Abstract
Objectives
Immunotherapy with immunostimulants is an attractive therapy against gliomas. C-type lectin receptors specific for galactose/N-acetylgalactosamine (GCLR) regulate cellular differentiation, recognition, and trafficking of monocyte-derived cells. A peptide mimetic of GCLR ligands (GCLRP) was used to activate blood monocytes and populations of myeloid-derived cells against a murine glioblastoma.
Methods
The ability of GCLRP to stimulate phagocytosis by human microglia and monocyte-derived cells of the brain (MDCB) isolated from a human glioblastoma was initially assessed in vitro. Induction of activation markers on blood monocytes was assayed by flow cytometry after administration of GCLRP to naive mice. C57BL/6 mice underwent stereotactic intracranial implantation of GL261 glioma cells and were randomized for tumor size by magnetic resonance imaging, which was also used to assess increase in tumor size. Brain tumor tissues were analyzed using flow cytometry, histology, and enzyme-linked immunosorbent assay with respect to tumor, peritumoral area, and contralateral hemisphere regions.
Results
GCLRP exhibited strong stimulatory effect on MDCBs and blood monocytes in vitro and in vivo. GCLRP was associated with an increased percentage of precursors of dendritic cells in the blood (P = 0.003), which differentiated into patrolling macrophages in tumoral (P = 0.001) and peritumoral areas (P = 0.04), rather than into dendritic cells, as in control animals. Treatment with GCLRP did not result in a significant change in survival of mice bearing a tumor.
Conclusions
In vitro and in vivo activation of monocytes was achieved by administration of GCLR to mice. GCLRP-activated blood monocytes were recruited to the brain and exhibited specific phenotypes corresponding with tumor region (glioma, peritumoral zone, and contralateral glioma-free hemisphere). GCLRP treatment alone was associated with increased glioma mass as the result of the infiltration of phagocytic cells. Regional specificity for MDCB may have significant tumor treatment implications.
Keywords: microglia, macrophages, peptide, brain tumor, glioblastoma, mouse, C-type lectin receptors, galactose/N-acetylgalactosamine, immunotherapy
Introduction
Stimulation of cells of the immune system is an attractive novel therapy against glioblastomas (GBM). Emphasis has been placed on the possibility of enhancing activity of antigen presenting cells (APC) by manipulating the processing of tumor-specific antigens, which should ultimately lead to an improvement in the specific killing of tumor cells while sparing normal tissue.1–5
The detection of an invading pathogen and subsequent activation of appropriate responses is often referred to as “pattern recognition,” in deference to the proposal that immunity is induced by signals from pattern-recognition receptors.6 Pattern-recognition receptors are comprised of a heterogeneous group of receptor subfamilies, among which are the Toll-like receptors and the C-type lectin receptors (CLRs), which have been most widely studied.6 CLRs are expressed as transmembrane proteins on myeloid cells and appear to be involved in the regulation of innate and adaptive immunity.6 This receptor family has recently received great attention because of potential implications as a therapy for immune diseases such as HIV and cancer.7–10 CLRs can be categorized into mannose/fucose- or galactose-recognizing lectins.9 CLRs specific for galactose (Gal)/N-acetylgalactosamine (GalNAc) play an important role in regulating cellular differentiation, activity, and trafficking of monocyte-derived cells.7,11 A peptide mimetic of Gal/GalNAc was identified that had biological activity. In our study, we designated this peptide as Gal/GalNAc-specific C-type lectin receptor ligand mimetic GCLRP.7
GCLRP stimulated release of interleukin-8 (IL-8) and IL-21, which is a member of the IL-2 cytokine family and a powerful immune-stimulating cytokine. IL-21 mediates proliferation or differentiation signals to T, B, or natural killer cells,12 has anti-tumor activity,13 and is required to control chronic viral infections by maintaining CD8+ T cells.14
We applied GCLRP in an experimental syngeneic murine model to determine whether such a peptide could elicit activity against a malignant glioma. We studied activation of blood monocytes and of the populations of myeloid-derived cells of the brain (MDCB) in naïve mice. The analyses of glioma-bearing brains were performed in a novel fashion with attention to the effects in discrete brain regions with respect to the MDCB populations. Tissue was harvested from various brain regions that included tumor, peritumoral, and the contralateral tumor-free hemisphere.
This study is described in two parts: Part 1 deals with the characterization of the MDCB populations related to the tumor and the effects of GCLRP treatment alone. Part 2 deals with the effects of GCLRP treatment with synergistic radiation treatment.
Materials and methods
GCLR-peptide synthesis
A phage display library was screened with the lectin from Helix pomatia, which is specific for GalNAc. The sequence identified by this method, GCLRP, was synthesized as previously described.7 With a C-terminal amide group, the structure of GCLRP was ((VQATQSNQHTPRGGGS)2K)2 K-NH2. The sequence −GGGS− was included as a spacer to extend the mimetic sequence from the core. GCLRP was purified on a preparative Jupiter Proteo C12 column (21.2 mm × 250 mm) (Phenomenex, Inc, Torrance, CA) using a gradient from 8% to 18% acetonitrile in water containing 0.1% trifluoroacetic acid (TFA), dried under vacuum, dissolved in sterile phosphate-buffered saline (PBS, pH 7.2), and passed through a Sephadex G-25 or G-15 column (1 × 45 cm) in PBS to remove trifluoroacetic acid. Concentration was determined by the bicinchoninic acid assay (Pierce Protein Biology Products, Thermo Fisher Scientific Inc, Rockford, IL) using known concentrations of a dansylated GCLRP as standard. Purity was checked by mass spectroscopy with a Voyager DE STR mass spectrometer (Applied Biosystems®, Life Technologies Corporation, Foster City, CA).
The purpose of this work was to find a peptide that would bind to CLEC10a/CD301, a type 2 transmembrane C-type lectin receptor that is expressed on immature myleloid dendritic cells and alternatively activated macrophages.11 This receptor is specific for glycans with a terminal GalNAc or Gal residue. Solid-phase assays showed that GCLRP bound with high avidity to recombinant CLEC10a. A control peptide, which was described previously,7 did not bind to GalNAc- or Gal-specific lectins and also did not bind to CLEC10a (data not shown).
In vitro assay
To test whether the mimetic peptide could activate MDCB, samples of surgically removed tumor tissue from human patients with GBM were cultured using standard techniques.15 In this system, MDCBs that had invaded the tumor were cultured along with the glioma cells, and thus provided the opportunity to examine a mixed culture. The tumor tissue was minced and cells were allowed to migrate out of the tumor explants. After 5 days, the tissue explants were removed and GCLRP was added to the remaining cells at a concentration of 10 nM. The culture was incubated for an additional 4 h at 37°C. Cells were then challenged with fluorescein-labeled Escherichia coli cells (BioParticles®, Molecular Probes®, Eugene, OR), and 1 h later the samples were washed and examined using a confocal microscope.
In vivo experiments
Animals
All experiments were performed in compliance with Institutional Animal Care and Use Committee approval.
Two sets of experiments were done to establish an effect of GCLRP on naïve mice and on animals with experimental GBM cells implanted. Thirty-six naïve animals received subcutaneous (SC) injections of 1 nmol GCLRP/g body weight. Animals were divided into four groups of nine animals each. Group 1 received a single dose of GCLRP 1 day before sacrifice; group 2 received four total doses, administered on alternate days, starting 7 days before sacrifice; group 3 received eight total doses, administered on alternate days, starting 15 days prior to sacrifice; and group 4 consisted of naïve control mice receiving sham saline injections on alternate days, starting 15 days prior to sacrifice.
For the second set of experiments, the murine GL261 glioma cell line was obtained from the National Institutes of Health cell repository (Bethesda, MD). Cells were harvested, washed in PBS, and resuspended in Dulbecco’s Modified Eagle Medium at a concentration of 1 × 107 cells/mL. For intracranial inoculation, 78 mice (female C57BL/6 mice, 6–8 weeks old) were anesthetized using intraperitoneal injection of xylazine (5 mg/kg) and ketamine (100 mg/kg). Animals were positioned into a standard small animal cranial stereotaxic frame and a hole was made using a 21-gauge needle; the hole was 2.3 mm lateral and 1 mm anterior from the intersection of the coronal and sagittal sutures (bregma). Cells (volume of 2 μL) were injected using a Hamilton syringe and Microsyringe Pump Controller (World Precision Instruments, Inc, Sarasota, FL). Burr holes were sealed with bone-wax. The scalp was sutured closed. Control animals received sham injections of saline while GCLRP-treated animals received SC injections of 1 nmol peptide/g on alternate days beginning on the 7th day and ending at death. Forty-eight animals were euthanized at day 20 for tissue investigations including fluorescence-activated cell sorting (FACS), immunohistochemistry, and histological staining.
Thirty animals were used to establish survival rate. Ten control animals received sham injections of saline and 20 GCLRP-treated animals received SC injections of 1 nmol peptide/g on alternative days beginning on the 7th day and ending at death. Animals used to establish the survival curve were observed four times a day and were euthanized at the occurrence of visible symptoms of impending death such as hunched posture, reduced mobility, and visible body weight loss.
MRI
Magnetic resonance imaging (MRI) scanning was first done for randomization at day 7 to assign animals into certain groups on the basis of matched tumor size to decrease bias; this was then repeated on day 19 after implantation. At day 7, the size of the tumors ranged for 1.4 mm to 2.3 mm. MRI was performed on a 3T GE LightSpeed Qx/i system (GE Medical Systems, Milwaukee, WI) using a 2 cm birdcage coil. Prior to imaging, animals were anesthetized using intraperitoneal injection of xylazine (5 mg/kg) and ketamine (100 mg/kg). Animals received an injection of a contrast agent (Bayer Healthcare Pharmaceuticals, Montville, NJ) (100 μL) into the tail vein and axial images of the brain were acquired within 10 min thereafter using the MRI scanner.
Isolation of blood monocytes
Blood was collected in heparin-coated tubes or in syringes containing 1.0 mL of PBS with 8 mM ethylenediaminetetraacetic acid. Blood monocytes were isolated from heparinized whole blood. After red blood cell lysis with BDPharm Lyse™ buffer (BD Biosciences, Becton, Dickinson and Company, San Jose, CA), cells were washed and filtered through 40 μm strainers.
Isolation of monocyte-derived cells of the brain
Brain tissue was harvested after perfusion with saline. To determine the nature and number of microglial and macrophage cells in the brain after GCLRP administration, brains were harvested from each treatment group, and single-cell homogenates were prepared for FACS analysis. The whole brains of naïve animals were harvested. With tumor-bearing animals, the tumor, peritumoral area, and contralateral tumor-free hemisphere were dissected separately. Brain tissues were finely minced with a razor blade, homogenized in 5 mL of PBS, and incubated for 1 h at 37°C with collagenase (Sigma-Aldrich Co, St Louis, MO). The homogenate was drawn up into a 5-mL syringe fitted with a 21-gauge needle and passed 10 times through the needle. The final, single-cell suspension was filtered through a 40-μm nylon cell strainer and centrifuged at 700 × g for 5 min. The supernatant containing fully digested tissue was removed. The pellet was re-suspended in 5 mL of 30% isotonic Percoll (Sigma-Aldrich Co, St Louis, MO) in Hanks Balanced Solution (Sigma-Aldrich), and overlaid onto 2 mL of 70% Percoll in Hanks Balanced Solution in 15 mL conical tubes. The sample was then centrifuged at 700 × g for 20 min. The cells at the 30%/70% Percoll interface, which contained the microglia and macrophages, were collected and washed once in the media. For cell surface staining, single cell suspensions were prepared and stained with fluorophore-tagged antibodies described below.
FACS analysis
The following antibodies were employed: CD115 (Pe-labeled), MHCII (PeCy5-labeled) (eBioscience, Inc, San Diego, CA); CD11b (PeCy7-labeled), CD11c (APC-labeled), and CD45 (FITC-labeled) (BD Biosciences, Becton, Dickinson and Company, San Jose, CA). Flow cytometric data were collected on a FACSAria™ (BD Biosciences) flow cytometer and analyzed with Diva™ software (Microsoft Corporation, Redmond, WA). Mononuclear cell subpopulations of the brain can be characterized on the basis of CD45 and CD11b.16,17 Microglia were described as CD45low/CD11b+ and monocytes/macrophages were identified as CD45high/CD11b+ populations. Because both CD11b and CD45 are not absolutely specific for the monocyte-derived cell linage,18 we used the CD115 marker to identify monocyte-derived cells in the brain. Since CD115 is a receptor for the macrophage colony-stimulating factor, virtually all of the monocytes and monocyte-derived cells should express this marker. This is currently considered to be one of the most reliable markers of monocyte-derived cells.18 The CD115-positive cell population was divided into microglial (CD45low/CD11b+) and monocytes/macrophages (CD45high/CD11b+) subsets.19–23
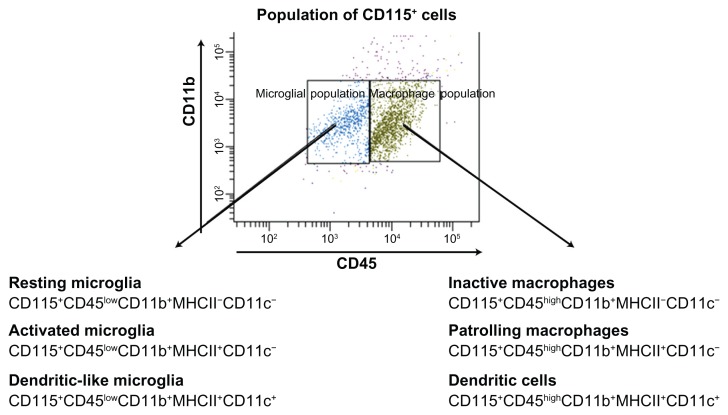
For analysis of tumor-associated MDCB, microglial cells (CD115+CD45low/CD11b+) were divided into subsets: resting microglial cells (RMi) (CD115+CD45lowCD11b+ MHCII−CD11c−), activated microglia (AMi) (CD115+ CD45lowCD11b+MHCII+CD11c−), and dendritic-like microglia (DLMi) (CD115+CD45lowCD11b+MHCII+CD11c+). The brain macrophagal population (CD115+ CD45high/CD11b+) was also divided into subsets: inactive macrophages (CD115+CD45highCD11b+MHCII−CD11c−), patrolling macrophages (PMa) (CD115+CD45highCD11b+MHCII+CD11c−), and dendritic cells (DC) (CD115+CD45highCD11b+ MHCII+CD11c+) (Figure 1).
Figure 1.
Microglial (CD45low/CD11b+) and macrophage (CD45high/CD11b+) populations were identified according to expression of CD45 within the whole CD115+ population upon FACS analysis.
Abbreviations: CD, cluster of differentiation; FACS, fluorescence-activated cell sorting.
Light microscopy and immunohistochemistry
For hematoxylin and eosin (HE) as well as immunohistochemical staining, animals at day 17 after tumor cell inoculation were perfused transcardially with 4% paraformaldehyde in PBS, pH 7.4. Whole brains were removed, embedded in paraffin, and cryosectioned parasagittally into 10 μm serial sections. Sections were HE stained for general morphology. The immunocytochemistry of tissue was then examined with anti-CD68 antibodies (Sigma-Aldrich Co, St Louis, MO) to visualize macrophagal/microglial cells.
Statistical analysis
Kaplan–Meier survival curves with a log-rank test were used to compare the survival of the two groups (control and GCLRP-treated animals). Statistical significance was determined using the Student’s t test. The asterisks in figures represent values significantly different between the two control and GCLRP-treated groups (P < 0.05).
Results
In vitro and animals without glioma
GCLRP increased phagocytosis in vitro and upregulated MHCII, CD11b and CD115 on blood monocytes and monocyte-derived cells of the brain
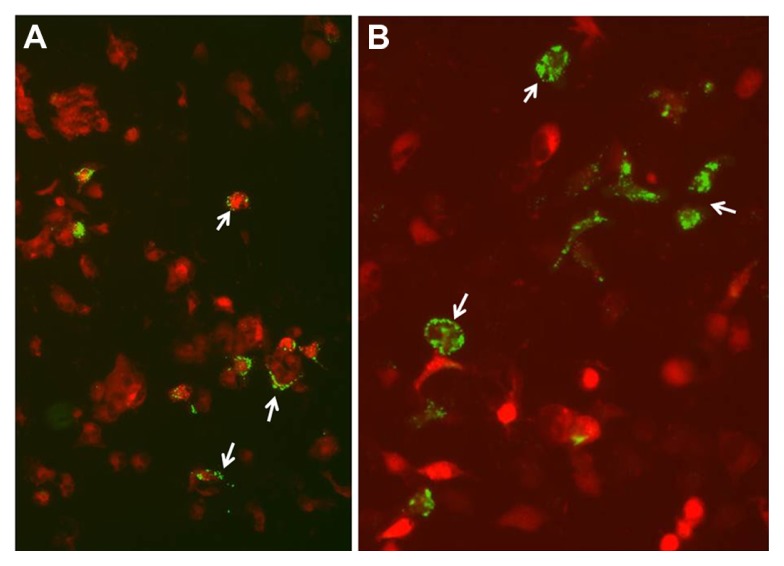
We initially evaluated the phagocytic activity GCLRP-treated MDCB considering that tumor antigen uptake by APC is a crucial event in the initiation of the anti-tumor immune response. This assay revealed that bacterial cells appeared to be associated with the surface of a few of the control cells that were presumed to be MDCBs based on their shape; however, little endocytosis of the bacterial cells was observed (Figure 2A). In contrast, these cells in cultures treated with the mimetic peptide internalized numerous bacterial cells (Figure 2B). Furthermore, there appeared to be diffuse fluorescence in the treated microglial cells, which suggested lysosomal digestion. This was in contrast to the punctate fluorescence noted on the cell surface from non-phagocytized E. coli in the untreated samples.
Figure 2.
MDCBs with bacterial cells in control cultures and cultures treated with GCLRP. (A) Control cultures show MDCBs with bacterial cells associated with their surface only. (B) Cultures treated 4 h with 10 nM GCLRP contain numerous MDCBs that internalized the fluorescently labeled bacterial cells indicating increased phagocytic activity (arrows indicate phagocytosis of the bacterial cells).
Abbreviations: MDCBs, monocyte-derived cells of the brain; GCLRP, peptide mimetic of a ligand of C-type lectin receptor.
In naïve mice treated with GCLRP, the number of monocytes in the blood was not changed. GCLRP exerted upregulatory effects on the expression of CD115 (on the 7th day compared to first day), CD11b (on the 7th day compared with control), and MHCII (on the 7th day compared with control). These data correlate with the increased phagocytic function of blood monocytes found in vitro when cultured with GCLRP (Eggink and Hoober, unpublished data).
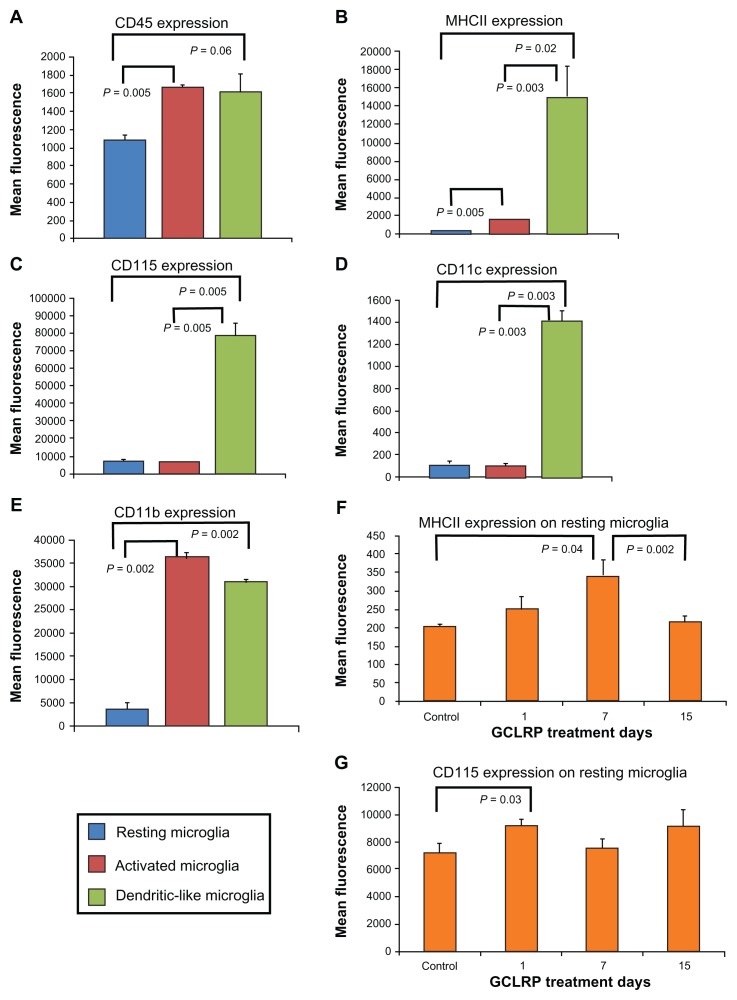
We analyzed the GCLRP effect on isolated microglial and macrophagal populations. Within the population of the microglial cells (CD115+CD45lowCD11b+), the majority of microglial cells (95.1% ± 2.6%) belonged to the RMi, while 3.7% ± 2.2% were Ami, and 1.0% ± 0.2% constituted the DLMi (Figure 3A–E).24,25 Although GCLRP did not change the number of cells in microglial subsets, it significantly increased the expression of CD115 (P = 0.03) and MHCII on RMi by the 7th day (P = 0.04); both of these constitute the majority of the microglial cell population (Figure 3F and G).
Figure 3.
Characterization of microglial population subsets in steady state according to selected cell surface markers. (A–E) Characterization of microglial population subsets in steady state according to selected cell surface markers. While DLMi show fairly consistent levels of expression across the markers, RMi and AMi show significantly variable levels of the same markers. (F and G) RMi exhibited increase in MHCII and upregulation of CD115 after GCLRP treatment initiation.
Abbreviations: CD, cluster of differentiation; DLMi, dendritic-like microglia; RMi, resting microglia; AMi, activated microglia; MHCII, major histocompatibility complex II; GCLRP, peptide mimetic of a ligand of C-type lectin receptor.
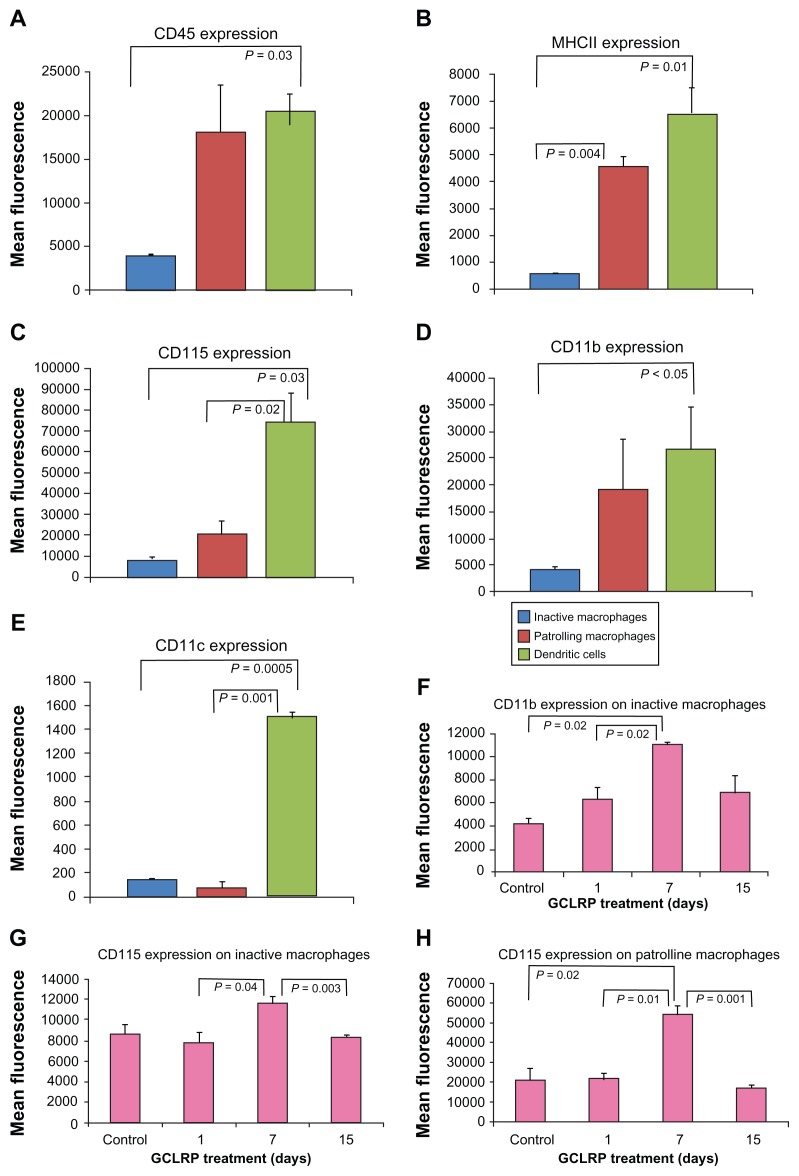
Among the macrophage population (CD115+CD45high CD11b+), IM constituted 22.6% ± 11.3%, PMa constituted 29.5% ± 8.15%, and dendritic cells (DC) constituted 31.6% ± 0.4% (Figure 4A–E). Response patterns of IM to GCLRP exposure led to increasing expression of CD115 and CD11b by 7th day (P = 0.04 and P = 0.003, respectively). PMa exhibited increased expression of CD115 by the 7th day as compared to control (P = 0.02) (Figure 4F–H). We found an increase in the amount of DC in the brain by day 15 compared to control (P = 0.01) (data not shown).
Figure 4.
Characterization of macrophagal populations in steady state according to selected cell surface markers. (A–E) Characterization of macrophagal populations in steady state according to selected cell surface markers. Dendritic cells show fairly consistent levels of expression across the markers, while inactive and PMa show significantly variable levels of the same markers. (F–H) Levels of selected surface markers on inactive and PMa are significantly increased by day 7 of GCLRP treatment while afterward levels fall.
Abbreviations: CD, cluster of differentiation; MHCII, major histocompatibility complex II; GCLRP, peptide mimetic of a ligand of C-type lectin receptor; PMa, patrolling macrophages.
Glioma-bearing animals
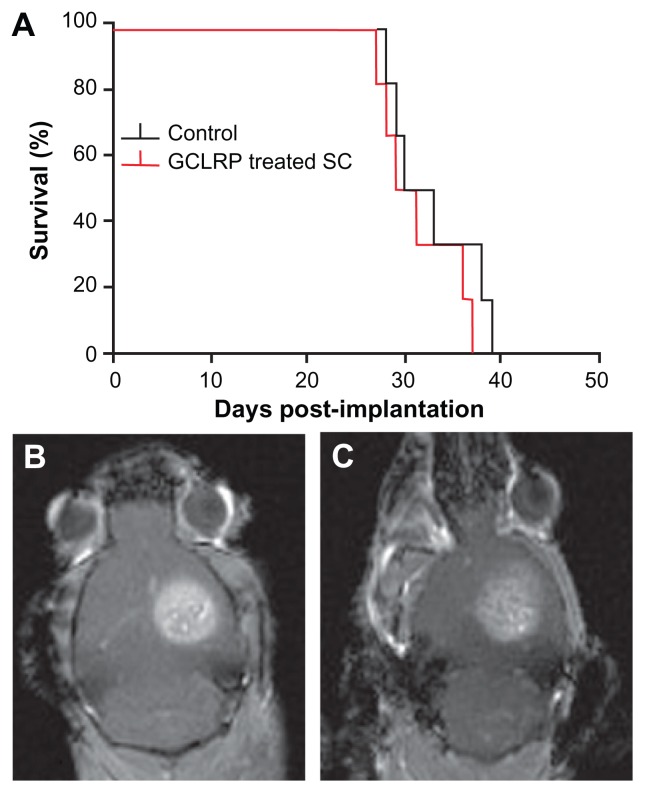
Survival of tumor-bearing control mice, sham-injected with saline, was compared to the animals treated with SC peptide administration. Survival of animals injected SC was not significantly different compared to control animals (30 days vs 31.5 days) (Figure 5A). Monitoring with contrast-enhanced MRI showed that tumor size in GCLRP-treated animals tended to be smaller (Figure 5C) compared to control animals (Figure 5B), but this was not statistically significant. GCLRP-treated tumors were notable for their infiltration of phagocytic cells.
Figure 5.
Kaplan–Meier curve showing median survival for control group vs GCLRP treatment group. (A) Survival curve for control and GCLRP-treated groups. MR images are shown of tumors (B) in a control animal and (C) in a GCLRP-treated animal.
Abbreviations: CD, cluster of differentiation; GCLRP, peptide mimetic of a ligand of C-type lectin receptor.
GCLRP upregulated CD115, MHCII, CD11b, and CD11c on blood monocytes
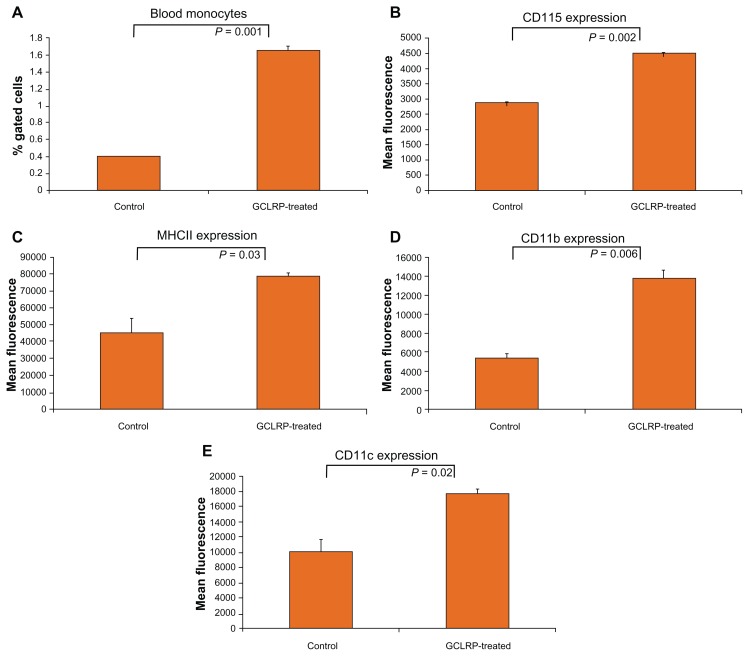
We isolated and performed flow cytometric analyses of blood monocytes in order to establish the effect of GCLRP administration on circulating peripheral blood monocytes, which are the main source of glioma-associated macrophages. We found that treatment with GCLRP resulted in a greater than four-fold increase of circulating peripheral blood monocytes in the blood (1.7% ± 0.2% vs 0.4% ± 0.0.1% in controls, P = 0.001) (Figure 6A). The total CD115+ population of cells had a uniformly significant increase in CD115, MHCII, CD11b, and CD11c expression (Figure 6B–E).
Figure 6.
In animals with implanted GBM, compared to control animals with tumors and no treatment, GCLRP treated animals showed increased monocytes (CD115+) in the blood (A) and significantly upregulated expression of key surface markers (B–E).
Abbreviations: GBM, glioblastoma; GCLRP, peptide mimetic of a ligand of C-type lectin receptor; CD, cluster of differentiation.
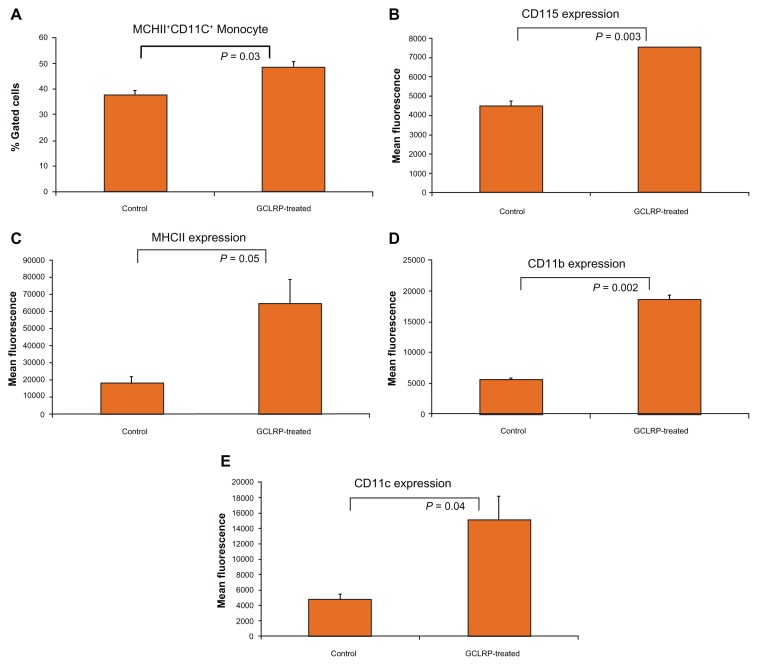
Further analysis of blood monocytes revealed an increasing number of MHCII+ CD11c+ positive cells (48.8% ± 1.2% vs 37.8% ± 1.7% in controls, P = 0.03), which are considered to be DC precursors in the blood after GCLRP treatment (Figure 7A) with upregulation of CD115, MHCII, CD11b, and CD11c (Figure 7B–E). These findings indicate that GCLRP is associated with strong activation of blood monocytes.
Figure 7.
MHCII+ CD11c+ positive monocytes (blood precursors of DCs) in animals with tumors treated with GCLRP compared to animals with tumors but no treatment. For MHCII+ CD11c+ positive monocytes (blood precursors of DCs), animals with tumors treated with GCLRP compared to animals with tumors but no treatment showed (A) increased number of MHCII+ CD11c+ positive monocytes in the blood, and significantly upregulated expression of key surface markers (B–E).
Abbreviations: MHCII, major histocompatibility complex II; CD, cluster of differentiation; DCs, dendritic cells; GCLRP, peptide mimetic of a ligand of C-type lectin receptor.
GCLRP-associated redistribution of macrophagal subpopulations in the glioma
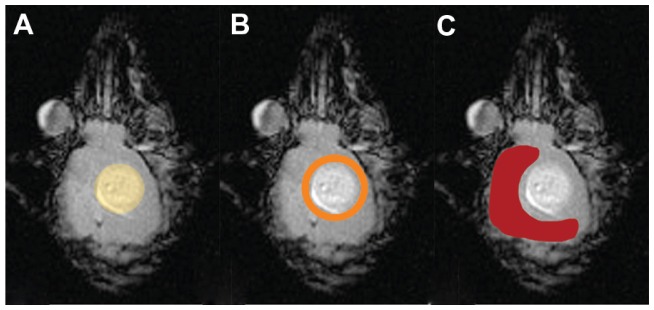
Within the murine brain, tissue analysis results originate from three discrete areas in relation to the tumor-glioma, peritumoral area, and the contralateral tumor free hemisphere (Figure 8).
Figure 8.
Schematic representation of brain tissue regions studied with relationship to the tumor. Tumor (A), peritumoral area (B), and contralateral tumor-free hemisphere (C).
Abbreviations: CD, cluster of differentiation; MHCII, major histocompatibility complex II.
Analysis of tissue within the glioma
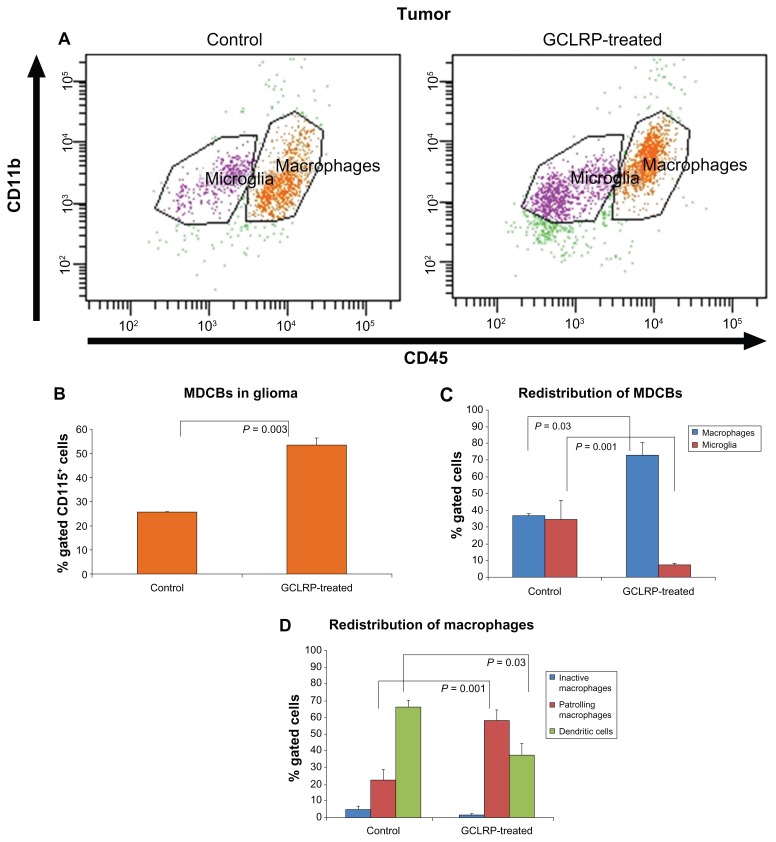
GCLRP treatment was associated with increasing numbers of MDCB within gliomas (53.7% ± 2.5% vs 25.6% ± 0.2% in controls, P = 0.003) (Figure 9A and B).
Figure 9.
FACS analysis of MDCBs within the glioma. (A) Dot-plots represent population of the CD115+ cells in control and GCLRP treated animals. Analysis of MDCB within glioma; (B) GCLRP treatment was associated with increased MDCBs within glioma; (C) proportional redistribution of microglial and macrophagal populations in the tumor area under GCLRP treatment; and (D) proportional redistribution of macrophagal subsets where DCs predominated in control samples, but PMa predominated in GCLRP treated samples.
Abbreviations: FACS, fluorescence-activated cell sorting; MHCII, major histocompatibility complex; MDCBs, monocyte-derived cells of the brain; CD, cluster of differentiation; GCLRP, peptide mimetic of a ligand of C-type lectin receptor; DCs, dendritic cells; PMa, patrolling macrophages.
Microglial populations
GCLRP treatment was associated with a decrease in the number of microglial cells in the tumor (7.4% ± 1.3% vs 34.8% ± 7.5% in controls, P = 0.001) (Figure 9C). However, we did not find any significant change in the distribution of microglial cell subpopulations in GCLRP-treated animals as compared with the control animals (data not shown).
Macrophagal populations
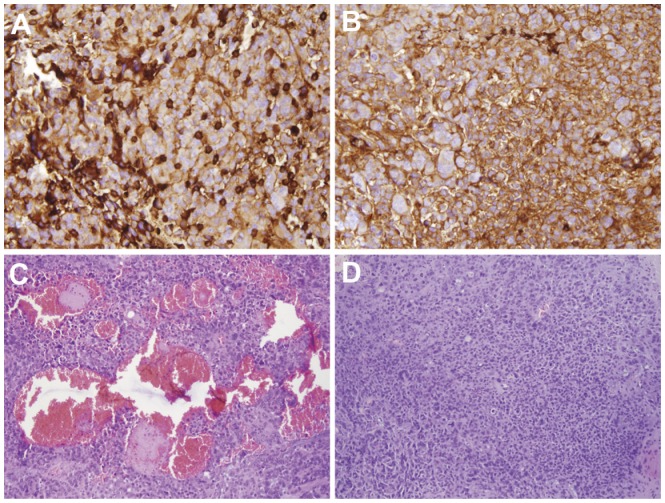
GCLRP treatment was associated with an increase in the number of macrophages (73.2% ± 11.5% vs 37% ± 1.3% in controls, P = 0.003) (Figure 9C), which was confirmed through immunohistochemical analysis of CD68 (Figure 10A and B). We verified uniform macrophagal infiltration of glioma tissue. There was no difference in the macrophagal density between the center and the peripheral areas of the tumor. An increase in the number of macrophagal cells was accompanied with the down-regulation of MHCII on PMa and DC in GCLRP-treated animals as compared to controls (P = 0.03 and P = 0.04, respectively, data not shown). Detailed analysis of macrophagal subsets indicated that GCLRP treatment was associated with an increase in the amount of PMa (58.2% ± 6.2% vs 22.5% ± 6.5% in controls, P = 0.001) along with a decrease of DC (37.3% ± 7.1% vs 66.4% ± 3.6% in controls, P = 0.03) (Figure 9D). Moreover, histological material stained with HE throughout the brain demonstrated an increase in the vascularization of the tumor and areas of hemorrhage (Figure 10C and D).
Figure 10.
Immunohistochemistry analysis of MDCBs within a glioma with anti-CD68 confirmed FACS data. The number of CD68-positive cells in tumor increased under GCLRP administration (A) compared to control samples (B) (×20). HE staining revealed increased tumor vascularization in GCLRP treated animals (C) compared to controls (D) (×10).
Abbreviations: MDCBs, monocyte-derived cells of the brain; CD, cluster of differentiation; FACS, fluorescence-activated cell sorting; GCLRP, peptide mimetic of a ligand of C-type lectin receptor; HE, hematoxylin and eosin.
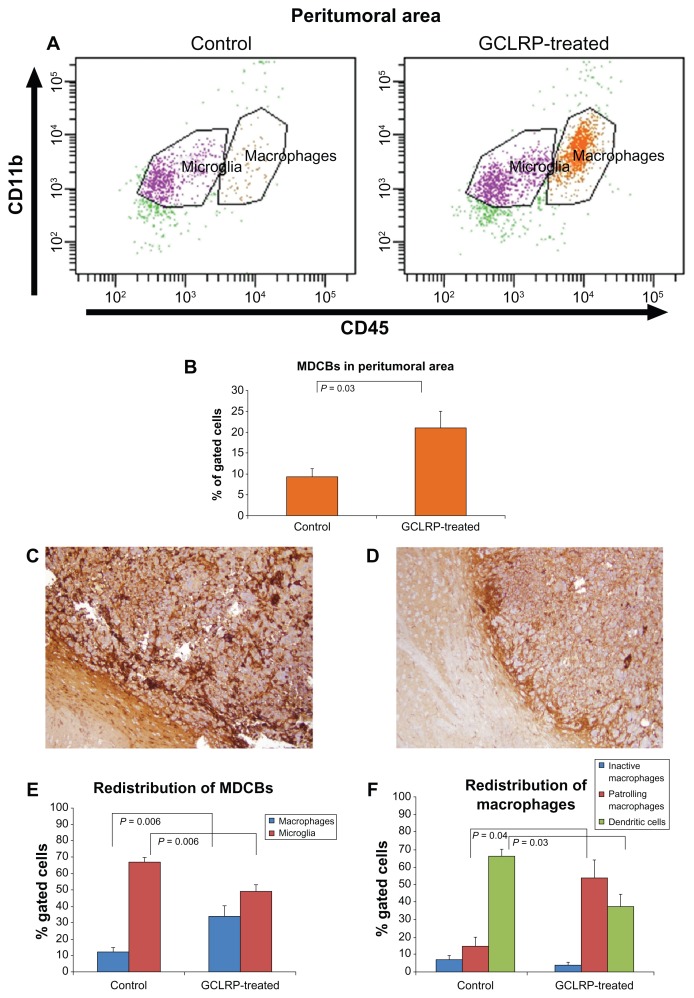
Analysis of tissue within peritumoral brain area
The number of MDCB cells in the peritumoral brain area increased in the GCLRP-treated animals (19.7% ± 4.3% vs 8.9% ± 2.7% in controls, P = 0.03) (Figure 11B) with an increasing amount of macrophagal cells (33.8% ± 5.7% vs 12.1% ± 1.9% in controls, P = 0.006) and a decreasing number of microglial cells (49.8% ± 2.4% vs 67.1% ± 2.1% in controls, P = 0.006) (Figure 11E).
Figure 11.
Analysis of the monocyte-derived cells in the peritumoral area under GCLRP treatment. (A) Dot-plots represent the population of CD115+ cells in controls compared to GCLRP-treated animals. (B) GCLRP increases the number of MDCBs within the peritumoral area. Immunohistochemistry with anti-CD68 confirmed FACS data: the number of CD68 positive cells in the peritumoral area increased under GCLRP administration (C) compared to the control sample (D) where DCs predominated (×10). (E) Microglial and macrophagal populations in the tumor area under GCLRP treatment showed proportional redistribution with the number of macrophages significantly increased and microglial cells decreased compared to controls. (F) Similarly, there was a proportional redistribution of macrophagal subsets under GCLRP treatment in the peritumoral area: DCs predominated in control samples but PMa predominated in GCLRP treated samples.
Abbreviations: GCLRP, peptide mimetic of a ligand of C-type lectin receptor; CD, cluster of differentiation; MDCB, monocyte-derived cells of the brain; FACS, fluorescence-activated cell sorting; DCs, dendritic cells; PMa, patrolling macrophages.
Microglial populations
The microglial population in the peritumoral area exhibited biological behavior similar to microglial cells within the glioma. There were no significant changes in microglial cell subpopulations in control tumors as compared with GCLRP-treated animals (data not shown).
Macrophagal populations
GCLRP-treated animals had a higher amount of PMa (53.8% ± 10.3%) than in the control group (14.7% ± 5.1%, P = 0.04), and lower numbers of DC (38.7% ± 7.7% vs 59.2% ± 4.9% in controls, P = 0.03) (Figure 11F).
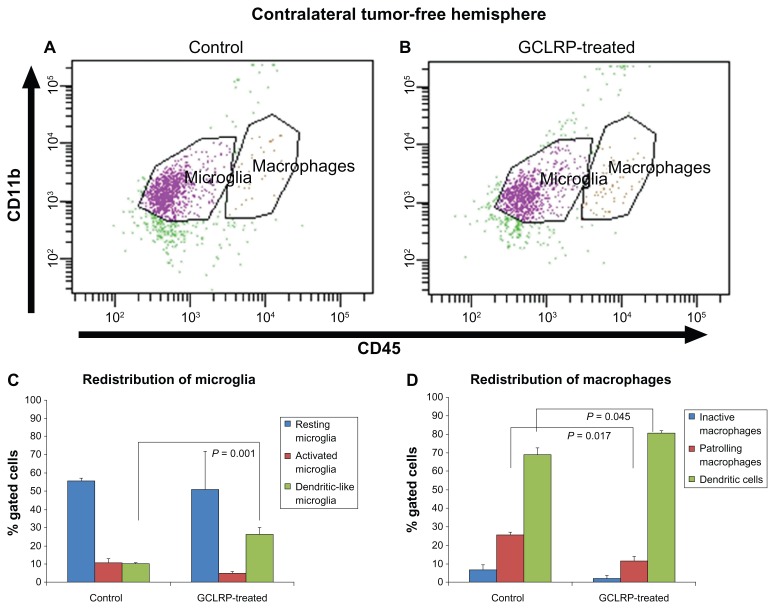
Analysis of the contralateral tumor-free hemisphere
Analysis of the contralateral tumor-free hemisphere of controls compared to that of the GCLRP-treated animals did not reveal significant differences in the number of MDCB cells (Figure 12A and B).
Figure 12.
Dot-plots represent populations of the CD115+ cells in controls and GCLRP-treated animals. Dot-plots represent populations of the CD115+ cells in controls (A) and GCLRP-treated animals (B) in the contralateral tumor-free hemisphere. (C) In the contralateral tumor-free hemisphere GCLRP treatment was associated with proportional increase in the DLMi. (D) Administration of GCLRP was associated with proportional decreased PMa population whereas an increase was found in the DC population as compared to controls.
Abbreviations: CD, cluster of differentiation; GCLRP, peptide mimetic of a ligand of C-type lectin receptor; DLMi, dendritic-like microglia; PMa, patrolling macrophages; DC, dendritic cell.
Microglial populations
GCLRP-treated animals showed an increase in the number of DLMi as compared with control animals (26.5% ± 3.6% vs 10.3 ± 0.4% in controls, P = 0.001) (Figure 12C) with upregulation of CD11c (P = 0.049) noted across these cells (data not shown).
Macrophagal populations
The contralateral tumor-free hemisphere of GCLRP-treated animals showed a decrease in PMa cells (11.6% ± 2.4%) as compared with controls (25.8% ± 1.5%, P = 0.017) (Figure 12D), whereas an increase was found in the DC population (80.6% ± 1.2% vs 69.1% ± 3.6% in controls, P = 0.045). GCLRP administration resulted in an increase in the density of CD45 and CD11c on macrophages (P = 0.04, P = 0.049, respectively) as compared with control animals. Also, the PMa population showed a higher expression of MHCII (P = 0.045), and DCs exhibited a higher expression of CD11c (P = 0.037).
Discussion
Recent treatments for GBM include manipulations of the activity of tumor-associated macrophages, such as inhibition of their recruitment to and survival in tumor tissue and restoration of their antitumor immunity.12,26–29 In this study, we investigated the effects of the activation of GCLRs in stimulating anti-glioma immunity based on previous work7,8 conducted with these receptors. We sought to determine whether such activation influenced monocytic activity in cancers and other immune-related diseases, and to determine whether there was potential for use of such treatments against a malignant glioma. In addition, favorable effects of GCLRP on naïve mice led us to examine whether the peptide could affect glioma growth in vivo in a murine syngeneic GBM model and increase survival.
In vivo administration of GCLRP in this tumor model scenario did not increase the duration of survival compared to control animals. However, the tumors of GCLRP-treated animals showed indications of more aggressive tumor behavior with more areas of abnormal vasculature and microhemorrhage found when compared to control animals. The analysis of the MDCBs of GCLRP-treated versus control animals was performed in a novel fashion, with attention focused on any potential effects in discrete brain regions (tumor, peritumoral, and contralateral tumor-free areas).
GCLRP caused an immunostimulatory effect on circulating blood monocytes in mice with tumors. In the presence of the tumors, GCLRP caused a stronger stimulatory effect on blood monocytes than that found in naïve mice, and this effect generated blood precursors of DC. GCLRP-treated animals with gliomas had a greater than four-fold increase in the number of circulating blood monocytes that over-expressed CD115, MHCII, CD11b, and CD11c.
We established that the phenotype of brain MDCB, recruited from activated monocytes, mostly depends on their area of recruitment. Two zones can be established: (1) within the tumor and peritumoral area where migrated MDCBs lose activated properties; and (2) within the contralateral tumor-free hemisphere where migrated MDCBs express an activated phenotype.
Results from our experiments showed that an increased number of activated monocytes were associated with an increased number of MDCBs. We hypothesize that the GCLRP mostly targets bone marrow precursors of the monocytes, which would explain the increasing number of these cells in the peripheral blood (in glioma-harboring animals) after peptide administration when compared to non-treated animals. As well, tumor tissue and its microenvironment secrete factors that recruit monocytes into the glioma, and these GCLRP-activated monocytes appear to respond more actively to the tumoral signals and more readily migrate to become part of the tumoral microenvironment. Our data show that significant GCLRP-mediated activation of the blood monocytes occurs only in the presence of growing glioma and tumoral factors in the blood. It may be that some of these factors provide a “permissive” effect on GCLRP-mediated activation of the monocytes.
GCLRP treatment was associated with the rearrangement of microglia and macrophage populations. Compared to controls, GCLRP-treated tumors contained nearly twice the percentage of macrophages, while the percentage of microglial cells decreased to only 20% of the control level. In the peritumoral area of treated animals, macrophages constituted a three-fold percentage increase compared to controls, while the level of microglia cells comprised 30% less than controls. Analysis indicated that most blood monocytes (without GCLRP treatment) recruited by the tumor (ie, within glioma tissue and peritumoral area) may become DCs, and the remainder of monocytes may become a PMa subset in the tumor and peritumoral areas. It seems that GCLRP-activated monocytes may not be able to overcome the immunosuppressive effect of the malignant glioma once recruited into the tumor and peritumoral area, and so these monocytes become PMa in the tumoral and in peritumoral areas, and comprise a much larger percentage of macrophagal cells in these areas. Our findings are consistent with results described by Komohara et al,30 who found that in patients, the number of macrophages in GBM (grade 4) was higher than that in grade 2 or 3 gliomas; this was closely correlated with the vascular density of the tumors.
In the contralateral hemisphere GCLRP treatment also caused redistribution of cell populations compared to the controls; the proportion of DCs was increased and the proportion of PMa cells decreased. The macrophagal population of GCLRP-treated animals had a higher expression of CD115, MHCII, CD11b, and CD11c in the contralateral hemisphere perhaps due to the peptide effect on the peripheral blood monocytes from which they were recruited. Our results also showed an increase in the DLMi compared to controls. They appeared as immature DCs in the contralateral tumor-free hemisphere, but not in the glioma or peritumoral area, perhaps related in part to the immunosuppressive effect of glioma. It is known that DLMi can differentiate into DCs.24,25,31 Microglial cells with high expression of CD11c and CD11b can maintain phagocytic activity and also might engage in a dialogue with T cells, promoting both neuronal survival and neural renewal.32 Our data suggest that the cell population rearrangements and perhaps the immunosuppressive effects of the glioma extend mainly within glioma tissue and peritumoral area, but not within the contralateral tumor-free hemisphere.
Of course, this study presents results within a murine syngeneic malignant glioma model, but many of the observations made here may be operational in humans as well. Currently, DC vaccination is considered to be a promising immunotherapeutic tool against malignant gliomas, but results of this strategy are controversial.33 DC vaccination is based on the idea that mature DCs may effectively present glioma antigens and trigger a strong immune response, especially since GBM reduces the ability of monocytes to differentiate into mature DCs which, in theory, could limit a potent antigenic response.34 Moreover, one of the major mechanisms of the DC vaccinations is explained by fusion of the DCs and glioma cells.35
The use of GCLRP in this study has shown that in relationship to the geography of the tumor, there are regionally relevant and unique behaviors associated with the varying subpopulations of MDCBs that influenced the growth and survival of the tumor. It appears that precursor DCs generated from blood monocytes under peptide influence were not able to overcome the glioma. This might explain some current pitfalls of immunotherapy using DCs for patients with GBM. An immunotherapeutic approach using the manipulation of GCLRs, as performed in this study, against an experimental immune-competent malignant glioma was not successful, even though in vitro study and evidence from the treatment of other cancers or immune-related diseases suggested potential for this technique. In this regard, Part 2 of this study discusses that GCLR-directed treatment may be beneficial when the compromised immune status is overcome, or when the tumor altered with an adjuvant treatment (such as with radiation therapy) and the resulting activated cells effect their specific anti-tumoral function.
Conclusion
A peptide mimetic of the ligand of a C-type lectin receptor specific for Gal/GalNAc exhibited upregulated expression of MHCII and phagocytic markers in peripheral blood monocytes and monocyte-derived cells of the brain in naïve mice. In this murine experimental syngeneic glioma, GCLRP-activated blood monocytes that were recruited to the brain exhibited a specific biological behavior corresponding with the area of their differentiation (glioma, peritumoral zone, or the contralateral glioma-free hemisphere). Such regional specificity for the cell population subtypes may have significant tumor treatment implications. GCLRP-activated blood monocytes recruited into the glioma mostly became PMa, rather than DC as was found in controls (ie, untreated, tumor-bearing animals). Although successful in vitro activation of blood monocytes and microglia was achieved, in vivo use of a peptide mimetic of GCLR ligands (GCLRP) used to activate blood monocytes and populations of myeloid-derived cells against a murine GBM was associated with more aggressive tumor characteristics and no apparent benefit in survival.
Acknowledgments
This study was supported by funds to Drs Preul, Hoober, and Eggink in a bi-institutional grant from the Arizona Biomedical Research Commission. Additional support was furnished by the Barrow Neurological Foundation and the Newsome Endowed Chair in Neurosurgery Research.
Footnotes
Disclosure
LLE and JKH declare that they are inventors of the technology contained in this report. Intellectual property has been licensed to Susavion Biosciences, Inc, in which these authors hold shares. Other authors have no conflicts to disclose.
References
- 1.Das S, Raizer JJ, Muro K. Immunotherapeutic treatment strategies for primary brain tumors. Curr Treat Options Oncol. 2008 Feb;9(1):32–40. doi: 10.1007/s11864-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 2.Luptrawan A, Liu G, Yu JS. Dendritic cell immunotherapy for malignant gliomas. Rev Recent Clin Trials. 2008 Jan;3(1):10–21. doi: 10.2174/157488708783330530. [DOI] [PubMed] [Google Scholar]
- 3.McBride WH. Combining radiation therapy with immunotherapy for treatment of brain tumors. In: Liau LM, Becker DP, Cloughesy TF, Bigner DD, editors. Brain Tumor Immunotherapy. 1st ed. New Jersey: Humana Press; 2001. pp. 345–362. [Google Scholar]
- 4.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008 Apr;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010 Jan;17(1):6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006 Dec;7(12):1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 7.Eggink LL, Hoober JK. A biologically active peptide mimetic of N-acetylgalactosamine/galactose. BMC Res Notes. 2009;2:23. doi: 10.1186/1756-0500-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggink LL, Salas M, Hanson CV, Hoober JK. Peptide sugar mimetics prevent HIV type 1 replication in peripheral blood mononuclear cells in the presence of HIV-positive antiserum. AIDS Res Hum Retroviruses. 2010 Feb;26(2):149–160. doi: 10.1089/aid.2009.0155. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. 2009 Jul;230(1):22–37. doi: 10.1111/j.1600-065X.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009 Jul;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashi N, Fujioka K, Denda-Nagai K, et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002 Jun 7;277(23):20686–20693. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- 12.Daga A, Orengo AM, Gangemi RM, et al. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. Int J Cancer. 2007 Oct 15;121(8):1756–1763. doi: 10.1002/ijc.22901. [DOI] [PubMed] [Google Scholar]
- 13.Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008 Mar;7(3):231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- 14.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009 Jun 19;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asch AS, Leung LL, Shapiro J, Nachman RL. Human brain glial cells synthesize thrombospondin. Proc Natl Acad Sci U S A. 1986 May;83(9):2904–2908. doi: 10.1073/pnas.83.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badie B, Schartner JM, Paul J, Bartley BA, Vorpahl J, Preston JK. Dexamethasone-induced abolition of the inflammatory response in an experimental glioma model: a flow cytometry study. J Neurosurg. 2000 Oct;93(4):634–639. doi: 10.3171/jns.2000.93.4.0634. [DOI] [PubMed] [Google Scholar]
- 17.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009 Mar;110(3):572–582. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008 Feb;20(1):52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graber DJ, Hickey WF, Harris BT. Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:8. doi: 10.1186/1742-2094-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Cabec V, Carreno S, Moisand A, Bordier C, Maridonneau-Parini I. Complement receptor 3 (CD11b/CD18) mediates type I and type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J Immunol. 2002 Aug 15;169(4):2003–2009. doi: 10.4049/jimmunol.169.4.2003. [DOI] [PubMed] [Google Scholar]
- 21.Mitrasinovic OM, Perez GV, Zhao F, Lee YL, Poon C, Murphy GM., Jr Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. J Biol Chem. 2001 Aug 10;276(32):30142–30149. doi: 10.1074/jbc.M104265200. [DOI] [PubMed] [Google Scholar]
- 22.Mitrasinovic OM, Robinson CC, Tenen DG, Lee YL, Poon C, Murphy GM., Jr Biolistic expression of the macrophage colony stimulating factor receptor in organotypic cultures induces an inflammatory response. J Neurosci Res. 2004 Aug 1;77(3):420–429. doi: 10.1002/jnr.20168. [DOI] [PubMed] [Google Scholar]
- 23.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem. 2006 May 26;281(21):14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butovsky O, Koronyo-Hamaoui M, Kunis G, et al. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santambrogio L, Belyanskaya SL, Fischer FR, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy BC, Maier LM, D’Amico R, et al. Dynamics of central and peripheral immunomodulation in a murine glioma model. BMC Immunol. 2009;10:11. doi: 10.1186/1471-2172-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE. Astrocytic regulation of human monocytic/microglial activation. J Immunol. 2008 Oct 15;181(8):5425–5432. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- 28.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011 Mar;59(3):472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 31.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001 Feb 15;166(4):2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005 Jun;4(3):281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 33.Kanaly CW, Ding D, Heimberger AB, Sampson JH. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg Clin N Am. 2010 Jan;21(1):95–109. doi: 10.1016/j.nec.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vleeschouwer S, Rapp M, Sorg RV, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery. 2006 Nov;59(5):988–999. doi: 10.1227/01.NEU.0000245595.38957.3E. [DOI] [PubMed] [Google Scholar]
- 35.Akasaki Y, Kikuchi T, Irie M, et al. Cotransfection of Poly(I: C) and siRNA of IL-10 into fusions of dendritic and glioma cells enhances antitumor T helper type 1 induction in patients with glioma. J Immunother. 2011 Mar;34(2):121–128. doi: 10.1097/CJI.0b013e3181e5c278. [DOI] [PubMed] [Google Scholar]