Abstract
The nuclear factor-κB (NF-κB) family of transcription factors has been shown to regulate proliferation in several cell types. Although recent studies have demonstrated aberrant expression or activity of NF-κB in human breast cancer cell lines and tumors, little is known regarding the precise role of NF-κB in normal proliferation and development of the mammary epithelium. We investigated the function of NF-κB during murine early postnatal mammary gland development by observing the consequences of increased NF-κB activity in mouse mammary epithelium lacking the gene encoding IκBα, a major inhibitor of NF-κB. Mammary tissue containing epithelium from inhibitor κBα (IκBα)-deficient female donors was transplanted into the gland-free mammary stroma of wild-type mice, resulting in an increase in lateral ductal branching and pervasive intraductal hyperplasia. A two- to threefold increase in epithelial cell number was observed in IκBα-deficient epithelium compared with controls. Epithelial cell proliferation was strikingly increased in IκBα-deficient epithelium, and no alteration in apoptosis was detected. The extracellular matrix adjacent to IκBα-deficient epithelium was reduced. Consistent with in vivo data, a fourfold increase in epithelial branching was also observed in purified IκBα-deficient primary epithelial cells in three-dimensional culture. These data demonstrate that NF-κB positively regulates mammary epithelial proliferation, branching, and functions in maintenance of normal epithelial architecture during early postnatal development.
INTRODUCTION
The mammary gland is an organ designed to deliver nourishment and passive immunity to infant mammals. It consists of an epithelium that synthesizes and secretes lipid and milk proteins, as well as a fatty stroma that provides support and local growth regulatory cues to the epithelium (reviewed by Medina, 1996). Although the mammary gland rudiment is established during embryogenesis, the majority of mammary gland development occurs postnatally. During puberty, the epithelium proliferates and branches in response to hormonal signals, eventually extending throughout the entire stroma. More extensive growth and differentiation of the epithelium occurs during each round of pregnancy. The distal tips of each epithelial branch proliferate and differentiate into lobuloalveoli, which synthesize and secrete milk during lactation. Upon cessation of nursing, the majority of the epithelium undergoes apoptosis in a process called involution (reviewed by Furth, 1999). After involution, the epithelium remains relatively quiescent until the next pregnancy, when the morphogenetic cycle is repeated.
The nuclear factor-κB (NF-κB) family of transcription factors regulates growth, differentiation, and apoptosis in several tissues, including lymphocytes, embryonic limb, lung and liver, skin, and bone (Beg et al., 1995; Klement et al., 1996; Boothby et al., 1997; Franzoso et al., 1997; Bushdid et al., 1998; Kanegae et al., 1998; Seitz et al., 1998; Bendall et al., 1999; Hu et al., 1999; Li et al., 1999a,b,c; Takeda et al., 1999; Tanaka et al., 1999; Chen et al., 2000a; Muraoka et al., 2000; Rudolph et al., 2000). In unstimulated cells, NF-κB dimeric complexes are sequestered in the cytoplasm by association with members of a family of specific inhibitors of κB (IκBs). Upon receipt of extracellular stimulators, such as growth factors, cytokines, or pathogenic agents, IκB is phosphorylated on evolutionarily conserved amino-terminal serine residues by specific IκB kinases. This phosphorylation event leads to polyubiquitination and proteosome-mediated degradation of IκB. Proteolytic degradation of IκB liberates the NF-κB dimer, allowing the active complex to translocate to the nucleus, bind specific DNA regulatory elements, and mediate changes in the expression of downstream target genes (reviewed by Verma et al., 1995; Ghosh et al., 1998; May and Ghosh, 1998).
The NF-κB family members p50 and RelA, as well as the IκB factors p105 and IκBα, are expressed in the murine mammary epithelium over the course of normal postnatal morphogenesis (Brantley et al., 2000; Clarkson et al., 2000). Moreover, maximal NF-κB activity is detected in the mammary gland during pregnancy, when the epithelium is proliferating, and also during involution, when the epithelium is undergoing apoptosis. These data suggest that NF-κB may regulate proliferation, apoptosis, or both during normal postnatal mammary epithelial morphogenesis. To ascertain the function of elevated NF-κB in the mammary epithelium during early postnatal morphogenesis, we have examined the morphology and development of mouse mammary epithelium lacking the gene encoding a major inhibitor of NF-κB, IκBα. Because IκBα-deficient mice die before the majority of mammary epithelial development occurs, a transplantation approach was taken. Mammary tissue was transplanted from IκBα-deficient neonatal mice into wild-type, gland-free mammary fat pads to circumvent neonatal lethality and to permit postnatal development of the mammary epithelium. We demonstrate that IκBα-deficient mammary epithelium is hyperplastic, displays increased lateral ductal branching, and contains decreased levels of extracellular matrix in virgin animals, suggesting that NF-κB modulates proliferation, branching, and normal structural development of the mammary epithelium during early postnatal morphogenesis.
MATERIALS AND METHODS
Mouse Strains
Generation and characterization of IκBα-deficient mice was described by Chen et al. (2000a). The phenotype of these mice is consistent with independently derived IκBα-deficient mice (Beg et al., 1995; Klement et al., 1996). The genotype of IκBα-deficient neonatal mice and littermates was confirmed by Southern analysis of genomic DNA isolated from tail biopsies. Genomic DNA (10 μg) was digested with BamHI and probed with a 1300-bp XbaI/NdeI fragment encompassing sequences from the 5′-flanking region of the iκbα gene present within the BamHI restriction site boundaries of the null allele (Chen et al., 2000a). Wild-type, heterozygous, or IκBα-deficient mice were housed in microisolators under identical conditions. Mice heterozygous for IκBα were bred with homozygous HLL mice, a transgenic mouse model harboring a luciferase transgene under the regulation of the NF-κB-responsive human immunodeficiency virus long terminal repeat (HIV-LTR; Blackwell et al., 2000; Brantley et al., 2000). Mice that were iκbα+/−, and positive for the HLL transgene were intercrossed to generate iκbα+/+, iκbα+/−, and iκbα−/− mice that were either positive or negative for HLL. Again, the genotype of these animals was confirmed by Southern analysis for iκbα wild-type and null alleles, as well as the hll transgene, by using genomic DNA from tail biopsy.
Tissue Luciferase Assay
Luciferase activity in the mammary tissue extracts prepared from all 10 neonatal whole mammary glands was quantified as previously described (Brantley et al., 2000), and protein concentrations in the extracts were determined by Lowry assay (Bio-Rad, Richmond, CA). All values are presented as relative light units (RLUs)/μg protein.
Mammary Tissue Transplantation
Isolation and transplantation of neonatal mammary epithelial tissue has been described previously (DeOme et al., 1959; Seagroves et al., 1998; Robinson et al., 1999). Briefly, number 4 inguinal mammary donor tissue located between the nipple region and lymph node, the region that contains the epithelial rudiment, was surgically removed from 6-d-old female wild-type, heterozygous, or IκBα-deficient neonatal mice and stored in DMEM (Mediatech, Herndon, VA) on ice before transplantation. The epithelium within the number 4 inguinal mammary glands of 3-wk-old virgin female C57Bl/6J recipient mice was surgically cleared by removing the portion of mammary tissue between the nipple region and the lymph node, the region in which all endogenous epithelium is contained at this stage of development. Neonatal donor tissue containing the epithelial rudiment (∼2 × 2 mm) was then implanted into an incision in the center of the remaining surgically cleared mammary fat pad. The mammary glands were analyzed 6 to 8 wk after transplantation. A portion of these samples was used for secondary rounds of mammary tissue reconstitution of wild-type cleared mammary stroma. These secondary recipient glands were collected 6 to 8 wk later, as well as the number 3 thoracic glands harboring unmanipulated host epithelium.
Histological Analyses
Whole-mount hematoxylin staining of reconstituted mammary glands, as well as native glands from host animals, was performed as described previously (Seagroves et al., 1998). Briefly, number 4 inguinal mammary glands were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.2, overnight at 4°C. The glands were washed in acetone, equilibrated into 95% ethanol, and stained in Mayer's hematoxylin solution (VWR Scientific, West Chester, PA) overnight at room temperature, light protected. The following day, the glands were destained in tap water and then further destained in 50% ethanol acidified with hydrochloric acid at a 0.05 M final concentration. The glands were then dehydrated in a graded ethanol series followed by xylenes, equilibrated into methyl salicylate (Sigma, St. Louis, MO), and photodocumented (Zeiss Stemi SV 11).
For analysis of the subcellular architecture, expression of matrix proteins, and expression of IκBα and RelA proteins, mammary glands were embedded in paraffin and 7-μm sections prepared. Hematoxylin and eosin staining was performed as described previously (Seagroves et al., 1998). Trichrome staining for visualization of the extracellular matrix was performed by the Vanderbilt University Skin Disease Research Center. Reconstituted glands were subjected to immunohistochemistry by using anti-IκBα and anti-RelA antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (Brantley et al., 2000). Specific immunoreaction was detected using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and 3′,3′-diaminobenzidine tetrahydrochloride horseradish peroxidase substrate (Zymed Laboratories, South San Francisco, CA) according to manufacturer's protocols. Sections were counterstained with hematoxylin before dehydration, mounting in Permount (Fisher Scientific, Pittsburgh, PA), and photomicroscopy (Olympus BX60). Mammary glands from 20 to 25 primary and secondary recipients from three to four primary donors per condition were analyzed.
Proliferation and Apoptosis Assays
For proliferation assays, a sterile solution containing 10 mg/ml bromodeoxyuridine (BrdU; Sigma) in PBS was injected intraperitoneally (100 μl/10 g of body weight). After 4 h, the mice were sacrificed, mammary glands were collected, fixed, and 7-μm sections prepared. BrdU incorporation was visualized by immunohistochemistry by using a BrdU detection kit (Zymed Laboratories) according to manufacturer's protocol, and nuclei were counterstained with hematoxylin. For quantification, 10 random fields per section at 40× magnification were documented by photomicroscopy, and the percentage of BrdU-positive epithelial cell nuclei relative to the total number of epithelial cell nuclei was calculated. The averages of 12 to 14 independent secondary reconstitution samples derived from three to four primary donor animals per genotype were quantified. The total number of nuclei was also quantified in each of these samples.
For apoptosis assays, mammary glands were collected, fixed, and 7-μm sections prepared as described. Fragmented DNA was labeled with a digoxigenin-conjugated UTP by using terminal deoxytransferase (Intergen, Purchase, NY). Positive nuclei were visualized by immunohistochemistry with an ApopTag labeling and detection kit (Intergen) according to manufacturer's protocol, and nuclei were counterstained with methyl green. For quantification, 10 random fields per section were documented by photomicroscopy, and the percentage of and terminal deoxynucleotide UTP nick-end labeling (TUNEL)-positive epithelial cell nuclei relative to the total number of epithelial cell nuclei was calculated. The averages of six independent primary or secondary reconstitution samples per genotype were determined.
Three-dimensional Mammary Epithelial Cell Culture
Primary mammary epithelial cells were isolated from mammary glands reconstituted with heterozygous/wild-type or IκBα-deficient mammary tissue and embedded in reconstituted matrix (Matrigel, growth factor-reduced; Becton Dickinson, Franklin Lakes, NJ). Briefly, the reconstituted glands were surgically removed, the lymph nodes excised, and the glands minced and digested in DMEM:F12 (Cellgro) supplemented with collagenase A (1.5 mg/ml; Roche Molecular Biochemicals, Indianapolis, IN) and hyaluronidase (100 U/ml; Sigma) at 37°C, shaking. The primary cells were then washed in PBS supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and embedded into growth factor-reduced Matrigel as per supplier's instructions. The three-dimensional cultures were maintained in DMEM:F12 supplemented with l-glutamine, antibiotics (Mediatech), and 10% fetal bovine serum, and the cultures were monitored over several days to observe in vitro tubulogenesis. The cultures were photodocumented and the number of lateral branches in 6 to 10 fields per culture quantified.
RESULTS
IκBα-deficient Mammary Epithelium Displays Increased Ductal Branching and a Disorganized Epithelial Architecture
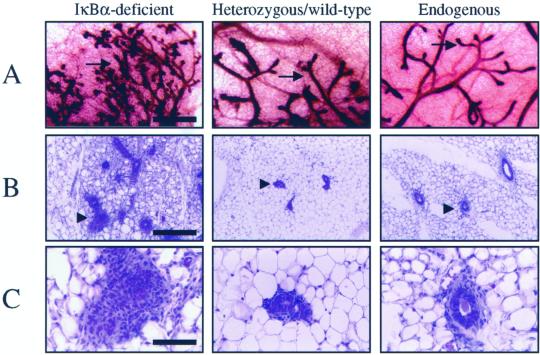
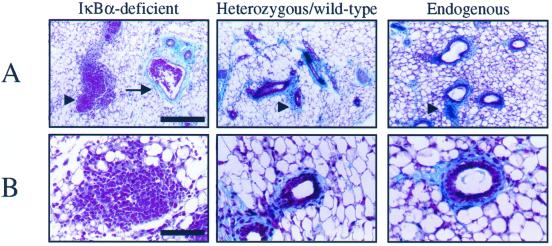
To ascertain the function of NF-κB in mammary epithelial development, mammary tissue lacking the gene encoding a major inhibitor of NF-κB, IκBα, was examined. Because IκBα-deficient mice die ∼9 d after birth (Beg et al., 1995; Klement et al., 1996; Chen et al., 2000a), postnatal development of mammary epithelium was enabled by transplanting epithelium-containing mammary tissue from neonatal IκBα-deficient female mice or heterozygous/wild-type littermates (n = 3–4 primary donors/genotype) into juvenile wild-type mouse mammary fat pads in which the endogenous epithelium was surgically removed (DeOme et al., 1959; Medina, 1996). Six to eight weeks after the initial transplantation, reconstituted mammary glands were isolated and used as donor tissue for a second round of reconstitution into several wild-type recipients (n = 6–8 recipients/original donor/genotype) to reduce the level of contaminating IκBα-deficient stroma introduced in the primary recipients and to provide more samples for analysis. These glands were then collected and analyzed 6 to 8 wk after transplantation. The epithelia of the reconstituted glands were stained in whole-mount with hematoxylin to visualize the gross morphology of the gland. Glands reconstituted with IκBα-deficient epithelium contained ductal branches that permeated the fat pad to the same degree and in a pattern similar to that observed in heterozygous/wild-type epithelium, as well as in native host epithelium (Figure 1A). However, an overall increase in the number of lateral ductal branches was observed in IκBα-deficient epithelium. These lateral branches emanated from a larger ductal network, and displayed numerous tertiary lateral branches in the IκBα-deficient epithelium that were not observed in control glands. In addition, the epithelium displayed pervasive intraductal hyperplasia, a phenotype that was confirmed by examining the cellular architecture in histological sections (Figure 1, B and C). Compared with control glands, mammary glands harboring IκBα-deficient epithelium presented a much higher epithelial-to-stromal cell ratio (Figure 1B). In many regions, the central ductal lumina were absent in IκBα-deficient epithelium or were surrounded by multiple epithelial cell layers rather than a single layer as seen in heterozygous/wild-type epithelium or host epithelium (Figure 1, B and C). Higher magnification revealed that the IκBα-deficient epithelial structure appeared to be disorganized (Figure 1C).
Figure 1.
Mammary glands harboring IκBα-deficient epithelium display increased lateral branching, pervasive intraductal hyperplasia, and abnormal epithelial morphology. (A) Whole-mount hematoxylin staining of mammary glands revealed that epithelial tissue derived from IκBα-deficient donors displayed an increase in the number of lateral ductal branches. Arrows indicate epithelium. Representative samples are shown and all samples are age-matched. Bar, 2 mm. (B) Hematoxylin and eosin-stained sections of glands reconstituted with IκBα-deficient, heterozygous/wild-type epithelium, and intact endogenous host glands revealed that IκBα-deficient epithelium displayed pervasive intraductal hyperplasia. Samples are shown at low (B) and high (C) magnification. Arrowheads denote epithelium. Bar, 500 μm (B) and 50 μm (C). Data are a representation of 20 to 25 independent glands per genotype.
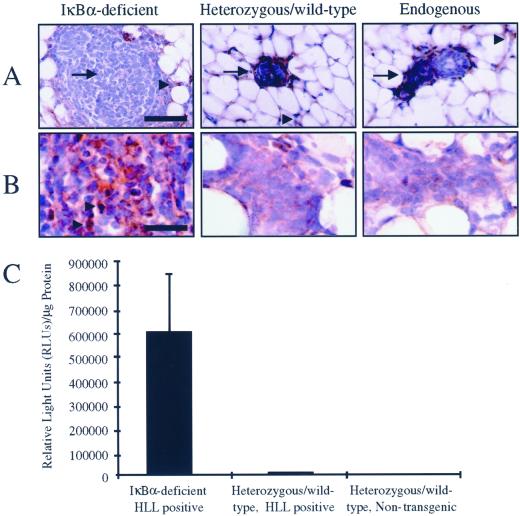
To confirm that IκBα protein was absent in the epithelium in mammary glands reconstituted with IκBα-deficient tissue, immunohistochemical analysis was performed. IκBα was not detected in mammary epithelium derived from null animals, although IκBα was present in the surrounding host stroma (Figure 2A). Consistent with previous studies, expression of IκBα was observed in mammary epithelium derived from heterozygous/wild-type littermates and also in host mammary epithelium from unmanipulated glands (Figure 2A; Brantley et al., 2000). These data suggest that loss of IκBα in the mammary is responsible for increased ductal branching and alteration of the histological structure of the epithelium derived from iκbα-defective donors.
Figure 2.
Lack of IκBα protein expression and enhanced transcriptional activity in IκBα-deficient mammary tissue. (A) Mammary glands reconstituted with IκBα-deficient tissue, heterozygous/wild-type tissue, or native host glands were subjected to immunohistochemistry for detection and localization of IκBα protein. Arrow indicates epithelium, arrowhead indicates surrounding host stroma. Samples shown are representative of IκBα expression for each genotype. Bar, 50 μm. (B) Mammary glands reconstituted with IκBα-deficient tissue, heterozygous/wild-type tissue, or native host glands were subjected to immunohistochemistry for detection and localization of RelA protein. Arrowhead indicates positive nuclear RelA expression. Bar, 10 μm. (C) Mammary glands from neonatal IκBα-deficient or heterozygous/wild-type littermates harboring a NF-κB-responsive luciferase reporter transgene (HLL) were analyzed for luciferase activity as a measure of in vivo NF-κB activity. An approximate 50-fold increase in luciferase activity was observed in IκBα-deficient, HLL-positive glands relative to controls. Data are a representation of three independent samples per genotype. Error bars represent SE of the mean, p < 0.03, χ2 analysis.
To assess expression levels and localization of RelA, a major transactivator within the NF-κB family that is present in mammary epithelium, immunohistochemical analysis was performed (Brantley et al., 2000). Compared with mammary glands reconstituted with heterozygous/wild-type epithelium or endogenous epithelium, glands reconstituted with IκBα-deficient epithelium displayed an increase in expression and nuclear localization of RelA (Figure 2B). These data confirm that loss of IκBα in the mammary epithelium results in increased nuclear import of the transactivator RelA.
To determine whether the morphological alterations of the IκBα-deficient epithelium are due to elevated NF-κB activity, iκbα+/− mice were mated to HLL mice, a transgenic mouse model expressing a luciferase reporter transgene under the regulation of the NF-κB-responsive HIV-LTR. Because the HIV-LTR contains two NF-κB enhancer elements that bind a broad range of homodimeric and heterodimeric NF-κB complexes (Kretzschmar et al., 1992; Liu et al., 1992; Doerre et al., 1993), HLL transgenic animals have been used as a model to quantify both constitutive and induced NF-κB activity in vivo in several organ systems, including the mammary gland (Blackwell et al., 2000; Brantley et al., 2000; Hao et al., 2000). Mice heterozygous for the targeted iκbα allele and hemizygous for the transgene were intercrossed to generate iκbα−/−, iκbα+/−, or iκbα+/+ offspring that also harbored the HLL transgene. Quantification of luciferase activity in neonatal mammary gland extracts collected from these mice revealed an ∼50-fold increase in luciferase reporter activity in IκBα-deficient mice compared with wild-type/heterozygous littermates or nontransgenic animals (IκBα-deficient, HLL-positive 596,849 ± 238,716 RLUs/μg protein; heterozygous/wild-type, HLL-positive 11,906 ± 2,454 RLUs/μg protein; heterozygous/wild-type, HLL negative 3,402 ± 1,964 RLUs/μg protein, p > 0.03, χ2 analysis; Figure 2C). The level of luciferase activity in heterozygous/wild-type, HLL-positive mammary extracts are consistent with previously published levels of luciferase activity in virgin HLL mammary glands (Brantley et al., 2000). Therefore, loss of IκBα results in elevated activity of NF-κB, suggesting that morphological abnormalities of IκBα-deficient mammary epithelium are due to increased NF-κB activity.
IκBα-deficient Mammary Epithelium Displays Increased Epithelial Proliferation, but no Change in Apoptosis
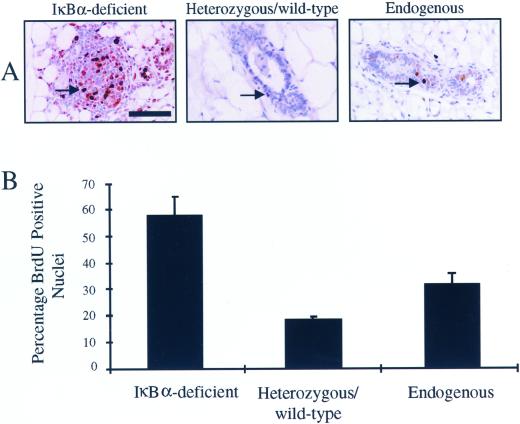
The observed increase in the epithelial content in mammary glands harboring IκBα-deficient epithelium may reflect an increase in the rate of epithelial proliferation and/or a decrease in the level of epithelial apoptosis. We tested both of these possibilities by quantifying BrdU incorporation and TUNEL assays, respectively. To assess the levels of proliferation in IκBα-deficient epithelium versus heterozygous/wild-type or host epithelium, reconstituted virgin animals were labeled with the thymidine analog BrdU. Based on immunohistochemical analysis, the number of BrdU-positive nuclei in IκBα-deficient mammary epithelium appeared to be greater than that in mammary glands reconstituted with heterozygous/wild-type tissue or in native mammary epithelium (Figure 3A). To quantify this increase, the percentage of BrdU-positive nuclei relative to the total number of nuclei was calculated. A two- to threefold increase in the percentage of BrdU-positive epithelial nuclei was observed in IκBα-deficient epithelium versus control epithelium (IκBα-deficient: 57 ± 7%; heterozygous/wild-type: 18 ± 1%; endogenous 30 ± 5%, p < 0.0004, χ2 analysis; Figure 3B). Because glands reconstituted with IκBα-deficient epithelium appear to contain a greater number of epithelial cells than age-matched controls, epithelial cell density was also quantified. Consistent with the higher level of proliferation in IκBα-deficient epithelium, there was a striking increase in the number epithelial cells per 40× field (IκBα-deficient: 276 ± 50 cells/field; heterozygous/wild-type: 93 ± 12 cells/field; endogenous: 105 ± 16 cells/field, p < 0.0004, χ2 analysis). These data demonstrate that mammary epithelium lacking IκBα is indeed hyperplastic in virgin animals.
Figure 3.
Mammary glands harboring IκBα-deficient epithelium display increased epithelial cell proliferation. (A) Immunohistochemical analysis of BrdU incorporation into glands reconstituted with IκBα-deficient tissue or with heterozygous/wild-type tissue, or endogenous host epithelium revealed hyperplasia in IκBα-deficient epithelium. Arrows indicate BrdU-positive nuclei. Bar, 50 μm. (B) Percentage of BrdU-positive nuclei was calculated according to the following formula: no. of positive nuclei/no. of total nuclei per 40× field. Values presented are the mean of a total of 10 individual fields from each section analyzed. A two- to threefold increase in the percentage of BrdU incorporation was observed in IκBα-deficient epithelium. Data are a representation of 12 to 14 independent secondary reconstitution samples derived from three to four primary donor animals per genotype. Error bars represent SE of the mean, p < 0.0004, χ2 analysis.
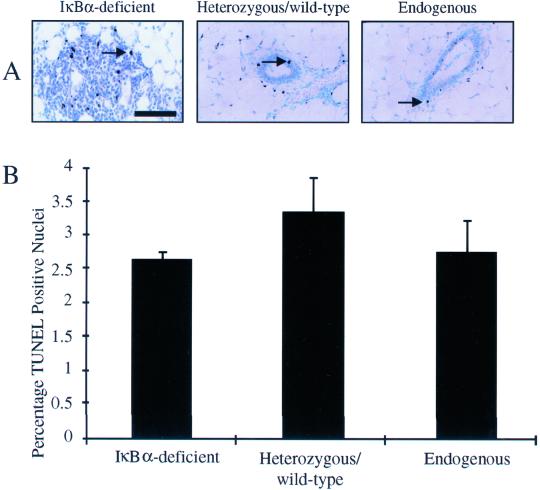
To assess the level of apoptosis in IκBα-deficient mammary epithelium, TUNEL analysis was performed on sections prepared from reconstituted or control virgin glands. TUNEL-positive nuclei were detected by immunohistochemistry. Based on immunohistochemical analysis, the percentage of TUNEL-positive nuclei in IκBα-deficient mammary epithelium was similar to the percentage in mammary glands reconstituted with heterozygous/wild-type tissue or in native mammary epithelium (Figure 4A). This was confirmed by quantification of the levels of apoptosis in the epithelium. As predicted based on immunohistochemical analysis, no statistically significant difference in the percentage of TUNEL-positive nuclei relative to the total number of nuclei was detected between IκBα-deficient mammary epithelium and epithelium derived from heterozygous/wild-type donors or native host epithelium (IκBα-deficient: 2.6 ± 0.13%; heterozygous/wild-type: 3.8 ± 0.42%; endogenous: 2.7 ± 0.30%, p > 0.7, χ2 analysis; Figure 4B). These data suggest that, although proliferation and cell density are increased, IκBα-deficiency in the mammary epithelium does not affect apoptosis in virgin animals.
Figure 4.
Mammary glands harboring IκBα-deficient epithelium display no significant alteration in epithelial apoptosis. (A) Immunohistochemical detection of TUNEL-positive nuclei in sections from mammary glands containing IκBα-deficient epithelium, heterozygous/wild-type epithelium, or endogenous host epithelium revealed no apparent difference in the level of apoptosis. Arrows indicate TUNEL-positive nuclei. Bar, 50 μm. (B) Percentage of TUNEL-positive nuclei was calculated according to the following formula: no. of positive nuclei/no. of total nuclei per 40× Values presented are the mean of a total of 10 individual fields from each section analyzed. No difference in the percentage of TUNEL-positive nuclei was observed in IκBα-deficient epithelium. Data are a representation of six independent samples per genotype. Error bars represent SE of the mean, p < 0.7, χ2 analysis.
IκBα-deficient Mammary Epithelium Displays Decreased Adjacent Extracellular Matrix
The epithelial architecture in mammary glands reconstituted with IκBα-deficient mammary tissue appeared disorganized and poorly confined by the extracellular matrix normally surrounding the ducts (Figure 1C). To examine this defect more closely, sections from mammary glands harboring IκBα-deficient mammary epithelium or heterozygous/wild-type epithelium and sections from host glands were subjected to trichrome staining to visualize extracellular matrix (ECM) proteins (Figure 5). Although trace amounts of ECM are detected, staining of ECM proteins (shown in blue) was strikingly reduced adjacent to IκBα-deficient donor epithelium, compared with heterozygous/wild-type donor epithelium and intact host epithelium (Figure 5, A and B). ECM expression surrounding the vascular endothelium of the reconstituted glands remained intact. Because the vasculature of the reconstituted gland is host-derived, it might be expected that vessel ECM expression is unaffected. This strongly supports the notion that alteration in ECM expression is specifically due to IκBα deficiency within the mammary epithelium, particularly because contaminating IκBα-deficient stroma cotransplanted with the epithelial rudiment is greatly reduced by transplantation into secondary recipients after initial transplantation from neonatal donors. Furthermore, these data suggest that IκBα/NF-κB plays a pivotal role in the proper structural development of the mammary gland.
Figure 5.
Mammary glands harboring IκBα-deficient epithelium display a reduction in ECM. (A) Trichome staining was used to visualize the ECM adjacent to IκBα-deficient epithelium, heterozygous/wild-type epithelium, or endogenous host epithelium. The ECM adjacent to IκBα-deficient epithelium was much reduced relative to the ECM in controls. Arrowheads indicate ECM stained in blue. This reduction was specifically associated with the epithelium, because ECM adjacent to blood vessels in IκBα-deficient samples was intact. Arrow indicates blood vessels. Bar, 100 μm. (B) Higher magnification further illustrates the reduction in ECM adjacent to IκBα-deficient epithelium relative to controls. Bar, 50 μm. Data are a representation of five independent samples per genotype.
Increased Branching in IκBα-deficient Epithelium Is Intrinsic to the Epithelium
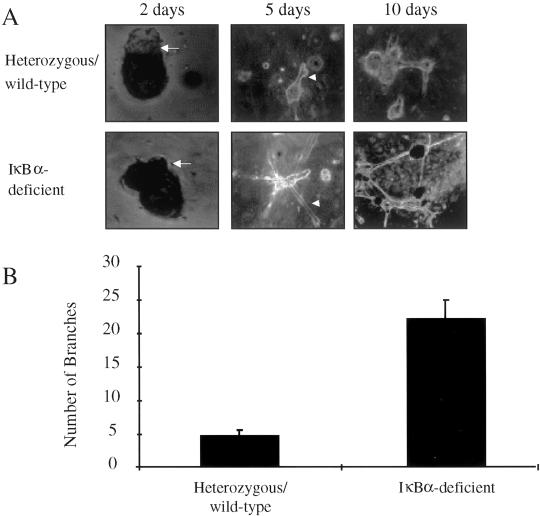
To determine whether the branching and proliferation defects are intrinsic to IκBα-deficient epithelium, purified epithelial cells from mammary glands reconstituted with IκBα-deficient tissue or with heterozygous/wild-type tissue were embedded within Matrigel, an artificial basement membrane. These purified epithelial cells were maintained in three-dimensional culture and monitored for growth and tubulogenesis. After 2 d in culture, both heterozygous/wild-type and IκBα-deficient cells formed epithelial protuberances that represent primordial tubules (Figure 6A). Although both heterozygous/wild-type and IκBα-deficient cells formed tubule branches by 5 d in culture, IκBα-deficient cells formed a greater number of these branches. The branches composed of IκBα-deficient cells were of greater length than controls. These observations were even more apparent after 10 d in culture, with an increase in the number of branches and in the structural complexity of this interconnecting tubule network. To quantify the increase in branching within IκBα-deficient cultures, the number of branches in heterozygous/wild-type cultures and in IκBα-deficient cultures was determined. A fourfold increase in the number of branches in IκBα-deficient cultures relative to wild-type cultures was observed after 10 d in culture (IκBα-deficient: 22.5 ± 2.9 branches/focal plane; heterozygous/wild-type: 5.2 ± 0.58 branches/focal plane, p < 0.003, χ2 analysis; Figure 6B). These data suggest that IκBα deficiency results in increased branching outgrowth of the mammary epithelium, and that these effects are intrinsic to epithelial cells.
Figure 6.
Mammary epithelial cells from IκBα-deficient glands display increased branching and tubulogenesis in three-dimensional culture. (A) Primary mouse mammary epithelial cells were isolated from glands reconstituted with IκBα-deficient tissue or with heterozygous/wild-type tissue and were maintained in three-dimensional Matrigel cultures. Cultures were assessed for tubulogenesis, branching, and outgrowth at 2, 5, and 10 d of culture and were photodocumented accordingly. Photographs shown are representative samples. Photographs taken on day 2 are 400×, whereas those taken on days 5 and 10 are 200×. Arrows indicate primordial tubules, arrowheads indicate branches. An apparent increase in the rate of branching and in the number/complexity of branching was observed in cultures containing IκBα-deficient epithelial cells. (B) Number of branches in IκBα-deficient epithelial cultures and in control cultures after 10 d was determined. A fourfold increase in the number of branches was observed in IκBα-deficient epithelial cultures. Values presented are the mean of three to six independent colonies per genotype. Data are a representation of 6 to 10 independent samples per genotype. Error bars represent SE of the mean, p < 0.003, χ2 analysis.
DISCUSSION
Post-natal morphogenesis of the mammary gland epithelium is tightly regulated by signal transduction cascades involving the activation of transcription factors that modulate changes in gene expression. By analyzing IκBα-deficient mammary tissue that lacks a major endogenous inhibitor of NF-κB transcriptional activity, we have been able to determine the consequences of elevated NF-κB activity on the growth and morphogenesis of mammary epithelium. Here, we provide novel, in vivo evidence that hyperactivation of NF-κB within the mammary epithelium perturbs normal growth and development of this organ. In previous studies, mice lacking IκBα exhibited elevated levels of NF-κB activity in several tissues, including B and T lymphocytes and skin (Beg et al., 1995; Klement et al., 1996; Chen et al., 2000a). We show here that the absence of IκBα protein also causes a remarkable elevation in NF-κB activity in mammary tissue, as well as elevated expression and nuclear localization of RelA. This is consistent with the known expression of NF-κB factors p50 and RelA in virgin mammary tissue (Brantley et al., 2000; Clarkson et al., 2000). In wild-type virgin mammary glands, NF-κB activity is low, even though p50 and RelA are expressed. This suggests that inhibition of NF-κB is accomplished by one of the family of IκBs, likely IκBα, which is also expressed in wild-type virgin mammary tissue.
Increased epithelial growth of IκBα-deficient epithelium was clearly evident by the number of epithelial cells present in the glands reconstituted with IκBα-deficient tissue, and BrdU labeling confirmed a significant increase in epithelial cell proliferation. This increase in proliferation may contribute to the altered morphology of the IκBα-deficient epithelium. For example, the increase in branching of the IκBα-deficient epithelium may be a direct result of increased proliferation, and the reduced levels of ECM expression may be due to heightened proliferation in the IκBα-deficient epithelium. Correlations between levels of proliferation and branching morphogenesis have been observed in other branching organ systems, including lung, another organ that uses NF-κB to establish epithelial branching patterns through epithelial–mesenchymal interactions (Serra et al., 1994; Muraoka et al., 2000). Alternatively, the reduction of ECM may be a direct effect of increased NF-κB activity. This hypothesis is also plausible, because several biochemical and in vivo studies have demonstrated that NF-κB can regulate the expression of ECM-degrading matrix metalloproteinases enzymes, such as MMP-1, MMP-3, MMP-9, and urokinase-type plasminogen activator (Hansen et al., 1992; Mohan et al., 1998; Bond et al., 1999; Wang et al., 1999; Yan et al., 2001). Increased expression of ECM-degrading enzymes and subsequent loss of ECM components could then permit increased branching and proliferation as a secondary effect in IκBα-deficient mammary epithelium. Precedence for this hypothesis comes from the observation that restoration of proper cell–ECM contacts in malignant breast cancer cells was found to restore normal tissue architecture and growth in three-dimensional culture, as well as reduced tumor formation in vivo, suggesting that loss of cell–ECM contacts are permissive for abnormal growth and malignancy (Weaver et al., 1997).
The effect of IκBα deficiency on the morphology of the mammary epithelium is profound and appears to be intrinsic to the epithelium. Increased branching and tubulogenesis were observed in purified mammary epithelial cell cultures. Stromal contamination in these cultures is absent; therefore, the possibility of indirect effects of the mesenchyme is eliminated. This is a critical consideration, because the mammary epithelium relies heavily on the corresponding stroma for signaling and support. These data demonstrate that NF-κB regulates both proliferation and the maintenance of the normal architecture of the mammary epithelium. Increasing evidence of NF-κB overexpression and elevated activity in various human breast cancer cell lines and primary tumors supports the implications of the results presented in this study.
We have demonstrated that elevated levels of NF-κB activity result in pervasive intraductal epithelial hyperplasia. Several studies have provided support for the function of NF-κB in regulating proliferation in vivo. Previous studies revealed that IκBα-deficient mice exhibited epidermal hyperplasia, with an increase in the number of proliferative keratinocytes relative to the number of differentiated keratinocytes (Klement et al., 1996; Chen et al., 2000b). Targeted disruption of the gene encoding IκB kinase α resulted in down-regulation of NF-κB transcriptional activity and perturbed normal proliferation and differentiation of the epidermis (Hu et al., 1999; Li et al., 1999; Takeda et al., 1999). Conversely, targeted disruption or inhibition of NF-κB family members in knockout/transgenic mice perturbed the proliferation and function of B and T lymphocytes (Kontgen et al., 1995; Sha et al., 1995; Weih et al., 1995; Boothby et al., 1997; Horwitz et al., 1997; Bendall et al., 1999; Grossmann et al., 1999). Moreover, inhibition of NF-κB activity blocked growth within the developing chick limb (Bushdid et al., 1998; Kanegae et al., 1998). These data provide precedence for positive regulation of cell proliferation by NF-κB.
Epithelial morphogenesis and lateral ductal branching of the mammary epithelium during puberty are regulated by interactions between the epithelium and the surrounding stroma (reviewed by Cunha and Hom, 1996; Robinson et al., 1999). NF-κB family members have been shown to modulate epithelial–mesenchymal interactions in embryonic limb and lung, suggesting that NF-κB may play a similar role in the regulation of growth and branching within the mammary epithelium. This is particularly intriguing because elevated NF-κB activity in the mammary epithelium results in increased branching and growth. NF-κB expression is induced in embryonic limb mesenchyme by signals derived from a specialized epithelial structure called the apical ectodermal ridge that is adjacent to the limb mesenchyme. Disrupting NF-κB activity in embryonic limb mesenchyme impaired limb outgrowth and resulted in aberrant morphology of the apical ectodermal ridge (Bushdid et al., 1998). Similar experiments in embryonic lung demonstrated that activity of NF-κB in lung mesenchyme is necessary for proper budding and growth of the adjacent lung epithelium (Muraoka et al., 2000). Based on data derived from these model systems, it is possible that NF-κB activity in the mammary epithelium is regulated by paracrine signals emanating from the adjacent stroma, or even neighboring epithelial cells, to control epithelial branching and proliferation during early mammary gland morphogenesis. In this scenario, loss of IκBα would constitutively activate NF-κB, mimicking the growth-activating signals from the stroma.
Loss of IκBα in the mammary gland epithelium did not result in any change in the level of epithelial apoptosis in virgin animals, which is somewhat surprising given that several biochemical and in vivo studies have demonstrated that RelA expression and activity confer protection against apoptosis (Beg et al., 1995; Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996; Li et al., 1999). Apoptosis of mammary epithelial cells during puberty is limited to small regions adjacent to terminal end buds during the process of lumen formation, and therefore it is possible any decrease in the level of apoptosis in IκBα-deficient mammary epithelium may be below the limits of detection at this stage of development. It has been noted that a large portion of the IκBα-deficient mammary epithelium does not form lumina. The cause of this phenomenon is unknown, but based on results presented here, it is does not appear to be a result of a decrease in apoptosis at this stage of development. It will be interesting to ascertain the function of NF-κB in mammary epithelial apoptosis in vivo, particularly during involution of the mammary epithelium. Very recently, nuclear RelA expression has been correlated with nonapoptotic epithelial cells within involuting mammary glands (Clarkson et al., 2000). Moreover, activation of NF-κB in a mammary cell culture model that mimics involution in vitro enhanced survival of these cells relative to controls. It is possible that NF-κB functions to block apoptosis only during involution while functioning to promote proliferation during early morphogenesis, because apoptosis is not affected in virgin IκBα-deficient mammary epithelium and proliferation is enhanced.
CONCLUSION
The NF-κB family of transcription factors has been shown to regulate proliferation in several cell types. Here, we present the first in vivo evidence that NF-κB activity regulates ductal branching and proliferation of the mammary epithelium and that increased activity disrupts the normal architecture of the epithelium. It would be of great interest to determine whether perturbations in NF-κB activity contribute to the development of breast adenocarcinoma. Several recent studies have demonstrated that many NF-κB family members are aberrantly expressed and/or activated in breast cancer cell lines and in primary tumors (Nakshatri et al., 1997; Sovak et al., 1997; Sovak et al., 1999; Newton et al., 1999). Amplification at the relA locus was observed in human breast adenocarcinomas (Matthew, et al., 1993). In addition, overexpression and enhanced nuclear localization of p50, p52, c-Rel, and Bcl-3 have been reported in breast cancer cell lines and in human tumors (Dejardin et al., 1995; Mukhopadhyay et al., 1995; Cogswell et al., 2000). In addition, the induction of NF-κB activity may be an early event in chemical carcinogenesis, because NF-κB activity was induced in vivo and in cell culture by 7,12-dimethylbenz[α]anthracene before neoplastic transformation (Kim et al., 2000). Further investigation is required to delineate precisely the role NF-κB plays in breast cancer and may lead to identification of new molecular targets for therapeutic interventions. We are currently monitoring mice reconstituted with IκBα-deficient or heterozygous epithelium for tumor development. The data presented in this study demonstrate that NF-κB plays a role in normal mammary epithelial proliferation during development, supporting a potential function for NF-κB in the pathology of breast cancer.
ACKNOWLEDGMENTS
We thank the Vanderbilt University Skin Disease Research Center for performing trichrome staining. Special thanks to Drs. Carlos Arteaga and Harold Moses for supplying reagents and valuable scientific discussions. We are grateful to many members of the Kerr lab, as well as Dr. Jin Chen, Dr. Timothy S. Blackwell, Dr. Chris Lamousin, Nikki Cheng, Linda Dzurek, and Karen Strunk at Vanderbilt University for review of this manuscript. D.M.B. is supported by a Department of Defense Breast Cancer Research Predoctoral Fellowship (DAMD17-97-1-7017). This work was supported by National Institutes of Health Grant R01GM51249 and by funding for Breast Cancer Research Project 9838 from the Susan G. Komen Breast Cancer Foundation to L.D.K.
REFERENCES
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bendall HH, Sikes ML, Ballard DW, Oltz EM. An intact NF-κB signaling pathway is required for maintenance of mature B cell subsets. Mol Immunol. 1999;36:187–195. doi: 10.1016/s0161-5890(99)00031-0. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Yull FE, Chen C-L, Venkatakrishna A, Blackwell TR, Hicks DJ, Lancaster LH, Christman JW, Kerr LD. Multi-organ nuclear factor κB activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- Bond M, Baker AH, Newby AC. Nuclear factor κB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley DM, Yull FE, Muroaka RS, Hicks DJ, Cook CM, Kerr LD. Dynamic expression and activity of NF-κB during post-natal mammary gland morphogenesis. Mech Dev. 2000;97:149–155. doi: 10.1016/s0925-4773(00)00405-6. [DOI] [PubMed] [Google Scholar]
- Bushdid PB, Brantley DM, Yull FE, Blaeuer GL, Hoffman LH, Niswander L, Kerr LD. Inhibition of NF-κB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature. 1998;392:615–618. doi: 10.1038/33435. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Singh N, Yull FE, Strayhorn D, Van Kaer L, Kerr LD. Lymphocytes lacking IκB-α develop normally, but have selective defects in proliferation and function. J Immunol. 2000a;165:5418–5427. doi: 10.4049/jimmunol.165.10.5418. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Yull FE, Cardwell N, Singh N, Strayhorn WD, Nanney LB, Kerr LD. RAG2−/−, IκB-α−/− chimeras display a psoriasiform skin disease. J Invest Dermatol. 2000b;115:1124–1133. doi: 10.1046/j.1523-1747.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, Watson CJ. NF-κB inhibits apoptosis in murine mammary epithelia. J Biol Chem. 2000;275:12737–12742. doi: 10.1074/jbc.275.17.12737. [DOI] [PubMed] [Google Scholar]
- Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-κB subunits in human breast cancer: potential roles for NF-κB2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hom YK. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Bonizzi G, Bellahcene A, Castronovo V, Merville MP, Bours V. Highly-expressed p100/p52 (NFKB2) sequesters other NF-κB-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–1841. [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ, Jr, Bern HA, Blair PE. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female CH3 mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- Doerre S, Sista P, Sun SC, Ballard DW, Greene WC. The c-rel protooncogene product represses NF-κB p65-mediated transcriptional activation of the long terminal repeat of type 1 human immunodeficiency virus. Proc Natl Acad Sci USA. 1993;90:1023–1027. doi: 10.1073/pnas.90.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth PA. Introduction: mammary gland involution and apoptosis of mammary epithelial cells. J Mammmary Gland Biol Neoplasia. 1999;4:123–127. doi: 10.1023/a:1018764922082. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Metcalf D, Merryfull J, Beg A, Baltimore D, Gerondakis S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA. 1999;96:11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SK, Nerlov C, Zabel U, Verde P, Johnsen M, Baeuerle PA, Blasi F. A novel complex between the p65 subunit of NF-κB and c-Rel binds to a DNA element involved in phorbol ester induction of the human urokinase gene. EMBO J. 1992;11:205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C-M, Yull F, Blackwell T, Komhoff M, Davis LS, Breyer MD. Dehydration activates an NF-κB driven, COX-2 dependent survival mechanism in renal medullary interstitial cells. J Clin Invest. 2000;106:973–982. doi: 10.1172/JCI9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz BH, Scott ML, Cherry SR, Bronson RT, Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-κB-deficient fetal liver cells. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Kanegae Y, Tavares AT, Izpisua Belmonte JC, Verma IM. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature. 1998;392:611–614. doi: 10.1038/33429. [DOI] [PubMed] [Google Scholar]
- Kim DW, Sovak MA, Zanieski G, Nonet G, Romieu-Mourez R, Lau AW, Hafer LJ, Yaswen P, Stampfer M, Rogers AE, Russo J, Sonenshein GE. Activation of NF-κB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Meisterernst M, Scheidereit C, Li G, Roeder RG. Transcriptional regulation of the HIV-1 promoter by NF-κB in vitro. Genes Dev. 1992;6:761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte J C, Verma IM. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999b;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999a;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med. 1999c;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Perkins ND, Schmid RM, Nabel GJ. Specific NF-κB subunits act in concert with Tat to stimulate human immunodeficiency virus type 1 transcription. J Virol. 1992;66:3883–3887. doi: 10.1128/jvi.66.6.3883-3887.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew S, Murty VV, Dalla-Favera R, Chaganti RS. Chromosomal localization of genes encoding the transcription factors, c-rel, NF-kappa Bp50, NF-kappa Bp65, and lyt10 by fluorescence in situ hybridization. Oncogene. 1993;8:191–193. [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- Medina D. The mammary gland: a unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996;1:5–19. doi: 10.1007/BF02096299. [DOI] [PubMed] [Google Scholar]
- Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998;273:25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-κB transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- Muraoka RS, Bushdid PB, Brantley DM, Yull FE, Kerr LD. Mesenchymal expression of nuclear factor-κB inhibits epithelial growth and branching in the embryonic chick lung. Dev Biol. 2000;225:322–338. doi: 10.1006/dbio.2000.9824. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TR, Patel NM, Bhat-Nakashatri P, Stauss CR, Goulet RJ, Jr, Nakashatri H. Negative regulation of transactivation function but not DNA-binding of NF-κB and AP-1 by IκBβ1 in breast cancer cells. J Biol Chem. 1999;274:18827–18835. doi: 10.1074/jbc.274.26.18827. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4:9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Pelton RW, Moses HL. TGF β1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development. 1994;120:2153–2161. doi: 10.1242/dev.120.8.2153. [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Sovak MA, Arsura M, Zanieski G, Kavanagh KT, Sonenshein GE. The inhibitory effects of transforming growth factor β1 on breast cancer cell proliferation are mediated through regulation of aberrant nuclear factor-κB/Rel expression. Cell Growth Differ. 1999;10:537–544. [PubMed] [Google Scholar]
- Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, Chiao PJ. Overexpression of urokinase-type plasminogen activator in pancreatic adenocarcinoma is regulated by constitutively activated RelA. Oncogene. 1999;18:4554–4563. doi: 10.1038/sj.onc.1202833. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky P, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV. collagenase expression by down-regulating NF-κB binding to the promoter as a consequence of IκBα-induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–1172. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]