Abstract
Interleukin 17 (IL-17)-producing TH17 cells are often present at the sites of tissue inflammation in autoimmune diseases, which has lead to the conclusion that TH17 are main drivers of autoimmune tissue injury. However, not all TH17 cells are pathogenic, in fact TH17 generated with TGF-β1 and IL-6 produce IL-17 but do not readily induce autoimmune disease without further exposure to IL-23. Here we show that TGF-β3, produced by developing TH17 cells, is dependent on IL-23, which together with IL-6 induces highly pathogenic TH17 cells. Moreover, TGF-β3-induced TH17 cells are functionally and molecularly distinct from TGF-β1-induced TH17 cells and possess a molecular signature that defines pathogenic effector TH17 cells in autoimmune disease.
Upon antigenic stimulation, naïve CD4+ T cells activate, expand and differentiate into different effector phenotypes 1. T helper type 1 (TH1) cells, induced by the transcription factor Tbx21 (T-bet) produce interferon-γ (IFN-γ), interleukin 2 (IL-2) and lymphotoxin (LT) and were shown to be crucial for clearing intracellular pathogens 2, 3. In contrast, TH2 cells that are generated by the transcription factor GATA-3 produce IL-4, IL-5, IL-13 and were shown to be critical for clearing extracellular pathogens 4, 5. An exaggerated TH1 response against self-antigens was implicated in inducing autoimmune diseases 6. However, the loss of IFN-γ or IFN-γ receptor (IFN-γR) did not induce resistance to developing autoimmune diseases 7, 8, in fact, mice deficient for these proteins were found to be highly susceptible to autoimmununity 7, 8. Paradoxically, loss of the TH1-specific transcription factor, T-bet, made these mice resistant to multiple autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), an animal model of the human disease multiple sclerosis (MS) 9. This raised the issue of what is the role of T-bet in inducing EAE, because the production of IFN-γ is not required for conferring encephalitogenicity to effector TH1 cells.
TH17 cells, which have been characterized as an additional effector T cell subset that produce IL-17A, IL-17F, IL-21 and IL-22, have been suggested to be the critical driver of autoimmune tissue inflammation 10–14. TH17 cells were observed to be expressed at the sites of tissue inflammation and have been associated with the induction of many human autoimmune diseases including psoriasis, inflammatory bowel disease (IBD), rheumatoid arthritis (RA), type 1 diabetes and MS 14. TH17 cells are differentiated by a combination of TGF-β1, IL-6 and IL-1 cytokines, which induces RORγt, a transcription factor required for their generation 10, 12, 13, 15. Whereas TGF-β1 plus IL-6 can induce TH17 cells, exposure to another cytokine, IL-23, was shown to be crucial for their stabilization and for their ability to induce autoimmune tissue inflammation in EAE16–18. IL-23R polymorphism has been genetically linked to many human autoimmune diseases including psoriasis, IBD and ankylosis spondylitis 19, 20. Exposure to IL-23 was shown to reduce the levels of the anti-inflammatory cytokine IL-10 in developing TH17 cells, thus making these cells pathogenic 21. However, this also raised the question whether there are cytokines or effector molecules dependent on IL-23 that make them pathogenic to induce inflammation in autoimmune disease.
Although TH17 cells were thought to be pathogenic11, 22, accumulating data indicates the existence of non-pathogenic IL-17 producing TH17 cells 23, 24. It remains unclear whether there is a differential requirement for their induction and what makes a TH17 cell pathogenic or nonpathogenic. Recent studies have shown that GM-CSF (CSF-2), which is produced by TH17 and transactivated by RORγt, is required for conferring pathogenicity to TH17 cells 25, 26. Indeed, GM-CSF-deficient mice are highly resistant to the induction of EAE 25, 27. However, it remains unclear why T-bet−/− mice are resistant to the induction of EAE and other autoimmune diseases, suggesting that T-bet must have additional roles in the induction of pathogenic TH17 cells in EAE 24. T-bet is expressed in TH17 cells24, but whether it plays a role in inducing pathogenic function of TH17 cells has not been addressed.
In this study we have identified an endogenous cytokine, TGF-β3, which is specifically produced by developing TH17 cells in an IL-23 dependent manner that is important for driving pathogenic TH17 phenotype. Whereas TGF-β1 plus IL-6 differentiate naïve T cells into TH17 cells, these T cells are not pathogenic unless they are further exposed to IL-23. We now show that IL-23 is critical for enhancing expression and/or maintaining the endogenous levels of TGF-β3 in developing TH17 cells. In fact, differentiation of TH17 cells in the presence of TGF-β3 and IL-6 makes TH17 cells pathogenic without any need for further exposure to IL-23. Using these subsets of pathogenic and non-pathogenic TH17 cells, we have identified a molecular signature associated with pathogenic TH17 cells.
Results
TGF-β3 induction in TH17 cells
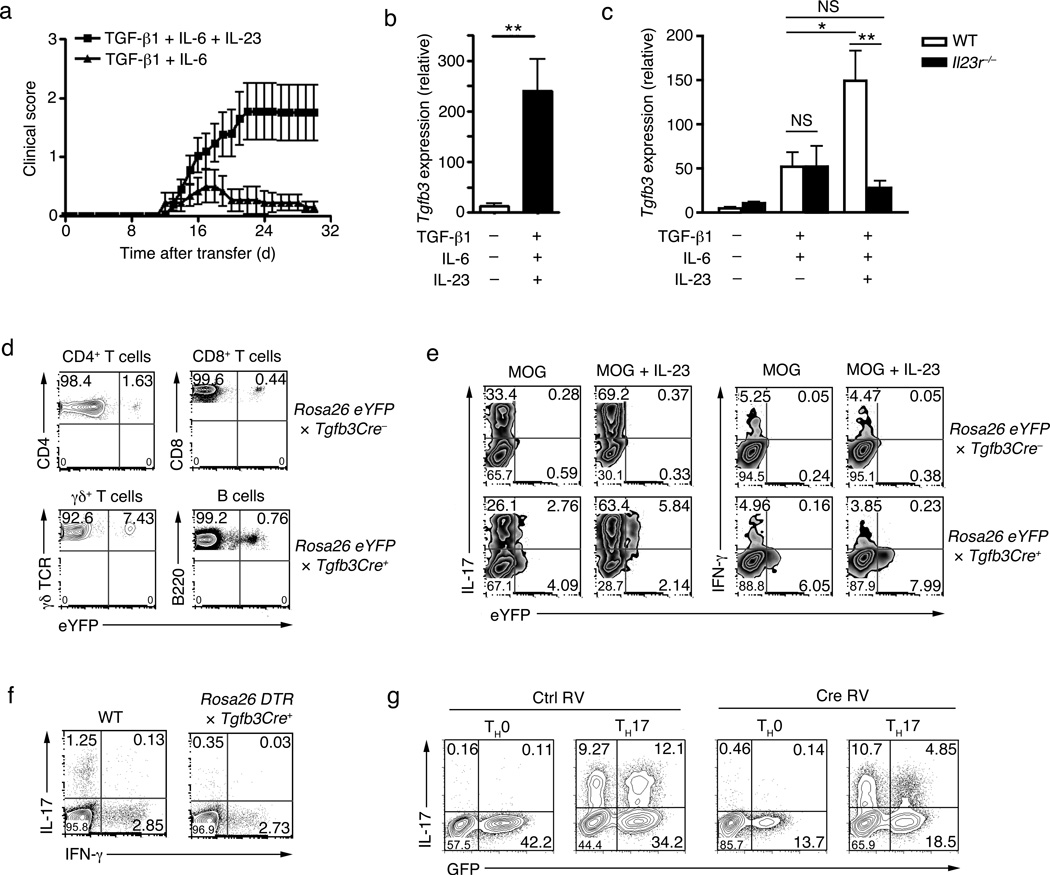
TGF-β1 and IL-6 are required for differentiation of TH17 cells10, 12, 13. However, TH17 cells induced by TGF-β1 plus IL-6 are not pathogenic, unless they are further exposed to IL-23 for a prolonged period of time 21, 22. In the EAE model, only adoptive transfer of myelin oligodendrocyte peptide (amino acid 35–55 (MOG35–55)) specific TCR transgenic CD4+ T cells (2D2) that were differentiated with TGF-β1-IL-6-IL-23 results in the induction of severe disease while T cells differentiated with TGF-β1-IL-6 develop mild to no disease, with majority of the recipient mice recovering rapidly within 24 days after the onset of clinical disease (Fig. 1a and Table 1). To investigate the role of IL-23 in the induction of pathogenic TH17 cells, we undertook detailed temporal microarray analysis that revealed a strong induction of TGF-β3 in developing TH17 cells exposed to TGF-β1-IL-6-IL-23 (Supplementary Fig. 1a), which was dependent on IL-23R signaling (Supplementary Fig. 1b). Quantitative RT-PCR confirmed the induction of TGF-β3 in TH17 cells (Fig. 1b). Naïve T cells activated in the presence of TGF-β1-IL-6 or TGF-β1-IL-6-IL-23 both expressed TGF-β3. However, IL-23R-deficient CD4+ T cells under TGF-β1-IL-6-IL-23 condition failed to enhance or maintain TGF-β3 expression (Fig. 1c). IL-23 was not required for the initial induction of TGF-β3, which was induced by IL-6 (Supplementary Fig. 1c), but the presence of IL-23R was required for maintaining TGF-β3 expression (Fig. 1c).
Figure 1. The induction of TGF-β3 in TH17 cells.
(a) Mean clinical scores (disease incidence) in wild-type recipients 30 days after transfer of naïve CD4+ T cells (5×106 cells) from 2D2 transgenic mice that were differentiated in vitro with TGF-β1-IL-6-IL-23 or TGF-β1-IL-6. (b) Quantitative RT-PCR analysis of Tgfb3 mRNA in naïve CD4+ T cells differentiated for four day in vitro with TGF-β1-IL-6-IL-23 or no cytokines. (c) Quantitative RT-PCR analysis of Tgfb3 mRNA in naïve CD4+ T cells from wild-type (WT) or Il23r−/− mice differentiated in vitro with TGF-β1-IL-6-IL-23 or no cytokines. (d) Flow cytometry analysis of TGF-β3-YFP expression in CD4+ T cells, CD8+ T cells, γδ T cells and B cells from the lymph nodes and spleen of TGF-β3crexRosa26yfp mice. (e) Intracellular cytokine staining showing co-expression of TGF-β3-YFP with either IL-17 or IFN-γ in naïve CD4+ T cells from MOG35–55+CFA immunized TGF-β3crexRosa26yfp mice stimulated with PMA+ionomycin four days post in vitro culture with or without IL-23. (f) Intracellular cytokine staining showing IL-17 and IFN-γ expression in PMA+ionomycin ex-vivo stimulated splenocytes from MOG35–55-immunized TGF-β3Cre+xRosaDTR and TGF-β3Cre-xRosaDTR littermate control (WT) mice treated with diptheria toxin on day 4 post-immunization. (g) IL-17 expression in naïve TGF-β3fl/fl CD4+ T cells cultured in vitro with IL-1β-IL-6-IL-23 (TH17) or no cytokines (TH0) then retrovirally transduced with Cre-GFP to delete TGF-β3. All data are a representative of more than three independent experiments with similar results. Statistical significance of *p<0.05, **p<0.01, or ***p<0.001 is indicated for the RT-PCR data. Error bars indicate mean ± s.d.
Table 1.
Induction of EAE following adoptive transfer of MOG specific TH17 cells and active immunization.
| Mice | Groups | Disease incidence (%) |
Mean score α |
Mean day of onsetα |
Mortality (%) |
Statistics | |
|---|---|---|---|---|---|---|---|
| 2D2 | IL-1β+IL-6+IL-23 | 20/21 (95%) | 3.7±0.29 | 12.2±0.35 | 10/21 (48%) | ns | |
| 2D2 | TGF-β3+IL-6 | 13/15 (86%) | 3.5±0.35 | 12.7±0.92 | 4/15 (26%) | p<0.001 | |
| 2D2 | TGF-β1+IL-6 | 10/17 (58%) | 2.6±0.45 | 14.1±1.77 | 0/17 (0%) | ||
| 2D2 | TGF-β1+IL-6+IL-23 | 11/21 (52%) | 3.6±1.53 | 14.8±2.40 | 5/21 (23%) | p<0.001χ | |
| 2D2xIL-23R−/− | IL-1β+IL-6+IL-23 | 0/8 (0%) | n/a | n/a | 0/8 (0%) | p<0.001β | |
| 2D2xIL-23R−/− | TGF-β3+IL-6 | 0/8 (0%) | n/a | n/a | 0/8 (0%) | p<0.001β | |
| Wt | Isotype control | 19/22 (86%) | 2.4±0.25 | 9±0.83 | 0/22 (0%) | p<0.001 | |
| Wt | Anti-TGF-β3 | 710 (70%) | 1.6±0.37 | 9.8±2.2 | 0/10 (0%) | ||
| 2D2xTbx21−/− | TGF-β3+IL-6 | 8/11 (73%) | 2.8±1.13 | 18.9±4.58 | 1/11 (1%) | p<0.001 | |
| 2D2xTbx21 | TGF-β1+IL-6 | 0/11 (0%) | n/a | n/a | 0/11 (0%) | ||
| 2D2xTbx21 | TGF-β1+IL-6+IL-23 | 0/7 (0%) | n/a | n/a | 0/7 (0%) | p<0.001β | |
The transferred 2D2 CD4+ cells were cultured in the presence of various combinations of IL-23, TGF-β, IL-1β, and IL-6.
Active EAE was induced with MOG35-55/CFA plus pertussis toxin.
Disease incidence, mean day of onset, and mortality are reported as number of mice affected/total number of mice
Mean calculated only from mice that developed clinical signs of experimental autoimmune encephaltomyelitis.
Compared to 2D2 controls with indicated cytokine conditions
Compared to 2D2 (TGF-β1+IL-6+IL-23) to 2D2 (TGF-β1+IL-6) conditions
n/a : Denotes: not applicable
ns: Denotes: not significant
It remains unknown if cells of the hematopoietic origin can produce TGF-β3. To investigate TGF-β3 protein expression, we generated TGF-β3-YFP fate reporter mice (TGF-β3CreR26ReYFP) by crossing mice that express the Cre recombinase in the TGF-β3 locus (TGF-β3Cre) 28 with reporter mice expressing eYFP under the Rosa26 promoter. Analysis of lymphoid tissue from the TGF-β3CreR26ReYFP mice revealed that TGF-β3 was produced in CD4+, CD8+, γσ T and B cells (Fig. 1d), but not by cells of the myeloid lineage (CD11b+ and/or CD11c+ cells) (data not shown). This observation suggested that TGF-β3 is endogenously produced by the hematopoietic cells. To address whether in vivo differentiating TH17 cells induces TGF-β3 expression and determine whether TGF-β3 expression is enhanced in response to IL-23, we immunized TGF-β3CreR26ReYFP mice with MOG35–55 emulsified in complete Freund’s adjuvant (CFA) and reactivated T cells in the presence of IL-23 in a recall assay. A small percentage of IL-17 expressing and non-expressing T cells produced TGF-β3 upon activation, but exposure to IL-23 largely restricted TGF-β3 expression to IL-17-producing TH17 cells. This was true in both CD4+ (Fig. 1e) and non-CD4+ T cell (Supplementary Fig. 1c) subsets. We also addressed whether TGF-β3 is co-produced by IFN-γ producing TH1 cells, which are also induced by immunization with MOG35–55+CFA. IFN-γ producing T cells did not co-express TGF-β3 (Fig. 1e). This was true for non-CD4+ T cells as well (Supplementary Fig. 1d), suggesting that TGF-β3 may be mostly expressed by IL-17 producing cells. In addition, deletion of TGF-β3+ cells in vivo in TGF-β3CreR26RDTR mice (TGF-β3Cre mice crossed to mice expressing the diphtheria toxin receptor (DTR) under the Rosa26 promoter) that were immunized with MOG35–55+CFA resulted in selective ablation of IL-17+ cells, while IFNγ+ T cell numbers remained unchanged upon diphtheria toxin (DT) treatment (Fig. 1f). CD4+ T cells derived from TGF-β3fl/fl mice in which TGF-β3 was deleted through the retroviral expression of a GFP-Cre, but not control retrovirus treated T cells, showed severely compromised IL-17 production in TH17 differentiation conditions (Fig. 1g). These findings reveal that TH17 cells produce TGF-β3 endogenously and that exposure to IL-23 largely restricted the expression of TGF-β3 to IL-17-producing cells.
TGF-β3-induced TH17 cells are highly pathogenic
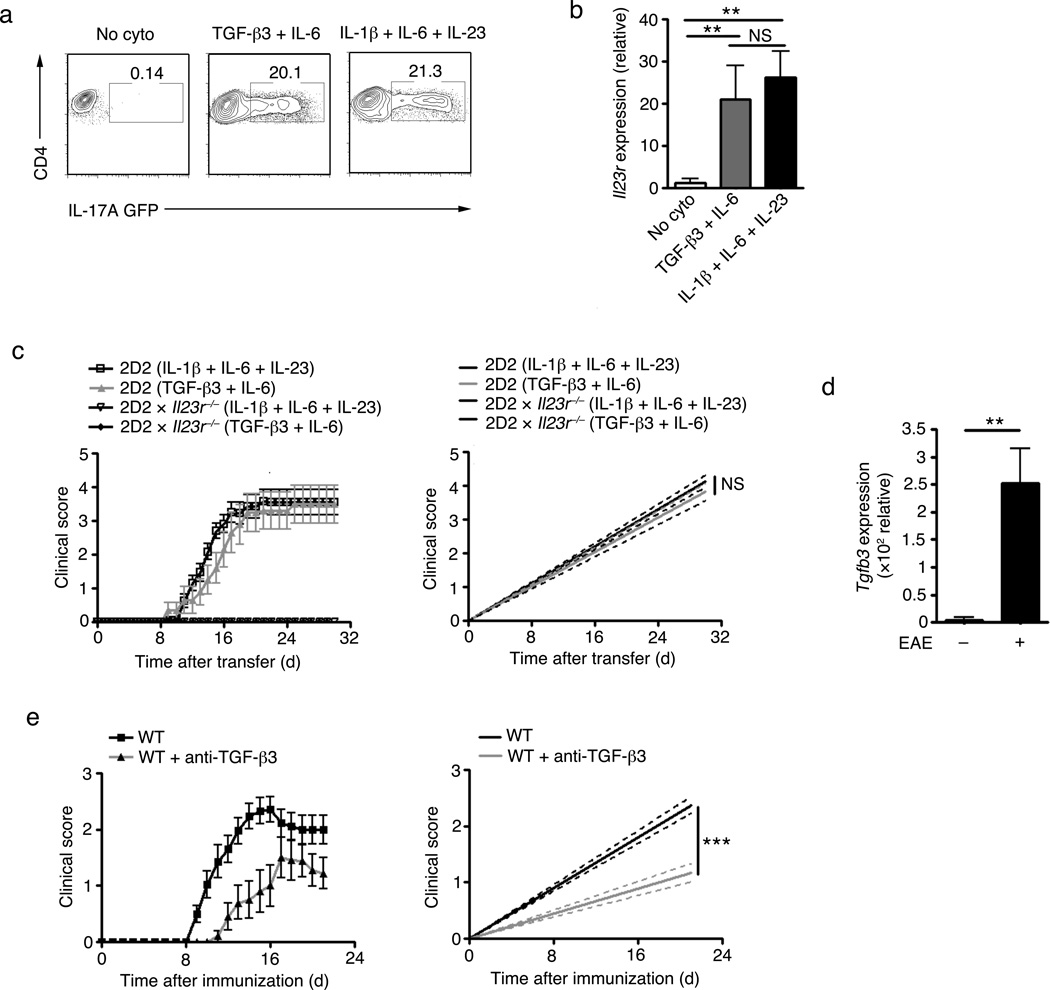
To determine whether TGF-β3 can induce TH17 cells, we polarized naive CD4+ T cells into TH17 cells with either TGF-β1-IL-6 or TGF-β3-IL-6 and compared the expression of IL-17 and other TH17 associated molecules. TGF-β3-IL-6-induced TH17 cells expressed comparable amount of IL-17 as TGF-β-IL-6-induced TH17 cells (Fig. 2a,b). Similar to TGF-β1-induced TH17 cells, TGF-β3-induced TH17 cells expressed Rorc, Il17a, Il17f, but no Ifnγ mRNA (Fig. 2c). However, TGF-β3-induced TH17 cells had significantly higher expression of Il23r and Il22 when compared to TGF-β1-induced TH17 cells (Fig. 2d). The higher expression of IL-23R protein in TGF-β3-induced TH17 cells was confirmed in IL-23R-GFP reporter mice16 (Fig. 2e).
Figure 2. TGF-β3-induced TH17 cells are highly pathogenic in inducing autoimmunity.
(a) Intracellular cytokine staining showing IL-17 expression of PMA+ionomycin stimulated naïve CD4+ T cells from C57BL/6 (wt) mice differentiated in vitro with TGF-β1-IL-6 or TGF-β3-IL-6 for four days. (b) ELISA showing IL-17 production from supernatants of naïve CD4+ T cells from C57BL/6 (wt) mice stimulated in vitro with TGF-β1, TGF-β3, TGF-β1-IL-6, TGF-β3-IL-6 for four days. (c,d) Quantitative RT-PCR of Rorc, IL-17a, IL-17f, IFNγ, Il23r and Il22 mRNA expression from wt naïve CD4+ T cells stimulated in vitro with TGF-β1-IL-6, TGF-β3-IL-6 or no cytokines for four days. (e) Flow cytometry analysis of IL-23R-GFP expression of naive CD4+ T cells from IL-23R-GFP knock-in mice differentiated in vitro with TGF-β1-IL-6, TGF-β3-IL-6 or no cytokines for four days (f) Mean clinical scores (disease incidence) in wild-type recipients 30 days after transfer of naïve CD4+ T cells (5×106 cells) from 2D2 transgenic mice that were differentiated in vitro with TGF-β1-IL-6 or TGF-β3-IL-6. Statistical analysis by linear regression curve was performed and graphed including the 95% confidence band of the regression line (***p<0.001) (g) Quantification of CNS lesions in the meninges and parenchyma from recipient mice sacrificed on day 30 (**p<0.01). Data is pooled from three independent experiments (n=6). (h) TGF-β signaling PCR array analysis of Smad 1, 3, 5 from wt naïve CD4+ T cells differentiated with TGF-β1-IL-6 or TGF-β3-IL-6 for 24 hours. cDNA was prepared and processed according to manufacturer’s instruction (Sabioscience, CA). The graph illustrates the relative fold change in the Smads of TGF-β3-IL-6 condition compared to TGF-β1-IL-6. The dotted line represents the normalized expression of TGF-β1-IL-6 set at one for each of the Smads. The data is pooled from three independent replicates. (i) Flow cytometry analysis showing p-smad1&5 or p-smad3 expression from wt naïve CD4+ T cells stimulated with TGF-β1 or TGF-β3 for 30 minutes. Adoptive transfer data from (f) is a representative of four independent experiments. All data are a representative of more than three independent experiments, unless otherwise mentioned, with similar results. Statistical significance of *p<0.05, **p<0.01, or ***p<0.001 is indicated for the RT-PCR data. Error bars indicate mean ± s.d.
Because IL-23 induces TH17 cells to become pathogenic and IL-23 can enhance and maintain expression of TGF-β3, we investigated the pathogenicity of TGF-β3-IL-6-induced TH17 cells. TH17 cells generated from naive 2D2 TCR transgenic (Tg) T cells with either TGF-β1-IL-6 or TGF-β3-IL-6 were transferred into naive recipients and development of EAE was monitored. In contrast to TGF-β1-IL-6-induced TH17 cells, which transferred a very mild disease (Fig. 2f and Table 1), TGF-β3-IL-6-induced TH17 cells transferred EAE with a high disease incidence, severity and mortality (Fig. 2f and Table 1). Histo-pathological quantification of CNS lesions showed significantly more parenchymal and meningeal inflammatory lesions in the TGF-β3-IL-6 groups in comparison to the recipients of TGF-β1-IL-6 TH17 cells (Fig. 2g), suggesting that TGF-β3-induced TH17 cells are highly pathogenic.
We next addressed how TGF-β1 and TGF-β3 can induce such a difference in the pathogenicity of TH17 cells, considering they both have been suggested to signal through a common TGF-βRII subunit29, 30. Mice expressing a dominant negative (DN) for of TGF-βRII (here called TGF-βRII DN mice) were previously shown to be to have reduced generation of TH17 cells and to be protected from EAE 13. We tested whether TGF-β3, similar to TGF-β1, requires TGF-βRII for the development of TH17 cells. To address this, we generated TGF-βRII DN × IL-17A-eGFP reporter mice and CD4+ T cells from these mice were differentiated with either TGF-β1-IL-6 or TGF-β3-IL-6 conditions. Both TGF-β1 and TGF-β3 were unable to induce the differentiation of TH17 cells in TGF-βRII DN T cells (Supplementary Fig. 2a), suggesting that both TGF-β1 and TGF-β3 signal through TGF-βRII for TH17 differentiation (Fig. 2d–f).
To test the possibility that TGF-βRII transmits different downstream signals upon ligation with TGF-β1 or TGF-β3, we performed a comprehensive TGF-β signaling PCR array analysis for TH17 cells generated with either TGF-β1 or TGF-β3. This array allowed us to determine the expression of approximately 90 known signaling molecules downstream of TGF-βRII and showed that TGF-β1 and TGF-β3 induced different expression profiles of target genes downstream of TGF-βRII engagement (Supplementary Fig. 2b). In particular, TGF-β3-IL-6-differentiated TH17 cells induced higher expression of Smad1 and Smad5 and lower expression of Smad3 when compared to TGF-β1/IL-6-induced TH17 cells (Fig. 2h). Consistent with the PCR array data, TGF-β3 induced less Smad2 and Smad3 phosphorylation than TGF-β1, while phosphorylation of Smad 1 and Smad5 was greatly enhanced (Fig. 2i, Supplementary Fig. 2c). Taken together, these data indicate that TGF-β3-IL-6-induced TH17 cells were functionally distinct from TGF-β1-IL-6-induced TH17 cells, and that the difference in the functionality of TH17 cells was supported by the differential signaling induced by TGF-β1 and TGF-β3 through TGF-βRII.
TGF-β3-IL-6 and IL-1β-IL-6-IL-23 TH17 cells are comparable
TGF-β-independent TH17 cells, which are induced by a combination of IL-1β/IL-6/IL-23 and are highly pathogenic compared to TGF-β1/IL-6-derived TH17 cells, have been recently described 31. We therefore compared the ability of TGF-β3-IL-6 and IL-1β-IL-6-IL-23 pathogenic TH17 cell populations to transfer EAE. TH17 cells differentiated with either TGF-β3-IL-6 or IL-1β-IL-6-IL-23 showed comparable IL-17 protein (Fig. 3a) and IL-23R mRNA (Fig. 3b) expression. Because the IL-23-IL-23R axis is essential for the development of pathogenic TH17 cells 16–18, we tested whether the high expression of IL-23R contributed to the pathogenicity of TGF-β3-IL-6-or IL-1β-IL-6-IL-23-differentiated TH17 cells. Adoptively transferred 2D2 T cells differentiated into TH17 cells with TGF-β3-IL-6 or IL-1β-IL-6-IL-23 into wild-type recipients transferred EAE with similar severity and incidence. Transfer of IL-23R-deficient 2D2 T cells failed to induce EAE in both TGF-β3-IL-6 and IL-1β-IL-6-IL-23 differentiating condition (Fig. 3c and Table 1). This indicates that IL-23-IL-23R pathway is absolutely critical for the development of pathogenic TH17 cells induced by either TGF-β3-IL-6 or IL-1β-IL-6-IL-23.
Figure 3. The pathogenicity of TGF-β3–IL-6 TH17 cells are highly comparable to IL-1β-IL-6-IL-23 TH17 cells.
(a) Flow cytometry analysis showing IL-17-GFP expression in naïve CD4+ T cells from the IL-17-GFP knock-in mice differentiated in vitro with IL-1β-IL-6-IL-23, TGF-β3-IL-6, or no cytokines for four days. (b) Quantitative RT-PCR analysis of Il23r mRNA expression from naïve CD4+ T cells from wt mice differentiated in vitro with IL-1β-IL-6-IL-23, TGF-β3-IL-6, or no cytokines for four days. (c) Mean clinical scores (disease incidence) in wild-type recipients 30 days after transfer of naïve CD4+ T cells (5×106 cells) from 2D2 transgenic or 2D2xIL-23R−/− mice that were differentiated in vitro with IL-1β-IL-6-IL-23 or TGF-β3-IL-6. Statistical analysis by linear regression curve was performed and graphed including the 95% confidence band of the regression line. (ns) denotes not significant. (d) Quantitative RT-PCR showing Tgfb3 mRNA expression of CNS-infiltrating CD4+ T cells isolated from MOG35–55+CFA+pertussis toxin (PT) immunized wt mice with either score 2–3 (peak of disease) or score 0 (no disease). Figure represents data of twelve individual mice pooled from three independent experiments. (e) Mean clinical scores (disease incidence) in wt mice immunized with MOG35–55+CFA+PT with either isotype control or anti-TGF-β3 neutralizing antibody in the emulsion then monitored for 21 days. Statistical analysis by linear regression curve was performed and graphed including the 95% confidence band of the regression line. Adoptive transfer data from (c) is pooled data from three independent experiments (n=8) and (e) is pooled data from two independent experiments (n=10). Cumulative data on the number of animals used are shown in Table 1. All in vitro experiments are a representative of at least three independent experiments unless specified differently. Error bars indicate mean ± s.d. (**p<0.01). (ns) denotes not significant.
Further analysis of CNS-infiltrating T cells showed that TGF-β3-IL-6-induced TH17 cells maintained higher IL-17 expression (~25%) and did not produce as much IFN-γ as IL-1β-IL-6-IL-23 induced TH17 cells (~15%) (data not shown). To address if TGF-β3 is involved in EAE induction following active immunization, we immunized mice with MOG35–55+CFA, which is known to induce EAE by generating MOG35–55-specific TH1 and TH17 cells. CD4+ T cells infiltrating the CNS in MOG35–55+CFA immunized mice showed a significantly higher expression of TGF-β3 in mice that developed the disease (score 2–3) compared to mice with no clinical signs of disease (score 0), even though there was infiltration of CD4+ T cells in the CNS of these mice (Fig. 3d). Local blockade of TGF-βs by adding neutralizing anti-TGF-β1,2,3 antibody in the immunizing emulsion was shown to prevent the development of TH17 cells and EAE 32, suggesting an in vivo role of TGF-β in the development of TH17 cells and the disease. However, the study did not reveal which TGF-β was required for the development TH17 cell, since anti-TGF-β1,2,3 neutralizes all three forms of TGF-β proteins. We therefore tested whether neutralizing TGF-β3 locally affects the development of EAE. The inclusion of anti-TGF-β3 antibody into the MOG35–55+CFA emulsion at the time of induction significantly inhibited the development of EAE (Fig. 3e and Table 1), suggesting that endogenous TGF-β3 contributes to the induction of EAE. Taken together, these data indicate that the pathogenic TH17 cells induced by TGF-β3 have a pathogenic and functional phenotype comparable to that of recently described IL-1β/IL-6/IL-23 TH17 cells.
The transcriptional signature of pathogenic TH17 cells
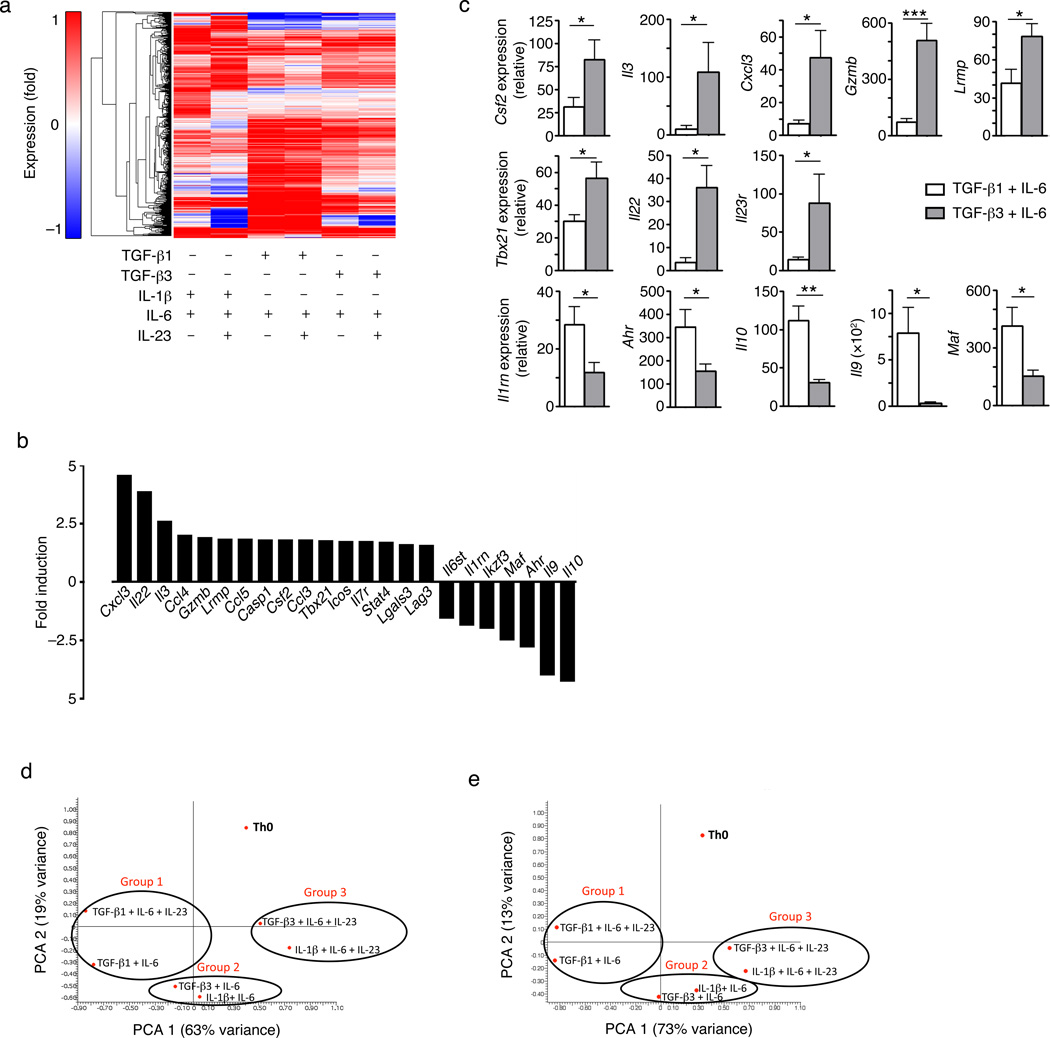
To characterize the molecular state associated with the different functionally of TGF-β1 and TGF-β3-induced TH17 cells, we measured the mRNA profiles of in vitro differentiated TH17 cells using whole genome microarrays. We compared the gene expression profiles of naïve CD4+ T cells differentiated into TH17 cell under six different polarizing conditions: IL-β1-IL-6, IL-β1-IL-6-IL-23, TGF-β1-IL-6, TGF-β1-IL-6-IL-23, TGF-β3-IL-6 and TGF-β3-IL-6-IL-23. Activated TH0 cells with the same TCR specificity that produce IL-4 and IFN-γ were used as controls. We found 434 genes that were differentially expressed, up-regulated or down-regulated at least 2 fold in TH17 cells in comparison to control TH0 cells (Fig. 4a). Across these 434 genes, the transcriptional profile of TH17 cells differentiated with IL-1β-IL-6 or IL-1β-IL-6-IL-23 was similar to that of TH17 cells differentiated with TGF-β3-IL-6 or TGF-β3-IL-6-IL-23, whereas the TH17 cells differentiated with TGF-β1-IL-6 or TGF-β1-IL-6-IL-23 had a distinct transcriptional profile (Fig. 4a). Direct comparison of the transcriptional profiles of TH17 cells differentiated with TGF-β1-IL-6 or TGF-β3-IL-6, as these two different differentiation conditions led to significant differences in the pathogenicity of TH17 cells, revealed 233 genes that were differentially expressed (Supplementary Table 1). Based on their biological function, we selected a representative subset of 23 genes (Fig. 4b) and validated their differential expression by qPCR (Fig. 4c).
Figure 4. Identification of transcriptional signature for pathogenic TH17 cells.
(a) A heat map of the microarray analysis showing differential expression of 434 genes that were up- or down-regulated (>2 fold; compared with TH0 cells) from wt naïve CD4+ T cells differentiated in vitro with IL-1β-IL-6, IL-1β-IL-6-IL-23, TGF-β1-IL-6, TGF-β1-IL-6-IL-23, TGF-β3-IL-6, or TGF-b3-IL-6-IL-23 for 60 hours. (b) Bar graph representing analysis of 23 genes selected from a pool of 233 targets that had greater than 1.5 fold difference between TGF-β3-IL-6 and TGF-β1-IL-6 differentiated TH17 cells. Data is represented as a fold change of TGF-β3-induced TH17 cells over TGF-β1-induced TH17 cells. Up-regulated genes denote a “pathogenic signature” from TGF-β3-induced TH17 cells while down-regulated genes are “nonpathogenic signature” from TGF-β1-induced TH17 cells. (c) Quantitative RT-PCR analysis showing mRNA expression of several targets highlighted in (b) from wt naïve CD4+ T cells differentiated in vitro with TGF-β1-IL-6 or TGF-β3-IL-6 for four days. Error bars indicate mean ± s.d., *p<0.05, **p<0.01, ***p<0.001). Data is pooled from at least four independent experiments. (d,e) Principal component analysis (PCA) of the 23 selected genes (d) or 233 genes (e) that were differentially expressed and had greater than 1.5 fold difference between the TGF-β3-IL-6 and TGF-β1-IL-6 differentiated TH17 groups.
TGF-β3-IL-6-induced TH17 cells were characterized by the higher expression of transcripts that could be broadly divided into 3 different modules: cytokines/chemokines (Cxcl3, Ccl4, Ccl5, Ccl3, Csf2, Il3, Il22 and Casp1, which is involved in the generation of mature IL-1β), transcription factors (Tbx21 and Stat4), and effector molecules (Gzmb, Lag3 and Lglas3 (encoding Galectin-3)). In addition, TGF-β3-IL-6-induced TH17 cells had downregulated, while TGF-β1-IL-6-induced TH17 cells had upregulated, molecules that are broadly associated with immune regulation (regulatory module), including Il10 and molecules involved in the regulation of IL-10 production (Ahr and Maf), Il9, Il1rn and the transcription factor Ikzf3 (Aiolos) (Fig. 4c).
Based on this molecular signature, we compared the profiles of each of these TH17 populations (Fig. 4d). Principal component analysis (PCA) revealed 3 groups: group I includes TGF-β1-IL-6-induced TH17 cells, regardless of whether they were exposed to IL-23. These cells show a mild or no pathogenic phenotype in vivo (Fig. 1a) and express little or no IL-23R (Fig. 2d,e). Group II includes TH17 cells induced with TGF-β3-IL-6 and IL-1β-IL-6. These cells are highly pathogenic in vivo (Figs. 2f) and express high levels of IL-23R (Fig. 2d,e). Group III includes TH17 cells induced with TGF-β3/IL-6/IL-23 and IL-1β/IL-6/IL-23, which also expressed high levels of IL-23R (Fig. 3b). Similar results were obtained when PCA was performed based on the expression of all 233 genes that are differentially expressed between TGF-β1-IL-6-induced and TGF-β3-IL-6-induced TH17 cells (Fig. 4e). These results show that TGF-β3-IL-6-induced TH17 cells are closely related to IL-1β-IL-6-induced TH17 cells, and differ from TGF-β1-IL-6-induced by TH17 cells. The 23-gene transcriptional signature of TH17 cells defined here provides a means to identify key molecules involved in pathogenicity of TH17 cells.
TGF-β3 overcomes lack of pathogenicity in Tbx21−/− TH17 cells
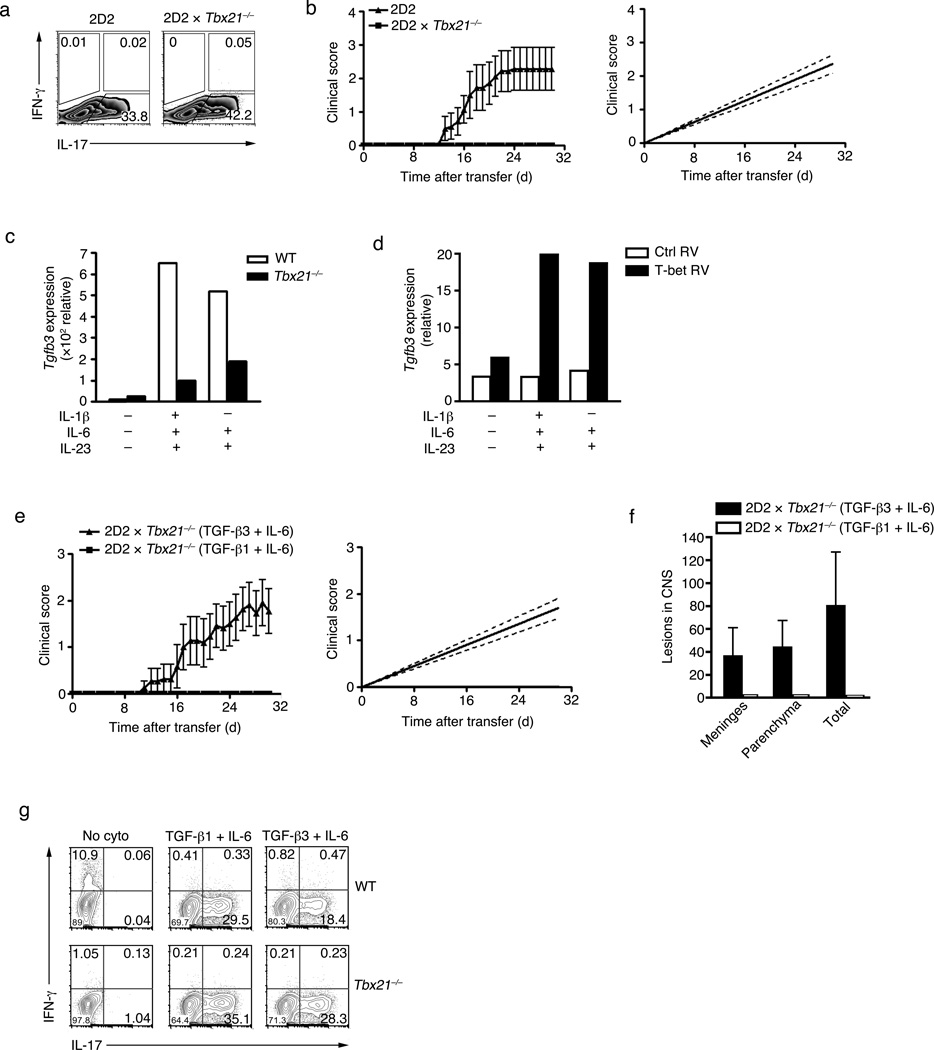
Our microarray analysis showed that TGF-β3-IL-6-induced TH17 cell expressed relatively higher level of the transcription factor Tbx21 (T-bet) (Fig. 4b). Because exposure of TH17 cells to IL-23 was shown to induce the expression of T-bet 33, we generated TGF-β1-IL-6-IL-23-induced TH17 cells from 2D2 Tbx21−/− and wild type T cells. Tbx21−/− TH17 cells induced higher expression of IL-17A as compared to wild-type TH17 cells (Fig. 5a), however 2D2 Tbx21−/−TH17 failed to transfer EAE, whereas wild-type 2D2 TH17 cells induced disease with high incidence and severity (Fig. 5b). This data indicates that T-bet plays a key role in inducing pathogenic functions of TH17 cells, and this is independent on IL-17 production. Because TGF-β3 is required for the induction of pathogenic signatures in TH17 cells, we tested whether the inability of T-bet-deficient T cells to induce disease was due to a decrease in TGF-β3 expression. In fact, TGF-β3 expression was decreased in T-bet-deficient TH17 cells (Fig. 5c). Conversely, retroviral over-expression of T-bet enhanced the expression of TGF-β3 (Fig. 5d). We next tested whether compensating for TGF-β3 in T-bet-deficient TH17 cells could induce EAE in an adoptive transfer system. Polarization of 2D2 Tbx21−/− CD4+ T cells in the presence of TGF-β3-IL-6, but not TGF-β1-IL-6-IL-23 or TGF-β1-IL-6, overcame the requirement of T-bet and were encephalitogenic (Fig. 5e and Table 1). Histological analysis showed that adoptively transferred TGF-β3-IL-6-induced 2D2 Tbx21−/− cells produced CNS lesions in the meninges and the parenchyma, while TGF-β1-IL-6-induced 2D2 Tbx21−/− cells produced no detectable lesions after transfer (Fig. 5f). T-bet-deficient T cells produced relatively higher amounts of IL-17 when differentiated with TGF-β1-IL-6-IL-23 or TGF-β3-IL-6 (Fig. 5g). Previous reports have suggested a requirement for IFN-γ-producing cells to breach the blood brain barrier in order to allow entry of IL-17 producing cells into the CNS 34. Analysis of peripheral or CNS isolated CD4+ T cells at the peak of disease revealed that TGF-β3-IL-6-induced 2D2 cells, but not 2D2 Tbx21−/− cells, expressed IFN-γ. This suggests that independent of IFN-γ production, TGF-β3-IL-6-induced 2D2 Tbx21−/− TH17 cells were pathogenic in vivo (Supplementary Fig. 3). These observations suggest that T-bet is part of the pathogenic TH17 signature and may regulate the endogenous expression of TGF-β3. Loss of pathogenicity in T-bet–deficient TH17 cells can be overcome by exogenous TGF-β3 and IL-6. It remains unclear whether T-bet is directly or indirectly required for the induction and/or maintenance of TGF-β3.
Figure 5. Exogenous TGF-β3 can overcome the absence of T-bet.
(a) Intracellular cytokine staining showing IL-17A and IFN-γ expression in naïve CD4+ T cells from 2D2 or 2D2 Tbx21−/− mice stimulated with PMA+ionomycin after in vitro differentiation with TGF-β1-IL-6-IL-23 for four days. (b) Mean clinical scores (disease incidence) in wild-type recipients 30 days after transfer of naïve CD4+ T cells (5×106 cells) from 2D2 transgenic or 2D2 Tbx21−/− mice that were differentiated in vitro with TGF-β1-IL-6-IL-23. Analysis by linear regression curve was performed and graphed including the 95% confidence band of the regression line. Data is pooled from two independent experiments (n=7). (c) Quantitative RT-PCR analysis of Tgfb3 mRNA expression from Tbx21−/− or wt naïve CD4+ T cells differentiated in vitro with IL-1β-IL-6-IL-23, IL-6-IL-23 or no cytokines for four days (d) Quantitative RT-PCR of Tgfb3 mRNA expression of wt CD4+ T cells differentiated in vitro with IL-1β-IL-6-IL-23, IL-6-IL-23 or no cytokines and retrovirally over-expressed with T-bet-RV or empty-vector-RV (both Thy1.1 marker) for four days. (e) Mean clinical scores (disease incidence) in wild-type recipients 30 days after transfer of naïve CD4+ T cells from 2D2 Tbx21−/− mice (5×106 cells) that were differentiated in vitro with TGF-β1-IL-6 or TGF-β3-IL-6. Analysis by linear regression curve was performed and graphed including the 95% confidence band of the regression line. Data is pooled from three independent experiments (n=11). (f) Quantification of CNS lesions in the meninges and parenchyma from recipient mice sacrificed on day 30 (***p<0.001). Data was pooled from three independent experiments, n=4. (g) Intracellular cytokine staining of IFNγ and IL-17 expression from naïve wt and Tbx21−/− CD4 T cells differentiated in vitro with TGF-β1-IL-6, TGF-β3-IL-6, or no cytokines for four days. All in vitro experiments are representative of at least three experiments with similar results. Adoptive transfer experiments are pooled from two to three independent experiments and cumulative data on the number of animals used are shown in Table 1.
Discussion
Although TH17 cells have been associated with induction of autoimmunity, emerging data suggests that not all TH17 cell are pathogenic and exposure to IL-23 is crucial for their ability to induce autoimmunity 16–18, 22. In this paper we show that TGF-β3 endogenously produced by developing TH17 cells plays a critical role in inducing pathogenic TH17 cells and T-bet a transcription factor normally associated with Th1 development is critical for the generation of pathogenic TH17 cells.
While IFNγ−/− and IFNγR−/− mice are highly susceptible to developing EAE 7, 8 mice lacking T-bet are protected from almost all autoimmune disease 9, 24. Our data begins to address this paradox by showing that T-bet is part of the pathogenic signature expressed by a subset of TH17 cells. Although it remains unclear how T-bet confers encephalitogenic potential of TH17 cells, we show that in the absence of T-bet, TH17 cells are not able to induce endogenous TGF-β3. Whether T-bet is directly transactivating TGF-β3 or is indirectly modulating other receptors/factors in TH17 cells is not clear at this stage. IL-23 signaling was shown to induce T-bet expression in TH17 cells and T-bet has been suggested to induce IL-23R expression35, suggesting a feed-forward loop between IL-23R-signaling and T-bet expression. Because T-bet expression in TH17 cells, it has been postulated that TH17 cells might produce IFN-γ and therefore, IL-17-IFN-γ double producers are the main pathogenic T cells that induce autoimmune disease. Our data suggests that the requirement for T-bet expression in pathogenic TH17 cells is independent of IFN-γ production, because IFN-γ-deficient TH17 cells induced with TGF-β3/IL-6 were still capable of inducing EAE (data not shown). One possible reason why we observe T-bet expression in TGF-β3/IL-6 induced TH17 cells is that, unlike TGF-β1, which suppresses T-bet 36 and IL-23R37, TGF-β3 promotes expression of these molecules, promoting the stability and pathogenicity of TH17 cells.
In addition to T-bet, several other factors were associated with the pathogenic signature of TH17, including GM-CSF, IL-23R and IL-7R. GM-CSF (csf2), which is transactivated by RORγt, was recently described as the key cytokine involved in the pathogenicity TH17 cells 25, 26 and our transcriptional analysis independently identified GM-CSF as a component of pathogenic TH17 cells. Indeed, GM-CSF-deficient mice are highly resistant to EAE and GM-CSF-deficient TH17 cells are incapable of transferring EAE 25, 26. This is consistent with our data that pathogenic TH17 cells induced by TGF-β3/IL-6 express GM-CSF. However, it remains to be determined whether TGF-β3/IL-6-induced TH17 cells require GM-CSF for encephalitogencity. Similarly, IL-23R is the key component of the pathogenic TH17 signature. IL-23R-deficient mice are resistant to EAE and IL-23R-deficient TH17 cells are unable to transfer EAE 16. The IL-7-IL-7R pathway has also been implicated in development of TH17 cells and genetic data link IL-7R to susceptibility to multiple sclerosis in patients 16, 38–41. However, the role of IL-7-IL-7R axis in the induction of pathogenic potential of TGF-β3/IL-6-induced TH17 cells remains to be determined. Whether IL-7 simply expands pathogenic TH17 cells or induces key molecules required for pathogenicity of TH17 cells has not been addressed as of yet. A systematic analysis of each of the molecules belonging to the pathogenic signature of TH17 cells will allow the identification of key nodes in the development of pathogenic TH17 cells.
Acquisition of a pathogenic phenotype by TH17 cells is not only the consequence of the upregulation of molecules that mediate pathology, but also of the downregulation of a number of other genes. We found that genes generally associated with immune-regulation, such as IL-10, Ahr and c-Maf, were significantly downregulated in pathogenic TH17 cells. Ahr and c-Maf are induced by TGF-β1/IL-6 conditions42–44, and they work together to transactivate IL-10 production 45. Failure to express these genes in TGF-β3/IL-6-induced TH17 cells might explain why these cells become highly pathogenic. Conversely, it will be interesting to determine whether TH17 cells induced by TGF-β1/IL-6 in the absence of Ahr, cMaf or IL-10 expression can attain pathogenic functions. IL-10 has been previously associated with non-pathogenic TH17 cells and exposure to IL-23 was shown to downregulate IL-10 production and increase their encephalitogenicity 21, 22. In addition to IL-10, nonpathogenic TH17 cells produced IL-1R antagonist (IL-1RN) and sIL-6R, both of which would presumably inhibit IL-1 and IL-6 signaling and thus further suppress differentiation of pathogenic TH17 cells. However, the relationship between IL-1R antagonism and IL-10 production by TH17 cells remains unclear at this point. It remains to be determined whether different subsets of TH17 cells, some that produces IL-17 and IL-10 and other that produces IL-17 and GM-CSF and express T-bet have evolved to clear different types of pathogens. Indeed, observations in human TH17 cells suggests that IL-17+IL-10+ TH17 cells are specific for S. aureus infection whereas IL-17+IFN-γ+ TH17 cells are specific for C.albicans infection 46. Similarly, our study suggests a pathogenic versus non-pathogenic subtypes of TH17 cells, and their development may be contingent on the type of TGF-β present during the initial differentiation stage.
Existing data suggest that both pathogenic and non-pathogenic TH17 cells exist in the repertoire. While the initial differentiation of IL-17-producing cells is not dependent on exposure to IL-23, it is now clear that IL-23 is required for attaining full pathogenic potential in TH17 cells by maintenance and stabilization of the TH17 phenotype, suppression of IL-10 production, promotion of GM-CSF production and other effector molecules and maintenance of TGF-β3 expression. The fact that enhancement and maintenance of TGF-β3 are dependent on IL-23 exposure is especially interesting given that TGF-β1/IL-6-differentiated TH17 cells cannot transfer disease, yet when exposed to IL-23 they acquire pathogenic potential and induce autoimmune disease. Thus, it is likely that the IL-23-dependent TGF-β3 production in TGF-β1/IL-6-differentiated TH17 cells is required for the induction of full pathogenic phenotype in TH17 cells to induce autoimmunity.
In summary, we report a critical role for TGF-β3 cell autonomously produced by TH17 cells in inducing pathogenic TH17 cells. Whereas both TGF-β1 and TGF-β3 can induce TH17 cells, TGF-β3 induced TH17 cells differ from TGF-β1 induced TH17 cells in multiple ways. TGF-β3 induced TH17 cells are not only pathogenic in inducing EAE but also very potent in inducing colitis in the adoptive transfer model (data not shown), supporting a broader role of TGF-β3 in inducing highly pathogenic and proinflammatory TH17 cells. Since TGF-β3 induces highly pathogenic TH17 in both EAE and colitis, TGF-β3 may provide a useful target for regulating tissue inflammation in multiple autoimmune diseases.
Materials and Methods
Mice
C57BL/6 wild-type (wt), TGF-βRII DN mice were from the Jackson Laboratory. IL-17A-GFP.KI mice were purchased from Biocytogen LLC (Worcester, MA). IL-23R-GFP.KI and IL-23R−/− mice were generated as described 16. TGF-β3fl/fl mice were generously provided by Dr. Ramireddy Bomireddy of University of Arizona. TGF-β3-YFP reporter mice were generated by crossing TGF-β3Cre 28 mice with Rosa26YFP. TGF-β3-RosaDTR were generated by crossing TGF-β3Cre 28 to Rosa26DTR mice. Wt, 2D2, 2D2xIL-23R−/−, 2D2xT-bet−/−, T-bet−/−, IL-23R-GFP.KI, IL-17A-GFP.KI mice10 were housed and maintained in a conventional pathogen-free facility at the Harvard Institute of Medicine in Boston, MA. All experiments were performed in accordance to the guidelines outlined by the Harvard Medical Area Standing Committee on Animals at the Harvard Medical School (Boston, MA).
Cell sorting, flow cytometry, p-smad and intracellular cytokine staining (ICC)
Cells were sorted with anti-CD4-PerCP, anti-CD62L-APC, anti-CD44-PE antibodies (all Biolegend, CA). Cells were stimulated with phorbol 12-myristate 13-aceate (PMA) (50ngml−1, Sigma-aldrich, MO) and ionomycin (1µgml−1, Sigma-aldrich, MO) and a protein transport inhibitor containing monensin (Goligistop) (BD Biosciences) for four hours prior to detection with staining with antibodies. Surface markers were stained in PBS with 1% FCS for 20 min in room temperature, then subsequently fixed in Cytoperm/Cytofix (BD Biosciences), permeabilized with Perm/Wash Buffer (BD Biosciences) and stained with cytokine antibodies (anti-IFN-γ-APC anti-IL-17A-alexa fluora 488 ab, all from Biolegend) diluted in Perm/Wash buffer as described previously 10. For psmad signaling flow cytometry, LN and SP cells from C57BL/6 wt mice were labeled with CD4 microbeads (Miltenyi Biotec, MA) then purified via the Automacs (Miltenyi Biotec, MA). Cells were stimulated in serum free media and serum starved overnight then subsequently cultured with either TGF-β1 or TGF-β3 (2ng/ml) for 30 min. Cells were fixed with Cytoperm/Cytofix (BD Biosciences), permeabilized with Perm/Wash Buffer (BD Biosciences), then stained with psmad2&3, psmad1&5, and isotype control antibodies (all Cell Signaling, MA). Data was collected using FACS Calibur or LSR II (Both BD Biosciences), then analyzed using Flow Jo software (Treestar, OR) 16, 47.
Active and passive EAE induction and disease analysis
For active EAE induction, mice were immunized with 100µg MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) in CFA injected subcutaneously, mice received pertussis toxin (List Biological Laboratory, CA) via intraperitoneally (i.p.). For local blockade of TGF-β3, 100ng/ml anti-TGF-β3 antibody (R&D, MN) was incorporated into the emulsion, and mice received 200ng of pertussis toxin on day 0 and 2. For passive EAE induction, cells were differentiated and adoptively transferred as previously described 16, 22. Animals were monitored and scored daily for development of EAE according to the following criteria: Animals were monitored daily for the development of classical and atypical signs of EAE according to the following criteria: 0, no disease; 1, decreased tail tone or mild balance defects; 2, hind limb weakness, partial paralysis or severe balance defects that cause spontaneous falling over; 3, complete hind limb paralysis or very severe balance defects that prevent walking; 4, front and hind limb paralysis or inability to move body weight into a different position; 5, moribund state22.
Analysis of CNS infiltrating mononuclear cells
At the peak of disease, mice were sacrificed for analysis of CNS infiltrating cells. Mice were perfused through the left ventrical of the heart with cold PBS. The brain and the spinal cord were flushed out with PBS by hydrostatic pressure. CNS tissue with minced with a sharp razor blade and digested with collagenase D (2.5mg/ml, Roche Diagnostics, IN) and DNaseI (1mg/ml, Sigma, MO) at 37°C for 20 minutes. Mononuclear cells were isolated by passing the tissue through a cells strainer (70um), followed by a percoll gradient (37% and 70%) centrifugation. Mononuclear cells in the interphase was removed, washed and resuspended in culture medium for analysis by intracellular cytokine staining.
In vitro T-cell differentiation
CD4+ T cells were purified from spleen and lymph nodes using anti-CD4 microbeads (Miltenyi Biotech) then further sorted for naïve CD4+ CD62Lhi CD44low T cells. Sorted cells were activated with plate bound anti-CD3 (2µg/ml) and anti-CD28 (2µg/ml) in the presence of cytokines (rmIL-23: R&D Systems, all other cytokines: Miltenyi Biotec). Anti-TGF-β3 (R&D Biosystems) were neutralized at 30µg/ml. For TH17 differentiation: 2ng/mL rhTGF-β1 and rhTGF-β3 (Miltenyi Biotec), 25ng/mL rmIL-6 (Miltenyi Biotec), 20ng/ml rmIL-23 (R&D Biosystems), and 20ng/ml rmIL-1β (Miltenyi Biotec). Cells were cultured for 4 days and harvested for RNA, intracellular cytokine staining, and for flow cytometry.
Cytokine analysis (ELISA) and real time PCR
Cytokines in culture supernatants were collected on day 4 and determined by enzyme-linked immunosorbent assay (ELISA) as previously described 10. On day 4 (unless otherwise noted) post culture, RNA was extracted with RNeasy kit (Qiagen), reverse transcribed using iscript cDNA synthesis kit (Bio-rad, CA), and was analyzed by quantitative RT-PCR with vii7 Real-time PCR systems (Applied Biosystems) for the gene of interest. Primer/probe mixtures were purchased from Applied Biosystems. The comparative threshold cycle method and an internal control (GAPDH) were use for normalization of the target genes. The list of primer and probes from Applied Biosystems: Cxcl3: Mm01701838_m1, Il3: Mm00439631_m1, Csf2: Mm01290062_m1, Il1rn: Mm01337566_m1, Lrmp: Mm00493168_m1, Gzmb: Mm00442834_m1, Tbx21: Mm00450960_m1, Il17a: Mm00439618_m1, Il17f: Mm00521423_m1, Ifng: Mm01168134_m1, Ahr: Mm00478932_m1, Il9: Mm00434305_m1, Il10: Mm00439614_m1, Maf: Mm02581355_s1, Il22: Mm00444241_m1, Il23r: Mm00519943_m1, Rorc: Mm00441144_g1, Tgfb1: Mm01178820_m1, Tgfb2: Mm00436955_m1, Tgfb3: Mm01307950_m1, GAPDH: 4352339E
TGF-β PCR array
TGF-β/BMP PCR array kit was purchased from Sabiosciences and cells were processed using manufacturer’s protocol. Naïve CD4 T cells (CD44neg CD62L+ CD4+) were sorted using BD Biosciences FACAria Cell sorter and cultured in vitro under TGF-β1/IL-6 or TGF-β3/IL-6 conditions. After 24 hours, cells were harvested and total RNA was purified using RNeasy mini kit (Qiagen, CA). RNA was converted to cDNA using manufacturer’s cDNA sythesis kit, RT2 First Strand Kit (Sabiosciences, CA). SYBR Green qPCR Master mix provided by the manufacturer was used and plated in pre-coated 96 well PCR array kit. RT-PCR was performed using the vii7 real time PCR system (Applied Biosystems, CA). Raw data was uploaded onto the manufacturer’s website and analyzed.
Histopathologic analysis
Mice were sacrificed 30 days after transfer and the CNS fixed in 10% neutral-buffered formalin and processed routinely for paraffin embedment. Inflammatory foci (>10 mononuclear cells) were quantified in the meninges and parenchyma by a pathologist in a double blind manner, such that disease status of the mice as well as the cell transfer groups were not revealed.
Microarray analysis
Wt naïve T cells were sorted and activated for 4 days with plate bound anti-CD3, anti-CD28 alone, or with the indicated cytokines. RNA was purified using the RNeasy kit (Qiagen, RD), amplified using the Ovation Biotin RNA Amplification and Labeling System (NuGEN, CA). The cDNA (10µg) was fragmented, labeled, and hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affymetrix). The data was normalized using the GenePattern software 48 with the Robust Multi Array (RMA) algorithm 49. Since the data was collected in two batches (SOM), we use the COMBAT software to remove batch effects 50. For our clustering analysis (Fig. 4a) we only considered probe sets that show at least two fold change (up or down) compared with the no-cytokine (TH0) state in at least one of the conditions (572 probe sets corresponding to 434 genes; when several repeats were available, we take the average fold change over all repeats). We clustered the genes using average linkage hierarchical agglomerative clustering.
To directly compare the gene expression profiles of TGF-β1/IL-6 and TGF-β3/IL-6 TH17 cells we considered genes that showed at least a 1.5 fold change (up or down) between these two conditions. From the 233 genes that showed at least a 1.5 fold change (Supplementary Table 1), we selected 23 representative genes based on their biological function for further validation and principal component analysis (PCA). Similar results were obtained when PCA was performed using all 233 genes that showed at least a 1.5 fold change or only the 23 representative genes (Fig. 4d and e).
Statistics
GraphPad Prism 4.0 was used for statistical analysis (linear regression with 95% confidence interval, and unpaired, two-tailed Student’s t test). Differences were considered statistically significant when p < 0.05. * denotes. *p<0.05, **p<0.01, ***p<0.001.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Bomireddy (University of Arizona) for generously providing the TGF-β3fl/fl mice and D. Kozoriz for cell sorting. This work was supported by the National Institutes of Health (NS030843, NS045937, AI073748 and AI045757 to V.K.K.) and grant RG2571 from the National MS Society, New York. AA was supported by a research grant from Crohn’s and Colitis Foundation of America, New York. FQJ was supported by grants AI075285, AI093903 from National Institutes of Health and RG4111A1 from the National Multiple Sclerosis Society. This work was also supported by the Merkin Foundation for Stem Cell Research at the Broad Institute, the NIH Pioneer Award, an NHGRI P01 and HHMI (to AR).
Footnotes
AUTHOR CONTRIBUTIONS
Y.L., A.A. designed the study, performed the experiments, analyzed the data, and wrote the manuscript. A.P., S.X., S.K., M.K. C.W did in vitro experiments. N.Y., F.J.Q., A.R. did the bioinformatics analysis of gene expression data. R.A.S did the immunohistochemical analysis. D.H provided discussion. V.K.K. and A.A. supervised the study and edited the manuscript.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 6.Kuchroo VK, et al. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 7.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 8.Wildbaum G, Youssef S, Grabie N, Karin N. Neutralizing antibodies to IFN-gamma-inducing factor prevent experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6368–6374. [PubMed] [Google Scholar]
- 9.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 18.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman P, et al. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 2008;58:1020–1025. doi: 10.1002/art.23389. [DOI] [PubMed] [Google Scholar]
- 21.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 22.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esplugues E, et al. Control of TH17 cells occurs in the small intestine. Nature. 475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codarri L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 26.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQualter JL, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang LT, Li WY, Kaartinen V. Tissue-specific expression of Cre recombinase from the Tgfb3 locus. Genesis. 2008;46:112–118. doi: 10.1002/dvg.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons RM, Miller DA, Graycar JL, Moses HL, Derynck R. Differential binding of transforming growth factor-beta 1, -beta 2, and -beta 3 by fibroblasts and epithelial cells measured by affinity cross-linking of cell surface receptors. Mol Endocrinol. 1991;5:1887–1896. doi: 10.1210/mend-5-12-1887. [DOI] [PubMed] [Google Scholar]
- 30.Graycar JL, et al. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- 31.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 33.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gocke AR, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 36.Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol. 2005;175:5666–5674. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 38.Walline CC, Kanakasabai S, Bright JJ. IL-7Ralpha confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 12:1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 40.Lundmark F, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 41.Gregory SG, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 42.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 43.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 44.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and T(H)-17 cells. Nat Immunol. 2008 doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielinski CE, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 47.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 48.Reich M, et al. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 49.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 50.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.