbow pain; and ringing in ears with dizziness. Seven of the events in the intervention group were deemed to be related to exercise participation, but did not result in withdrawals: bruising or blistering in the groin area (two incidents, due to harness placement – resolved with change in placement and padding); loss of footing on the treadmill, no fall; fall on treadmill; sharp pain in left heel/ankle; and pain and discomfort in the hip/groin area.
Table 3.
Body composition outcomes used per-protocol analyses, reported as mean (SD), for intervention and control groups at baseline, after 4 months of intervention/control activities and at 12-month follow-up
| Baseline |
4 months |
12 months |
||||
|---|---|---|---|---|---|---|
| FES (n = 17) | Control (n = 17) | FES (n = 16) | Control (n = 12) | FES (n = 16) | Control (n = 11) | |
| Calf muscle CSA (mm2) | 7017 (1019) | 6763* (1642) | 7275 (1133) | 6356* (1831) | 7269 (975) | 6349** (1405) |
| Calf fat CSA (mm2) | 2917 (1506) | 2784* (1719) | 2831 (1567) | 3196* (1934) | 2929 (1555) | 3325** (1896) |
| Total body fat mass (kg) | 25.4 (9.7) | 23.2 (10.8) | 24.1 (9.7) | 24.0 (12.7) | 24.9 (9.3) | 26.2 (11.3) |
| Leg lean mass (kg) | 14.9 (5.8) | 16.3 (3.5) | 17.1 (3.1) | 16.0 (4.4) | 17.0 (3.1) | 16.1 (4.7) |
*Two pQCT scans were not performed at this site due to spasms or oedema, so n is 2 less than noted.
**Three pQCT scans were not performed at this site due to spasms or oedema, so n is 3 less than noted.
Discussion
The key message of our study is that FES-assisted walking may not increase muscle size or reduce total body fat mass in the short term, but it may help prevent muscle loss in the long term, even after the training has ceased. The strengths of our study include a randomized controlled trial design with arguably the largest sample size to date for this type of trial, and a 12-month follow-up to evaluate further changes or maintenance of effects, with relatively good retention. There were no significant between-group differences in any body composition measure between FES and control groups after 4 months of training. Our finding that FES appeared to better preserve muscle area 8 months after training had ceased when compared with a standard exercise program is promising, but should be interpreted with caution given the substantial variability across participants and the disproportionate loss to follow-up in the control group.
The bulk of the literature to date on muscle and fat adaptations to FES consists of case series reporting positive benefits of FES training on muscle mass or CSA, with less conclusive evidence that it can reduce body fat.7,8,16,18,19,23–25,33,34 Only one other randomized trial of FES training in the lower extremities of individuals with SCI has been reported; FES-assisted quadriceps strength training over 8 weeks (n = 10 per group) demonstrated small increases in strength, but the authors speculated that they may not be large enough to be clinically important.35 Knowledge syntheses and physical activity guidelines promote FES as a tool for increasing physical activity based on health-related benefits, including improved body composition.36–38 FES has the added theoretical benefit of improving function, and patients and their families often have high expectations of FES therapy. It is critical to establish realistic expectations regarding the effects of FES in all of its forms using best evidence, so that users are not disappointed and cease efforts to exercise all together. It is also equally important to report the potential harms. Adverse events are infrequently reported, and do occur; we reported several side effects and one hospital visit directly attributable to exercise, and a case report of a fracture during FES has been reported previously.39 The hospital visit was a result of orthostatic hypotension and a fall that occurred during the first session; it resulted in additional assessment and withdrawal from the study, speaking to the need for rigorous screening of individuals with SCI prior to initiating exercise. Future studies should systematically collect these data and report them as outcomes to enhance awareness and efforts to prevent them. The findings of the current study are in contrast with previous reports and convention that FES-training in general results in significant, long-term increases in muscle size or reduced fat mass.
Although FES-assisted walking is functionally relevant, our intervention may not have created the right kind of stimulus or implemented it long enough to see appreciable changes in muscle size in the short term. FES-assisted walking may promote muscular endurance, but may not induce large-enough loads to stimulate muscle hypertrophy. Reports of increased muscle size after FES-training have typically implemented resistance training regimens aimed at increasing strength, or FES-cycling, which often requires moderate-to-high force production.7,18,19,23,25,33,34,35 Our intervention may also have been too short to demonstrate a noticeable effect of FES-assisted walking on muscle size. Although a few case series have reported increases in muscle size after 8 weeks of intervention,7,12,14 case reports of improved body composition after 6 or 12 months of FES training are more credible.16,19,33 It is expected that increases in strength would precede increases in size, and small increases in strength have been observed after 8 weeks of FES-induced quadriceps training in individuals with SCI.35 However, the intent was to investigate whether a short-term intervention could improve walking function, and whether improvements in walking function would lead to longer-term benefits, such as improved body composition. Therefore, if our hypotheses had been correct we should have observed differences at the 12-month follow-up. Although we did not observe significant group differences when group means were compared, the significant group-by-time interaction and the large variability in muscle area across subjects at baseline prompted an analysis of change scores, which revealed a significant between-group difference in favour of FES after 12 months. These findings should be interpreted with caution given the large variability across participants and because of attrition, which was greater in the control group. The group-by time interaction, graphically depicted, and the analysis of change scores suggest that muscle area in the FES group was changing in a positive direction whereas it was moving in a negative direction for the control group. It is possible that the changes were not large enough, or the variability was too great to observe a significant between-group difference. Variability across SCI participants in outcomes is often inherent in research given the diversity in participant characteristics such as age, severity and level of injury, or gender. Ideally, we would recruit or stratify based on potential confounders or effect modifiers, but the difficulty in recruiting large numbers of individuals with SCI make it impossible. It is also possible that between-group differences were diminished because we used an active control group, or because the characteristics of our intervention were distinctly different than other studies that have shown benefit. It is also plausible that the differences are at least in part attributable to differences in trial design and the high risk of bias in previously published case series. There is a need for large, well-designed trials to verify if indeed muscle area decreases over time in individuals with chronic SCI and if FES can prevent or reverse these changes.
We acknowledge that our conclusions are limited by several factors. We were not able to control for lifestyle factors, which are important determinants of body composition. We did not measure or control diet in our participants, which could have added variability in the estimate of effect. Our methodology was designed in 2004, when initiatives aimed at improving the conduct and reporting of clinical trials, such as CONSORT (http://www.consort-statement.org/), were less widely known. Therefore, although our design is likely more rigorous than many previous studies, there is room for improvement. For example, central randomization is a preferred method over sealed envelopes. We did not pre-specify one primary outcome. A few instances of missing data for our pQCT outcomes may have limited our ability to detect true differences. However, the bias that these limitations would have introduced would have been more likely to sway the findings to a positive effect of our intervention.
Conclusion
In summary, a 4-month FES-assisted walking intervention did not result in a significant increase in muscle mass or CSA, nor did it reduce body fat compared to a standard exercise program. Our data suggest that FES may result in long-term preservation of muscle when compared with exercise, but these findings should be interpreted with caution given the loss-to-follow-up that was higher in the control group and the variability across participants in muscle area. Our data support the need for a larger trials to confirm if indeed individuals with chronic SCI lose muscle over time and if FES might prevent or reverse this. There is a need for international multicentre collaboration to achieve rigorous randomized controlled trials to establish the efficacy of FES-training with respect to functional and health-related benefits for individuals with SCI.
Acknowledgements
This article is based on the presentation at the 5th National SCI Conference in Toronto, Ontario, Canada in October 2012. The Ontario Neurotrauma Foundation funded the clinical trial. We thank Lesley Beaumont and Andrea Brown for assistance with outcome assessment, Kent Campbell for assistance with statistical analysis, and the participants for their dedication to the research.
References
- 1.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross- sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 1999;80(4):373–8 [DOI] [PubMed] [Google Scholar]
- 2.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 1995;33(11):674–7 [DOI] [PubMed] [Google Scholar]
- 3.Nuhlicek DN, Spurr GB, Barboriak JJ, Rooney CB, el Ghatit AZ, Bongard RD. Body composition of patients with spinal cord injury. Eur J Clin Nutr 1988;42(9):765–73 [PubMed] [Google Scholar]
- 4.Sedlock DA, Laventure SJ. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia 1990;28(7):448–54 [DOI] [PubMed] [Google Scholar]
- 5.Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC, et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 2006;87(6):772–8 [DOI] [PubMed] [Google Scholar]
- 6.Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998;36(7):463–9 [DOI] [PubMed] [Google Scholar]
- 7.Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 1999;80(4):394–6 [DOI] [PubMed] [Google Scholar]
- 8.Kagaya H, Shimada Y, Sato K, Sato M. Changes in muscle force following therapeutic electrical stimulation in patients with complete paraplegia. Paraplegia 1996;34(1):24–9 [DOI] [PubMed] [Google Scholar]
- 9.BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil 1996;75(1):29–34 [DOI] [PubMed] [Google Scholar]
- 10.Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 2000;81(8):1090–8 [DOI] [PubMed] [Google Scholar]
- 11.Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 1996;19(1):61–8 [DOI] [PubMed] [Google Scholar]
- 12.Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord 1999;37(4):264–8 [DOI] [PubMed] [Google Scholar]
- 13.Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev 1994;31(1):50–61 [PubMed] [Google Scholar]
- 14.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997;273(3 Pt 2):R1072–9 [DOI] [PubMed] [Google Scholar]
- 15.Leeds EM, Klose KJ, Ganz W, Serafini A, Green BA. Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil 1990;71(3):207–9 [PubMed] [Google Scholar]
- 16.Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 1997;35(1):1–16 [DOI] [PubMed] [Google Scholar]
- 17.Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci (Lond) 1988;75(5):481–7 [DOI] [PubMed] [Google Scholar]
- 18.Ragnarsson KT. Physiologic effects of functional electrical stimulation-induced exercises in spinal cord-injured individuals. Clin Orthop 1988;(233):53–63. http://www.ncbi.nlm.nih.gov/pubmed/3261220. [PubMed] [Google Scholar]
- 19.Skold C, Lonn L, Harms-Ringdahl K, Hultling C, Levi R, Nash M, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med 2002;34(1):25–32 [DOI] [PubMed] [Google Scholar]
- 20.Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest 2003;33(5):412–9 [DOI] [PubMed] [Google Scholar]
- 21.Gallien P, Brissot R, Eyssette M, Tell L, Barat M, Wiart L, et al. Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia 1995;33(11):660–4 [DOI] [PubMed] [Google Scholar]
- 22.Shimada Y, Sato K, Abe E, Kagaya H, Ebata K, Oba M, et al. Clinical experience of functional electrical stimulation in complete paraplegia. Spinal Cord 1996;34(10):615–9 [DOI] [PubMed] [Google Scholar]
- 23.Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D, Jr, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86(7):1502–4 [DOI] [PubMed] [Google Scholar]
- 24.Rodgers MM, Glaser RM, Figoni SF, Hooker SP, Ezenwa BN, Collins SR, et al. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev 1991;28(4):19–26 [DOI] [PubMed] [Google Scholar]
- 25.Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil 1999;80(12):1531–6 [DOI] [PubMed] [Google Scholar]
- 26.Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Brain Res Rev 2002;40(1–3):274–91 [DOI] [PubMed] [Google Scholar]
- 27.Ladouceur M, Barbeau H. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: longitudinal changes in maximal overground walking speed. Scand J Rehabil Med 2000;32(1):28–36 [DOI] [PubMed] [Google Scholar]
- 28.Ladouceur M, Barbeau H. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: changes in the kinematics and physiological cost of overground walking. Scand J Rehabil Med 2000;32(2):72–9 [DOI] [PubMed] [Google Scholar]
- 29.Graupe D, Davis R, Kordylewski H, Kohn KH. Ambulation by traumatic T4–12 paraplegics using functional neuromuscular stimulation. Crit Rev Neurosurg 1998;8(4):221–31 [DOI] [PubMed] [Google Scholar]
- 30.Smith B, Peckham PH, Keith MW, Roscoe DD. An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomed Eng 1987;34(7):499–508 [DOI] [PubMed] [Google Scholar]
- 31.Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil 1999;80(12):1577–83 [DOI] [PubMed] [Google Scholar]
- 32.Popovic MR, Curt A, Keller T, Dietz V. Functional electrical stimulation for grasping and walking: indications and limitations. Spinal Cord 2001;39(8):403–12 [DOI] [PubMed] [Google Scholar]
- 33.Ashe MC, Eng JJ, Krassioukov AV, Warburton DE, Hung C, Tawashy A. Response to functional electrical stimulation cycling in women with spinal cord injuries using dual-energy X-ray absorptiometry and peripheral quantitative computed tomography: a case series. J Spinal Cord Med 2010;33(1):68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22 [DOI] [PubMed] [Google Scholar]
- 35.Harvey LA, Fornusek C, Bowden JL, Pontifex N, Glinsky J, Middleton JW, et al. Electrical stimulation plus progressive resistance training for leg strength in spinal cord injury: a randomized controlled trial. Spinal Cord 2010;48(7):570–5 [DOI] [PubMed] [Google Scholar]
- 36.Stein RB, Chong SL, James KB, Kido A, Bell GJ, Tubman LA, et al. Electrical stimulation for therapy and mobility after spinal cord injury. Prog Brain Res 2002;137:27–34 [DOI] [PubMed] [Google Scholar]
- 37.Martin Ginis KA, Hicks AL, Latimer AE, Warburton DER, Bourne C, Ditor DS, et al. Physical activity guidelines for adults with spinal cord injury. 2008. Available from: http://www.sciactioncanada.ca/guidelines/ [DOI] [PubMed]
- 38.Wolfe DL, Martin Ginis KA, Latimer AE, Foulon BL, Eng JJ, Hicks A, et al. Physical activity and spinal cord Injury. 2010. Available from: http://www.scireproject.com/rehabilitation-evidence/physical-activity/effects-of-physical-activity .
- 39.Hartkopp A, Murphy RJ, Mohr T, Kjaer M, Biering-Sørensen F. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Arch Phys Med Rehabil 1998;79(9):1133–6 [DOI] [PubMed] [Google Scholar]
- 40.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng 2005;7:327–60. [DOI] [PubMed] [Google Scholar]
- 41.Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006;44(3):143–51 [DOI] [PubMed] [Google Scholar]
- 42.Popovic MR, Kapadia NM, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011;25(5):433–42 [DOI] [PubMed] [Google Scholar]
- 43.Kapadia NM, Zivanovic V, Furlan J, Craven BC, McGillivray C, Popovic MR. Toronto Rehabilitation Institute's functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs 2011;35(3):2126. [DOI] [PubMed] [Google Scholar]
- 44.Thrasher TA, Flett HM, Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord 2006;44(6):357–61 [DOI] [PubMed] [Google Scholar]


