Abstract
BACKGROUND
We previously reported early results of a randomized trial of whole-body hypothermia for neonatal hypoxic–ischemic encephalopathy showing a significant reduction in the rate of death or moderate or severe disability at 18 to 22 months of age. Long-term outcomes are now available.
METHODS
In the original trial, we assigned infants with moderate or severe encephalopathy to usual care (the control group) or whole-body cooling to an esophageal temperature of 33.5°C for 72 hours, followed by slow rewarming (the hypothermia group). We evaluated cognitive, attention and executive, and visuospatial function; neurologic outcomes; and physical and psychosocial health among participants at 6 to 7 years of age. The primary outcome of the present analyses was death or an IQ score below 70.
RESULTS
Of the 208 trial participants, primary outcome data were available for 190. Of the 97 children in the hypothermia group and the 93 children in the control group, death or an IQ score below 70 occurred in 46 (47%) and 58 (62%), respectively (P = 0.06); death occurred in 27 (28%) and 41 (44%) (P = 0.04); and death or severe disability occurred in 38 (41%) and 53 (60%) (P = 0.03). Other outcome data were available for the 122 surviving children, 70 in the hypothermia group and 52 in the control group. Moderate or severe disability occurred in 24 of 69 children (35%) and 19 of 50 children (38%), respectively (P = 0.87). Attention–executive dysfunction occurred in 4% and 13%, respectively, of children receiving hypothermia and those receiving usual care (P = 0.19), and visuospatial dysfunction occurred in 4% and 3% (P = 0.80).
CONCLUSIONS
The rate of the combined end point of death or an IQ score of less than 70 at 6 to 7 years of age was lower among children undergoing whole-body hypothermia than among those undergoing usual care, but the differences were not significant. However, hypothermia resulted in lower death rates and did not increase rates of severe disability among survivors. (Funded by the National Institutes of Health and the Eunice Kennedy Shriver NICHD Neonatal Research Network; ClinicalTrials.gov number, NCT00005772.)
Moderate or severe neonatal hypoxic–ischemic encephalopathy is associated with a high incidence of death or motor and sensory disability in children.1-5 Children with encephalopathy are at risk for cognitive deficits even in the absence of functional deficits. Survivors without disability have delayed entry into primary school and fine-motor dysfunction and behavioral abnormalities.
Hypothermia to 33 to 34°C for 72 hours, when initiated within 6 hours after birth among infants of more than 35 weeks’ gestational age with hypoxic–ischemic encephalopathy, has been shown to reduce the risk of death or disability and increase the rate of survival free of disability at 18 to 24 months of age.6-12 In the earlier report of our randomized, controlled trial of whole-body hypothermia for neonatal hypoxic–ischemic encephalopathy,7 the rate of death or moderate or severe disability at 18 to 22 months of age was 62% in the control group versus 44% in the hypothermia group (P = 0.01), with mortality of 37% and 24%, respectively (P = 0.08). As compared with the control group, there was no significant increase in major disability among survivors in the hypothermia group; the rates of moderate or severe cerebral palsy were 30% in the control group versus 19% in the hypothermia group, with corresponding rates of blindness of 14% versus 7% and hearing impairment of 6% versus 4%. However, longer-term data have not been available to assess whether the benefits of hypothermia for neonatal hypoxic–ischemic encephalopathy persist after 2 years of age.
We designed the present study to assess rates of death, cognitive impairment, and other neurodevelopmental and behavioral outcomes associated with whole-body hypothermia at 6 to 7 years of age, at which time outcomes of neonatal interventions are believed to be more definitive.
Methods
Study Conduct
Data were collected at participating sites of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) and were transmitted to the Research Triangle Institute, the data coordinating center for the NRN, which stored, managed, and analyzed the data for this study. The principal investigators at the participating sites (see the Supplementary Appendix, available with the full text of this article at NEJM.org) are responsible for the integrity of the data, and the data center is responsible for the accuracy of the data analysis. The first author wrote the manuscript, with input from a subcommittee of the authors. The other authors reviewed the manuscript and provided input. All the authors made the decision to submit the manuscript for publication.
Study Design
Participants were recruited between July 2000 and May 2003. Infants were eligible if they had either moderate or severe encephalopathy within 6 hours after birth, with severe acidosis or resuscitation at birth after an acute perinatal event. Infants were randomly assigned either to undergo whole-body hypothermia at 33.5°C for 72 hours or to usual care. All surviving children were evaluated at 6 to 7 years of age; the families of those who did not return for follow-up were contacted by telephone to obtain information about the primary outcome. The protocol, available at NEJM.org, was approved by the institutional review board at each site, and written informed consent was obtained from the parents of all trial participants.
Study Outcomes
A training session for research personnel was held to standardize all study procedures. The primary outcome of this study was death or an IQ score below 70 at 6 to 7 years of age. The secondary outcomes included death or severe disability, components of disability, motor function, higher cognitive function, severe cognitive delay, and psychosocial health.
Cerebral palsy was classified on the basis of the Surveillance of Cerebral Palsy in Europe (SCPE) Network decision tree.13 Functional activity was graded according to the Gross Motor Function Classification System14 (GMFCS) levels: I, walking without restriction; II, walking without an assistive mobility device; III, walking with an assistive device; IV, self-mobility with limitations; and V, severe limitation of mobility.
IQ scores were measured with the use of the Wechsler Preschool and Primary Scale of Intelligence III in the 96 children at or under 7 years 3 months of age and the Wechsler Intelligence Scale for Children IV in the 18 older children. These two tests address verbal comprehension, perceptual organization, and processing speed, yielding verbal and performance IQ scores that are combined to give a full-scale IQ score; a mean (±SD) score of 100±15 is normal. Higher cognitive function (i.e., attention and executive function and visuospatial processing) were evaluated by means of the Developmental Neuropsychological Assessment (NEPSY), on which a score of 100±15 is normal. The Child Health Questionnaire, which has good psychometric properties for children with and those without cerebral palsy,15 was completed by the parents to evaluate the physical, emotional, and social well-being of the study children and to assess the effect of the child’s health on the parents. All IQ and psychometric assessments were performed by examiners unaware of the assignment status of the children.
Severe disability was defined as an IQ score more than 3 SD below the mean score (i.e., <55), a GMFCS level of IV or V, or bilateral blindness. Moderate disability was defined as an IQ score 2 to 3 SD below the mean score (i.e., 55 to 69), a GMFCS level of III, bilateral deafness (with or without amplification), or refractory epilepsy (defined as clinical or electroencephalographic seizure disorder requiring anticonvulsant therapy). Mild disability was defined as an IQ score 1 to 2 SD below the mean score (i.e., 70 to 84) or a GMFCS level of I or II. No disability was defined as an IQ score of more than 84 (i.e., >1 SD below the mean) with no cerebral palsy, hearing or visual deficits, or epilepsy. Among nondisabled survivors, the detailed neurologic examination assessed everyday motor function (ability to walk), complex motor function (heel-to-toe test, ability to hop and stand on one foot, or Romberg’s test), and fine-motor-function tests of coordination (including finger-to-nose test, rapid alternation of hands, thumb–index finger apposition, thumb–four fingers apposition sequentially, heel-to-shin test, and foot tapping).
Statistical Analysis
The baseline characteristics at the time of randomization were compared between the children for whom data were and those for whom data were not available for the primary outcome at 6 to 7 years of age. The primary and secondary outcome data were analyzed for differences between the two groups, including the use of robust Poisson regression to produce relative risk estimates for treatment effect with adjustment according to center. The primary outcome was also examined after adjustment for severity of encephalopathy at baseline in addition to center. Multinomial cumulative logistic regression was used to produce adjusted P values for ordinal outcomes: level of disability among survivors, physical health scores, and scores reflecting emotional impact on parents. Some secondary outcomes could not be adjusted according to center because of their low prevalence. These unadjusted results were analyzed by means of robust Poisson regression or Fisher’s exact test for categorical variables (motor-function scores among nondisabled survivors) and Cochran–Armitage trend tests for ordinal variables (child’s self-esteem level).
All reported P values are two-sided and were not adjusted for multiple comparisons. The concordance between the degree of disability at 18 to 22 months and 6 to 7 years was assessed with the use of analyses of sensitivity, specificity, and positive and negative predictive values.
Results
Study Participants
Our study involved 208 infants with moderate or severe encephalopathy who were randomly assigned at less than 6 hours of age to undergo hypothermia (102 infants) or usual care (106 infants). The follow-up visits at 6 to 7 years of age were conducted between August 2006 and August 2010. The median age at follow-up was 6.7 years in the hypothermia group and 6.8 years in the control group.
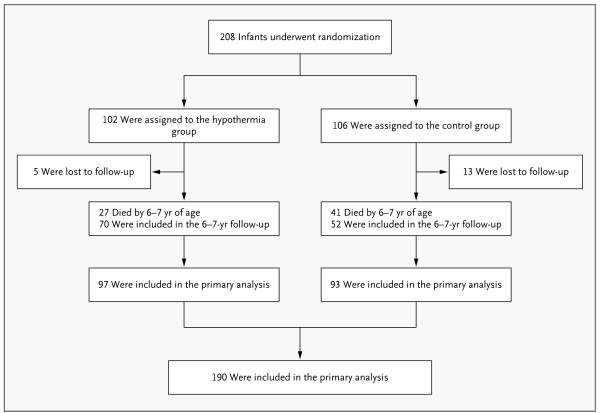
Primary outcome data were available for 190 of the 208 children (91%) enrolled in the trial: 97 of the 102 (95%) in the hypothermia group and 93 of the 106 (88%) in the control group (Fig. 1). A total of 27 children in the hypothermia group and 41 in the control group died before reaching the 6-to-7-year visit; 3 in each group had died after the 18-month visit. Five children in the hypothermia group were lost to follow-up (2 with moderate and 2 with severe hypoxic–ischemic encephalopathy, as well as 1 with seizures without a neurologic examination), as were 13 children in the control group (12 with moderate hypoxic–ischemic encephalopathy and 1 with severe hypoxic–ischemic encephalopathy).
Figure 1.
Enrollment, Randomization, and Follow-up of the Study Participants.
The baseline characteristics of the 190 children with primary outcome data were similar to those of the 18 without primary outcome data (Table 1 in the Supplementary Appendix), except that mothers of children with primary outcome data were more likely to be white (37% vs. 11%) and married (59% vs. 29%, P = 0.02) and, at birth, the children were more likely to have a lower umbilical cord pH (6.8±0.2 vs. 7.0±0.2, P = 0.05). Likewise, the baseline characteristics were similar between the 97 children in the hypothermia group and the 93 in the control group with primary outcome data, except that the frequency of maternal antepartum hemorrhage was lower in the hypothermia group (9% vs. 20%, P = 0.04) (Table 1).
Table 1.
Maternal and Neonatal Baseline Characteristics, According to Treatment Group.*
| Characteristic | Hypothermia (N = 97) |
Control (N = 93) |
|---|---|---|
| Maternal | ||
| Race — no. (%)† | ||
| Black | 31 (32) | 35 (38) |
| White | 39 (40) | 31 (33) |
| Age — yr | 27±6 | 27±6 |
| Education less than high school — no. (%)‡ | 20 (21) | 27 (29) |
| Complications of pregnancy — no. (%) | ||
| Chronic hypertension | 11 (11) | 12 (13) |
| Antepartum hemorrhage§ | 9 (9) | 19 (20) |
| Intrapartum complications — no. (%) | ||
| Fetal heart-rate deceleration¶ | 69 (71) | 68 (74) |
| Cord prolapse | 22 (23) | 13 (14) |
| Uterine rupture | 16 (16) | 11 (12) |
| Shoulder dystocia | 11 (11) | 7 (8) |
| Maternal hemorrhage | 6 (6) | 8 (9) |
| Emergency cesarean delivery — no. (%) | 68 (70) | 72 (77) |
| Neonatal | ||
| Age at randomization — hr | 4.3±1.3 | 4.3±1.2 |
| Transferred from another hospital — no. (%) | 45 (46) | 42 (45) |
| Male sex — no. (%) | 48 (49) | 58 (62) |
| Apgar score ≤5 — no. (%)∥ | ||
| At 5 min | 87 (91) | 86 (92) |
| At 10 min | 76 (84) | 66 (79) |
| Birth weight — g | 3391±620 | 3358±587 |
| Intubation in the delivery room — no. (%) | 93 (96) | 86 (92) |
| Continued resuscitation at 10 min — no. (%) | 90 (93) | 88 (95) |
| Cord-blood pH | 6.9±0.2 | 6.8±0.2 |
| Base deficit — mmol/liter | 18.5±6.8 | 20.5±8.6 |
| Seizure — no. (%) | 41 (42) | 48 (52) |
| Encephalopathy — no. (%) | ||
| Moderate | 67 (69) | 53 (57) |
| Severe | 30 (31) | 40 (43) |
| Use of anticonvulsant agent — no. (%)** | 39 (43) | 39 (48) |
Plus–minus values are means ±SD. Percentages are based on the numbers of mothers or children for whom data were available. Baseline characteristics did not differ significantly between the groups, except as noted.
Maternal race was self-reported.
Data were missing for 24 mothers in the hypothermia group and 27 in the control group.
P = 0.04.
Data were missing for 1 infant in the control group.
The Apgar score at 5 minutes was missing for 1 infant in the hypothermia group; the Apgar score at 10 minutes was missing for 7 infants in the hypothermia group and 9 in the control group.
Data were missing for 7 infants in the hypothermia group and 11 in the control group.
Study Outcomes
The primary outcome of death or an IQ score below 70 at 6 to 7 years of age occurred in 46 of the 97 children (47%) in the hypothermia group and 58 of the 93 (62%) in the control group (relative risk after adjustment according to center, 0.78; 95% confidence interval [CI], 0.61 to 1.01) (Table 2). The relative risk for death or an IQ score below 70, after adjustment also for the degree of severity of encephalopathy at randomization, was 0.84 (95% CI, 0.66 to 1.07). There were a total of 27 deaths in the hypothermia group and 41 in the control group (relative risk after adjustment according to center, 0.66; 95% CI, 0.45 to 0.97). Among the 122 survivors, 19 of 70 children (27%) in the hypothermia group and 17 of 52 (33%) in the control group had an IQ score below 70 (unadjusted relative risk, 0.83; 95% CI, 0.48 to 1.44). The mean IQ scores were 82 and 75 (P = 0.22), respectively, with median values of 84 and 81 (P = 0.70), respectively. Primary outcome data stratified according to the initial severity of encephalopathy (moderate or severe) are shown in Table 2.
Table 2.
Relative Risks of Outcomes with Hypothermia versus Usual Care Among Study Children at 6 to 7 Years of Age.*
| Variable | Hypothermia (N = 97) |
Control (N = 93) |
Relative Risk (95% CI) |
P Value |
|---|---|---|---|---|
| no./total no. (%) | ||||
| Death or IQ score <70 (primary outcome) | 46/97 (47) | 58/93 (62) | 0.78 (0.61–1.01) | 0.06 |
| Among children with moderate hypoxic–ischemic encephalopathy† |
22/67 (33) | 25/53 (47) | 0.70 (0.45–1.09) | 0.11 |
| Among children with severe hypoxic–ischemic encephalopathy† |
24/30 (80) | 33/40 (82) | 0.97 (0.77–1.22) | 0.79 |
| Death‡ | 27/97 (28) | 41/93 (44) | 0.66 (0.45–0.97) | 0.04 |
| Death or moderate or severe disability | 51/97 (53) | 60/93 (65) | 0.84 (0.66–1.06) | 0.14 |
| Death or severe disability | 38/93 (41) | 53/89 (60) | 0.72 (0.54–0.97) | 0.03 |
| Death or IQ score <55 | 38/93 (41) | 53/89 (60) | 0.72 (0.54–0.97) | 0.03 |
| Death or cerebral palsy | 39/96 (41) | 56/93 (60) | 0.71 (0.54–0.95) | 0.02 |
| Survival | 70/97 (72) | 52/93 (56) | ||
| IQ score <70 among survivors† | 19/70 (27) | 17/52 (33) | 0.83 (0.48–1.44) | 0.51 |
| Attention and executive function score <70 among survivors† |
2/48 (4) | 4/32 (13) | 0.33 (0.06–1.71) | 0.19 |
| Visuospatial score <70† | 2/53 (4) | 1/36 (3) | 1.36 (0.13–14.4) | 0.80 |
| Level of disability among survivors | 0.87 | |||
| Moderate or severe disability | 24/69 (35) | 19/50 (38) | ||
| Mild disability | 17/69 (25) | 10/50 (20) | ||
| None | 28/69 (41) | 21/50 (42) | ||
| Cerebral palsy† | 12/69 (17) | 15/52 (29) | 0.60 (0.31–1.18) | 0.14 |
| Hearing impairment† | 3/63 (5) | 1/50 (2) | 2.38 (0.26–22.2) | 0.45 |
| Bilateral blindness† | 1/67 (1) | 2/50 (4) | 0.37 (0.03–4.00) | 0.42 |
| Seizures† | 7/67 (10) | 8/50 (16) | 0.65 (0.25–1.68) | 0.38 |
| Motor-skill abnormalities among nondisabled survivors | 0.93 | |||
| Every day | 0/27 | 1/21 (5) | ||
| Complex | 3/27 (11) | 2/21 (10) | ||
| Fine | 3/27 (11) | 2/21 (10) | ||
| None | 21/27 (78) | 16/21 (76) | ||
For IQ scores, the Spanish version of the Wechsler Intelligence Scale for Children IV was administered to 3 of the 18 children assessed by means of this tool. The P value for level of disability among survivors was calculated with the use of multinomial cumulative logistic regression with adjustment for center. The P value for motor-skill abnormalities was calculated with the use of Fisher’s exact test.
Results for relative risk were not adjusted for center.
In the hypothermia group, there were 24 deaths before and 3 after 18 months, and in the control group, 38 deaths before and 3 after 18 months.
The rates of the secondary composite outcomes of death or severe disability and of death or cerebral palsy were significantly lower in the hypothermia group than in the control group. Among survivors at 6 to 7 years, in the hypothermia group and control group, the rates of cerebral palsy were 17% and 29%, respectively, the rates of blindness were 1% and 4%, and the rates of hearing impairment (requiring aids) were 5% and 2% (unadjusted P value for all comparisons, >0.05). The proportion of children with attention and executive function scores or visuospatial scores below 70 also did not differ significantly between the two groups.
There were no significant between-group differences in the level of disability among all survivors or in motor function among the 49 nondisabled children. There were also no significant differences in parental assessments of the child’s health or self-esteem between the two groups or in ratings of the emotional impact of the child’s well-being on the parents (Table 2 in the Supplementary Appendix).
Among children who had moderate or severe disability at 18 months, the corresponding rates at 6 to 7 years of age were 88% in the hypothermia group and 95% in the control group. All the children who had moderate-to-severe cerebral palsy at 18 months continued to be affected at 6 to 7 years in both groups. Details of the concordance of outcomes at 18 to 22 months and 6 to 7 years are shown in Table 3 in the Supplementary Appendix.
Discussion
We previously reported beneficial outcomes from treatment with hypothermia for neonatal hypox-ic–ischemic encephalopathy at 18 to 22 months of age, including significant reductions in the rate of combined outcome of death or moderate or severe disability.7 Other trials of whole-body or head-only cooling with whole-body hypothermia for neonatal encephalopathy have shown beneficial outcomes of hypothermia at 18 to 24 months of age in the entire study cohorts10-12 and in selected subgroups.6,9 In the present study assessing children at 6 to 7 years of age, the difference in rates of the composite outcome of death or an IQ score below 70 between hypothermia and usual care did not achieve statistical significance (P = 0.06); however, the previous finding of reduced mortality with hypothermia was maintained, with no appreciable increase in the risk of neurodevelopmental deficits among survivors. These results are reassuring since hypothermia is being used extensively around the world and currently is recommended by health care policy-makers.16,17 The 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care16 and the 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations17 state that during the postresuscitation period in neonates who are at 36 weeks’ gestational age or older with progressing moderate-to-severe encephalopathy, hypothermia should be offered in the context of clearly defined protocols similar to those used in published clinical trials.
The main limitation of our study is that it was not powered to evaluate secondary outcomes such as individual components of disability, cognitive and motor outcomes, and overall physical and psychosocial health. We cannot rule out the possibility of effects on the results of loss to follow-up, as there were some differences in the baseline characteristics of the group with and the group without primary outcome data. However, primary outcome data were available for most participants in both groups.
The majority of follow-up studies of children with birth asphyxia were published before intervention with hypothermia.1-5 Disability at 5 years of age was reported in 6 to 21% of children with moderate encephalopathy after acute perinatal asphyxia and in 42 to 100% of those with severe encephalopathy. Nondisabled children have delays in reading, spelling, arithmetic and language, memory, and sensorimotor perception scores,2,3 as well as increased rates of attention deficit–hyperactivity disorder.18 In the present study, we examined both gross and fine-motor outcomes to evaluate subtle benefits of hypothermia. We found a nonsignificant decrease (from 29% to 17%) in the rate of moderate or severe cerebral palsy in the hypothermia group as compared with the control group. We did not find a decrease in the risk of abnormalities in motor function among the non-disabled children in the hypothermia group as compared with those in the control group.
In a previous study assessing the relationship between early and later childhood neurologic assessments, the sensitivity of the 1-year evaluation in predicting the 5-year neurologic outcome was 96%, and the sensitivity of the Mental Developmental Index of the Bayley Scales of Infant Development to predict the IQ score was 87%.5 We likewise found a high concordance between assessment of moderate-to-severe disability at 18 to 22 months of age and similar assessment at 6 to 7 years of age.
Children with cerebral palsy have markedly poor health; however, the psychosocial health of children and the emotional impact of the child’s health on the parents tend to be similar regardless of the level of disability.19 We found only nonsignificant differences between the hypothermia group and the control group in the frequency of parental ratings of the child’s health as excellent or very good and of their ratings of concern about the child’s emotional well-being or behavior.
The significant difference in the rate of the primary outcome between the hypothermia group and the control group seen at 18 to 22 months of age in our previous report7 and the borderline significant difference in the rate of this outcome at 6 to 7 years of age in our current report were largely driven by deaths, with the majority occurring within the first 18 months of life. Two other trials10,11 have shown a reduction in mortality among infants 18 to 22 months of age undergoing hypothermia as compared with usual care. A concern with any therapy that reduces mortality among infants at high risk of death and disability is the possibility of an increase in the number of children who survive with disabilities. As reported here, there was no evidence of increased rates of an IQ score below 70, severe disability, or cerebral palsy at 6 to 7 years of age among surviving children treated with hypothermia; in our previous report, the frequency of ad-verse events was similar in the hypothermia and control groups both during the 72-hour study intervention period and during the neonatal-hospitalization period.7
In summary, whole-body hypothermia did not significantly reduce the rate of a composite end point of death or an IQ score below 70 at 6 to 7 years of age. However, whole-body hypothermia did reduce the rate of death and did not increase the rates of a low IQ score or severe disability among survivors. These data extend our previous support for the use of hypothermia in term and near-term infants with hypoxic–ischemic encephalopathy.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health and the Eunice Kennedy Shriver NICHD Neonatal Research Network.
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Appendix.
The authors’ affiliations are as follows: the Department of Pediatrics, Wayne State University, Detroit (S.S., A.P., R.B.); the Statistics and Epidemiology Unit, Research Triangle Institute, Research Triangle Park, NC (S.A.M., A.D., J.H.), and Rockville, MD (C.M.P.H.); the Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, RI (B.R.V., T.M.L.); the Department of Pediatrics, Stanford University School of Medicine, Palo Alto, CA (S.R.H.); the Department of Pediatrics, University of Cincinnati, Cincinnati (K.Y.); the Department of Pediatrics, Duke University, Durham, NC (K.E.G., R.F.G.); the Department of Pediatrics, University of Texas Medical School at Houston, Houston (C.G., J.E.T., P.W.E.); the Department of Pediatrics, Yale University School of Medicine, New Haven, CT (R.A.E.); the Division of Neonatology, University of Alabama at Birmingham, Birmingham (M.P.-C.); the Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas (R.J.H.); the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland (D.E.W.-C.); the Department of Pediatrics, University of California at San Diego, San Diego (Y.E.V.); the University of Miami Miller School of Medicine, Miami (C.R.B.); the Department of Pediatrics, Indiana University School of Medicine, Indianapolis (A.M.D.); Emory University School of Medicine, Department of Pediatrics, and Children’s Healthcare of Atlanta, Atlanta (I.A.-C.); University of Rochester School of Medicine and Dentistry, Rochester, NY (R.G.); University of New Mexico Health Sciences Center, Albuquerque (L.-A.P.); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network, National Institutes of Health, Bethesda, MD (R.D.H.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The authors’ affiliations are listed in the Appendix.
References
- 1.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–F224. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 2.Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90:F380–7. doi: 10.1136/adc.2004.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson CMT. Long-term follow-up of term infants with perinatal asphyxia. In: Stevenson DK, Benitz WE, Sunshine P, editors. Fetal and neonatal brain injury. 3rd ed Cambridge University Press; Cambridge, United Kingdom: 2003. pp. 829–858. [Google Scholar]
- 4.Gonzalez FF, Miller SP. Does perinatal asphyxia impair cognitive function with-out cerebral palsy? Arch Dis Child Fetal Neonatal Ed. 2006;91:F454–F459. doi: 10.1136/adc.2005.092445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25:135–48. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [Erratum, N Engl J Med 2010;362:1056.] [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 11.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4):e771–e778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 12.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Cans C. Surveillance of cerebral palsy in Europe: a collection of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 14.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the Gross Motor Function Classification System. Dev Med Child Neurol. 2006;48:424–8. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 15.Wake M, Salmon L, Reddihough D. Health status of Australian children with mild to severe cerebral palsy: cross-sectional survey using the Child Health Questionnaire. Dev Med Child Neurol. 2003;45:194–9. doi: 10.1017/s0012162203000379. [DOI] [PubMed] [Google Scholar]
- 16.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal Resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126(5):e1400–e1413. doi: 10.1542/peds.2010-2972E. [Erratum, Pediatrics 2011;128:176.] [DOI] [PubMed] [Google Scholar]
- 17.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5):e1319–e1344. doi: 10.1542/peds.2010-2972B. [DOI] [PubMed] [Google Scholar]
- 18.Moster D, Lie RT, Markestad T. Joint association of Apgar scores and early neonatal symptoms with minor disabilities at school age. Arch Dis Child Fetal Neonatal Ed. 2002;86:F16–F21. doi: 10.1136/fn.86.1.F16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnaud C, White-Koning M, Michelsen SI, et al. Parent-reported quality of life of children with cerebral palsy in Europe. Pediatrics. 2008;121:54–64. doi: 10.1542/peds.2007-0854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.