Abstract
Background
Several irregular red blood cell alloantibodies, produced by alloimmunization of antigens in transfusions or pregnancies, have clinical importance because they cause hemolysis in the fetus and newborn and in transfused patients.
Objective
a prospective analysis of patients treated by the surgical and clinical emergency services of Hospital de Clínicas of the Universidade Federal do Triângulo Mineiro (HC/UFTM), Brazil was performed to correlate alloimmunization to clinical and epidemiological data.
Methods
Blood samples of 143 patients with initial negative antibody screening were collected at intervals for up to 15 months after the transfusion of packed red blood cells. Samples were submitted to irregular antibody testing and, when positive, to the identification and serial titration of alloantibodies. The Fisher Exact test and Odds Ratio were employed to compare proportions.
Results
Fifteen (10.49%) patients produced antibodies within six months of transfusion. However, for 60% of these individuals, the titers decreased and disappeared by 15 months after transfusion. Anti-K antibodies and alloantibodies against antigens of the Rh system were the most common; the highest titer was 1:32 (anti-K). There was an evident correlation with the number of transfusions.
Conclusions
Given the high incidence of clinically important red blood cell alloantibodies in patients transfused in surgical and clinical emergency services, we suggest that phenotyping and pre-transfusion compatibilization for C, c, E, e (Rh system) and K (Kell system) antigens should be extended to all patients with programmed surgeries or acute clinical events that do not need emergency transfusions.
Keywords: Blood transfusion, Blood group antigens, Hemolysis, Immunophenotyping, Emergencies
Introduction
Red blood cell (RBC) alloimmunization is an immune response against foreign RBC antigens; this generally occurs after sensitization due to blood transfusions and pregnancies(1). Rh, Kell, Kidd and Duffy alloantibodies have a high clinical importance; they react at 37ºC and cause hemolysis in transfused patients, fetuses and newborns(1-3).
With the increase in life expectancy and technological developments, increases in the number of chronic-degenerative diseases and more complex surgeries which require a higher number of blood transfusions have been observed. Thus, the frequency of RBC alloantibodies that do not belong to theABO system has been increasing(4-7). This often results in difficulties in finding compatible blood and a higher risk of delayed hemolytic transfusion reactions(6,8). However, in most blood transfusion services, phenotyping and pre-transfusion compatibilization of the most immunogenic antigens are normally applied to patients with chronic diseases. Additionally, RBC alloimmunization investigations are often preformed only after transfusion events and many alloantibodies may not be detected as no further transfusions are required or because the titer of antibodies decreases over time and reaches a non-detectable level prior to testing (9). In cases of patients who require further transfusions and receive an antigen that had already caused sensitization, on the other hand, a much faster secondary immune response may occur with the possibility of a severe hemolytic reaction (6,8,10). Over the last few years in the United States, irregular RBC alloantibodies have been linked to the majority of fatal hemolytic transfusion reactions reported to the Food and Drug Administration (FDA) and are considered the second main cause of transfusion-related deaths(11).
These facts motivated the present study, whose objectives were: to screen for RBC alloantibodies in patients treated for surgical and medical emergencies and who received packed RBC transfusions; to evaluate the time interval between the transfusion and detection of alloantibodies; to identify the antibodies and correlate the type of response with clinical and epidemiological data; and to measure the titers, monitor their drop and see how long they persist.
Methods
From July 2008 to March 2010, the purpose of this study was explained to adult patients treated for surgical or clinical emergencies in Hospital de Clínicas of the Universidade Federal do Triângulo Mineiro (HC/UFTM), Brazil. Post-transfusion blood samples were drawn from those who consented. A total of 143 patients, who initially had negative results for RBC antibodies, were enrolled in the study. Individuals with oncohematology diseases (leukemia and myelodysplastic syndrome), hemoglobinopathies (sickle cell disease and thalassemia) and recipients of multiple transfusions who were already phenotyped, were excluded from the study. Of the remaining participants, the mean number of packed RBC transfusions was 3.89/patient. Samples, collected from these patients at intervals, were submitted to irregular antibody screening by the tube technique using the DiaCell I and II reagents (Diamed-Biorad)(6,8,12). For the samples with positive serological results, the alloantibodies were identified, utilizing a commercial panel of 11 papainized and 11 non-papainized RBCs (Diamed-Biorad)(6,8,12). Subsequently, initial titers were determined and further (periodic) titrations were performed. Of the participants, 30 had histories of transfusions with possible previous sensitization, but were maintained in the study, because their samples did not presented irregular RBC antibodies at the time of transfusion.
The following data were collected from each patient: gender, age, ethnicity, ABO/Rh blood group, type of acute disease or medical emergency, history of transfusion and pregnancies (including the number of pregnancy partners) and the number of transfusions at HC/UFTM, as well as the results of screening and identification of irregular antibodies. When possible, the irregular antibody screening was repeated every month for 6 months and the detected alloantibodies were titered at 3-to 6-month intervals for up to 15 months after transfusion. Statistical analysis was performed using the software R with the Fisher Exact test and Odds Ratio (OR). The level of significance was set at 5%(13). Alloantibody analysis (specificity, titer, time of appearance and persistence) was only descriptive. This study was submitted to and approved by the Research Ethics Committees of UFTM (#1138) and Fundação Hemominas (#246).
Results
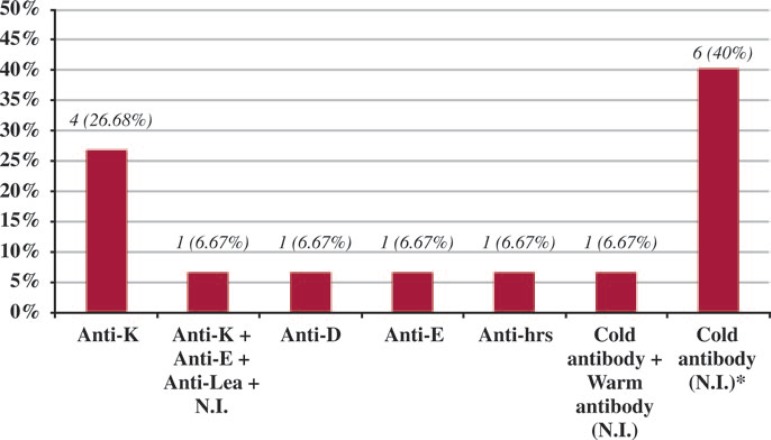
Of the 143 individuals with negative results for irregular antibody screening before the packed RBC transfusion, 15 (10.49%) developed irregular RBC alloantibodies. Among the alloimmunized individuals, four (26.68%) developed only anti-K antibodies, three (20%) presented antibodies against different antigens of the Rh system in isolation, one (6.67%) against antigens of the Rh, Kell and Lewis systems and an unidentified antibody (multiple). Six individuals (40%) presented cold alloantibodies and one (6.67%) developed a cold antibody associated to a warm alloantibody whose specificities could not be determined (Figure 1).
Figure 1.
A - The type of alloantibody of alloimmunized patients who received packed RBC transfusions for surgical and medical emergencies
*N.I. (Not identified).
No difference was observed in the rates of alloimmunization in respect to gender, age, ethnicity or type of disease or medical emergency and, on calculating the OR, no significant risk or protection was identified in relation to the occurrence of alloimmunization according to the different clinical and epidemiological data (Table 1).
Table 1.
Occurrence of post-transfusion alloimmunization of 143 patients treated for surgical and clinical emergencies in respect to epidemiological and clinical data
| Patient | Total | Non-alloimmunized | Alloimmunized | OR (95% CI) | p-value | |||
| profile | n | (%) | n | (%) | ||||
| Gender | ||||||||
| Male | 74 | 66 | (89.19) | 8 | (10.81) | |||
| Female | 69 | 62 | (89.85) | 7 | (10.15) | 0.932 (0.319-2.722) | 1.000 | |
| Age | ||||||||
| 18-49 years | 64 | 57 | (89.06) | 7 | (10.94) | |||
| > 50 years | 79 | 71 | (89.87) | 8 | (10.13) | 0.918 (0.314-2.683) | 1.000 | |
| Ethnicity | ||||||||
| Non-Caucasians | 60 | 52 | (86.67) | 8 | (13.33) | |||
| Caucasians | 83 | 76 | (91.57) | 7 | (8.43) | 0.599 (0.205-1.753) | 0.412 | |
| Diagnosis | ||||||||
| SWGS | 05 | 04 | (80.00) | 1 | (20.00) | |||
| AHDC | 12 | 10 | (83.33) | 2 | (16.67) | 0.800 (0.056-11.512) | 1.000 | |
| DSS | 12 | 10 | (83.33) | 2 | (16.67) | 0.800 (0.056-11.512) | 1.000 | |
| ST | 15 | 13 | (86.67) | 2 | (13.33) | 0.615 (0.043-8.709) | 1.000 | |
| HS | 19 | 17 | (89.47) | 2 | (10.53) | 0.471 (0.034-6.572) | 0.521 | |
| Trauma | 44 | 40 | (91.91) | 4 | (9.09) | 0.400 (0.036-4.503) | 0.431 | |
| Others | 23 | 21 | (91.30) | 2 | (8.70) | 0.381 (0.028-5.277) | 0.459 | |
| GO | 13 | 13 | (100.00) | 0 | (0.00) | 0.111 (0.004-3.246) | 0.278 | |
Fisher’s Exact Test; OR (Odds Ratio)
SWGS: Stab or gunshot wound; AHDC: Acute hemorrhages of differing causes (duodenal or gastric ulcers, intestinal parasitosis, voluminous epistaxis and others); DSS: Digestive tract surgery; ST: Solid tumors (uterine myomatosis, intestine carcinoma, cervical cancer etc.); HS: Heart surgery; GO: Gynecology and obstetrics.
A higher proportion of alloimmunized individuals was found among O positive blood group patients (25%). However, no significant risk or protection was identified by the OR; the occurrence of alloimmunization according to blood group was not proven (Table 2).
Table 2.
Occurrence of post-transfusion alloimmunization in 143 patients treated for surgical and clinical emergencies according to the blood group (ABO/RhD)
| Blood group | Total | Non-alloimmunized | Alloimmunized | Antibodies | OR (95% CI) | p-value | |||
| (ABO/Rh) | n | (%) | n | (%) | |||||
| O negative | 8 | 6 | (75.00) | 2 | (25.00) | Anti-D; | |||
| Anti-K | |||||||||
| Anti-E; | 0.692 | ||||||||
| B positive | 16 | 13 | (S1.25) | 3 | (1S.75) | Cold Ab | 1.000 | ||
| (0.091-5.295) | |||||||||
| (N.I.) x2) | |||||||||
| Anti-K (x2); | 0.375 | ||||||||
| Cold Ab + | |||||||||
| A positive | 45 | 40 | (SS.S9) | 5 | (1111) | (0.059-2.3SS) | 0.283 | ||
| Warm Ab (N.I.); | |||||||||
| Cold Ab (x2) | |||||||||
| Anti-K; | |||||||||
| Anti-E + Anti-K + Anti-Lea | 0.306 | 0.220 | |||||||
| O positive | 54 | 49 | (90.74) | 5 | (9.26) | + N.I.; | |||
| (0.048-1.940) | |||||||||
| Anti-hrs; | |||||||||
| Cold Ab (N.I.) (x2) | |||||||||
| A negative | 11 | 11 | (100.00) | 0 | (0.00) | - | 0.113 | 0.164 | |
| (0.005-2.734) | |||||||||
| AB positive | 7 | 7 | (100.00) | 0 | (0.00) | - | 0.173 | 0.467 | |
| (0.007-4.311) | |||||||||
| B negative | 2 | 2 | (100.00) | 0 | (0.00) | - | 0.520 | 1.000 | |
| (0.018-15.111) | |||||||||
Fisher's Exact Test; OR (Odds Ratio); N.I.: Not identified
We observed that 13.33% of the individuals with history of transfusions produced alloantibodies and there was a higher proportion of alloimmunization among patients who had been submitted to more than 10 transfusions at HC/UFTM (50%). On calculating the OR, a relationship between a higher risk of alloimmunization and a higher number of transfusions was verified as alloimmunized patients had received a mean of 6.2 RBC transfusions/patient, while in non-alloimmunized patients the mean was just 3.62 transfusions/patient (Table 3).
Table 3.
Occurrence of post-transfusion alloimmunization in 143 patients treated for surgical and clinical emergencies in relation to the history of transfusions and number of transfusions at HC/UFTM
| Parameter | Total | Non-alloimmunized | Alloimmunized | OR (95% CI) | p-value | |||
| n | (%) | n | (%) | |||||
| NT | ||||||||
| > 10 | 6 | 3 | (50.00) | 3 | (50.00) | |||
| 4 to 10 | 48 | 42 | (87.50) | 6 | (12.50) | 0.143 | 0.051 | |
| (0.023-0.877) | ||||||||
| 1 to 3 | 89 | 83 | (93.26) | 6 | (6.74) | 0.072 | 0.010 | |
| (0.012-0.438) | ||||||||
| HT | ||||||||
| No | 111 | 100 | (90.09) | 11 | (9.91) | |||
| Yes | 30 | 26 | (86.67) | 4 | (13.33) | 0.715 | 0.525 | |
| (0.210-2.430) | ||||||||
| N.I. | 2 | 2 | ( (100.00) | 0 | (0.00) | 1.178 | 1.000 | |
| (0.048-28.818) | ||||||||
Fisher’s Exact Test; OR (Odds Ratio)
NT: Number of transfusions at HC/UFTM; HT: History of transfusion; N.I.: Not identified
Thirty percent of nulliparous women presented alloimmunization versus 6.78% of women who had had pregnancies; however, there was no statistical difference between these two groups. The rates of occurrence of alloantibodies were independent of the number of pregnancies. There was no significant difference in alloimmunization between women with conceptuses of different partners and those of just one partner (18.18% versus 4.17%, respectively - Table 4).
Table 4.
Occurrence of post-transfusion alloimmunization in 69 women treated for surgical and clinical emergencies according to the history of pregnancies, number of pregnancies and number of pregnancy partners
| Parameter | Total | Non-alloimmunized | Alloimmunized | OR (95% CI) | p-value | |||
| n | (%) | n | (%) | |||||
| HP | ||||||||
| Yes | 59 | 55 | (93.22) | 4 | (6.78) | |||
| No | 10 | 7 | (70.00) | 3 | (30.00) | 0.170 | 0.0574 | |
| (0.031-0.921) | ||||||||
| NP | ||||||||
| 1 to 3 | 29 | 27 | (93.10) | 2 | (6.90) | |||
| > 3 | 30 | 28 | (93.33) | 2 | (6.67) | |||
| PP | ||||||||
| One partner | 48 | 46 | (95.83) | 2 | (4.17) | |||
| Different | 11 | 9 | (81.82) | 2 | (18.18) | 0.196 | 0.154 | |
| partners | (0.024-1.576) | |||||||
Fisher’s Exact Test; OR (Odds Ratio)
HP: History of pregnancy; NP: Number of pregnancies; PP: Pregnancy partners
The alloantibody of one patient (6.67%) was identified within 4 days of the transfusion; of one (6.67%) within 15 days; of three (20%) within three months and ten (66.67%) within six months. The highest titer was 1:32 (anti-K) and the alloantibodies disappeared for nine (60%) of the 15 alloimmunized individuals within a maximum of 15 months after the transfusion (Table 5).
Table 5.
Distribution of the alloimmunized patients who received packed RBC transfusions for surgical and medical emergencies, according to gender, time of appearance and persistence, specificity and titer of the alloantibodies
| Patient | Gender | 4 days | 15 days | 3 months | 6 months | 9 months | 12 months | 15 months |
| 1 | F | ** | ** | Anti-D (1:4) | Anti-D (1:4) | Anti-D (1:2) | Anti-D (1:1) | Neg. |
| 2 | M | ** | ** | Anti-K (1:8) | ** | Anti-K (1:8) | Anti-K (1:1) | Neg. |
| 3 | F | ** | ** | Neg. | Anti-K (1:4) | Anti-K (1:1) | Neg. | |
| 4 | F | ** | ** | Neg. | Cold Ab | Cold Ab | Neg. | |
| (N.I.)* (1:2) | (N.I.)* (1:1) | |||||||
| 5 | M | ** | ** | ** | Anti-K (1:32) | Anti-K (1:2) | Neg. | |
| Anti-K, | Anti-K, | Anti-K, | Anti-K, | |||||
| Anti-E, | Anti-E, | Anti-E, | Anti-E, | |||||
| 6 | M | ** | ** | ** | Anti-Lea | Anti-Lea | Anti-Lea | Anti-Lea |
| + N.I.* | + N.I.* | + N.I.* | + N.I.* | |||||
| (1:16) | (1:4) | (1:2) | (1:2) | |||||
| 7*** | M | ** | ** | Anti-hrs (1:2) | ||||
| Cold Ab, | ||||||||
| 8*** | M | Warm Ab | ||||||
| (N.I.)* | ||||||||
| (1:4) | ||||||||
| 9 | M | Neg. | Anti-E § | ** | Anti-E (1:16) | ** | Anti-E (1:4) | ** |
| 10 | F | ** | ** | ** | Anti-K (1:2) | Anti-K (1:2) | Anti-K (1:2) | ** |
| 11 | F | ** | Neg. | Neg. | Cold Ab | Neg. | ||
| (N.I.)* (1:1) | ||||||||
| 12 | F | ** | Neg. | Neg. | Cold Ab | Neg. | ||
| (N.I.)* (1:2) | ||||||||
| 13 | M | ** | Neg. | Neg. | Cold Ab | ** | Neg. | |
| (N.I.) (1:2) | ||||||||
| 14 | M | ** | ** | ** | Cold Ab | Neg. | ||
| (N.I.)* (1:2) | ||||||||
| 15 | F | ** | ** | Neg. | Cold Ab | ** | ** | ** |
| (N.I.)* (1:2) | ||||||||
*N.I.: Not identified;
**Not investigated;
***Death after the last collection;
§ Not titered
Discussion
The present study shows that, of the 143 patients treated in the Surgical and Clinical Emergency Departments of HC/UFTM and who received packed RBC transfusions, 15 (10.49%) developed irregular RBC alloantibodies.
The frequency of alloimmunization was higher than that found by other authors (8.4% and 2.1%) who studied 452 and 5690 patients treated for surgery and clinical emergencies, respectively(5,6). However, in the latter study, all the individuals were studied only during hospitalization, a time that is generally insufficient for the development of alloantibodies(9).
The higher occurrence of alloantibodies against antigens of Rh and Kell systems is in agreement with the literature; both systems have highly immunogenic antigens(5,6,12,14). In a previous study, evaluating patients with chronic and acute diseases, 53.76% and 13.87% of the alloimmunized patients produced antibodies against Rh and Kell antigens, respectively(14). AStudy of Cozac(15), carried out in 722 patients, most of whom had oncohematological diseases and hemoglobinopathies, reported 59.42% and 21.01% of alloimmunization against Rh and Kell antigens, respectively.
In the current study, one alloimmunized patient had anti-hrs alloantibodies (Rh system). The patient had no history of transfusions and received 15 ABO and RhD compatible transfusions. The corresponding antigen (hrs) is a variant of the e antigen of the same system(16).
The development of anti-D alloantibodies by one individual was surprising and required a special investigation. The alloantibody was detected three months after the transfusion in a 50-year-old female patient during the first post-transfusional collection. This patient was O RhD negative and had suffered uterine myomatosis; she received two units of O RhD negative packed RBC transfusions, that is, without the D antigen. It was not possible to check if there was an increase in the post-transfusion hemoglobin, but she did not develop any detectable hemolytic reaction. Although she had not received previous transfusions, she had had 3 gestations (from 2 different partners). Her youngest son, born 27 years previously, had the O RhD positive blood type. The donors of the RBC units were O RhD negative and screening for the weak D antigen was negative. A molecular investigation showed that one donor did not have the RHD gene and had the RhD negative phenotype, while the other had the same phenotype and RHDψ/RHCE*ccee genotype, which is not capable of expressing the RhD protein(17,18).
As the patient probably already had been sensitized against the D antigen from her Rh positive son or in her 2 other gestations, a possible explanation for the development of the anti-D alloantibody is that the transfusions could have induced a secondary immune response, even though the transfused RBC units did not express the respective antigen. Although rare, this re-stimulation might occur in cases of RBC transfusion that do not contain the antigen that caused a previous sensitization. Consequently, the individual produces specific alloantibodies against the same antigen, but in low titers (Castilho SL, 2010; Cozac AP, 2010 - personal communication).
Although some authors have reported a higher rate of women becoming alloimmunized(6), the percentages were similar in respect to gender, thus supporting the results of two other studies(5,8). Santos et al (6) reported a significantly higher rate in women and suggested that the risk of alloimmunization might be influenced by the gender of the recipient, in particular due to gestations.
In the current study, no significant differences were found on comparing age with alloimmunization. However, the mean age in alloimmunized patients was 47.4 years and hence lower than the study of Redman et al. (5) (≈ 65.3 years) even though the authors reported that most patients were over 65 years old. Although without statistical difference, a higher occurrence of alloimmunization was seen in non-Caucasian patients (13.33%). On considering the diagnosis or surgical/clinical procedure that led to transfusions, variations in the alloimmunization rates were probably due to the small number of patients in each of the different groups.
Additionally, similar to other authors, no correlation was observed between alloimmunization and the different blood types of the ABO system(6).
As expected, there was a higher occurrence of alloimmunization in individuals with history of transfusion. Despite the absence of alloantibodies before transfusions, these patients could, theoretically, present a secondary immune response to some RBC antigens. However, considering that in these cases the antibodies are already detectable between 24 and 48 hours after the new antigenic stimulation(10), in only one case it is possible to suggest an anamnestic response, while in three, this hypothesis is not possible because the antibodies were detected six months after transfusions, with all the previous tests being negative.
Similar to other authors(12), this study shows that the higher the number of transfusions, the higher the risk of alloimmunization; the mean number of transfusions was higher in individuals that produced RBC alloantibodies; similar to the results of two other studies(6,12).
Even though the antiglobulin test was negative at the time of emergency care, the four alloimmunized women who had histories of pregnancies might have presented secondary immune responses due to fetal-maternal sensitization. Thus, the fact that women without history of pregnancies presented a higher production of alloantibodies was unexpected, because these women must have been sensitized for the first time with the transfusion in the emergency service. However, of the three nulliparous women that became alloimmunized, two had reported histories of transfusions.
The occurrence of alloimmunization was higher, albeit without statistical significance, in women with more than one pregnancy partner. We believe that this increases the possibility of fetal-maternal alloimmunization due to the different genetic profiles of each partner and, consequently, of the concept.
There is a hypothesis that the time to alloimmunization was shorter in some individuals who were not tested for alloantibodies at all predetermined time intervals. However, the results of the current study are similar to two other studies; most alloantibodies were detected within 6 months of transfusions(5,9).
Cases of early alloimmunization have already been described in the literature. In a similar study, the time to alloantibody production varied from three to 97 days(6). In another work, 16.68% of the patients became alloimmunized within 14 days of transfusions and 2.3% within three days(9). According to the authors, these results suggest a secondary immune response; this probably occurred in one male patient of the current study with a history of transfusions, whose alloantibodies were detected on the fourth post-transfusion day. For another male patient, the irregular alloantibody test was positive on the 15th day in his first post-transfusion evaluation; however, the individual did not mention previous transfusions.
The low alloantibody titers and their fast decline should be mentioned; for 60% of the alloimmunized patients the alloantibodies disappeared within 15 months of the transfusion. A gradual decline in the levels of alloantibodies, despite a long persistence, was also observed in another study, with 25% being detectable after 192 months(19). Other authors demonstrated that several alloantibodies (including 45% of patients with anti-K alloantibodies) continued evident for more than 5 years after their detection(9). In the current study, the anti-K alloantibody was detected in two patients 12 and 15 months after transfusions.
This study demonstrates a high frequency of alloimmunization in non-chronic recipients of packed RBC (10.49%). Most alloantibodies have clinical significance and are directed against antigens of the Rh and Kell systems, in agreement with the literature. We also observed that alloimmunization is correlated to the number of transfusions, the alloantibodies were in low titers and most disappeared within 15 months after transfusions. Faced with these facts, we suggest that phenotyping and compatibilization for the C, c, E, e (Rh system) and K (Kell system) antigens should be extended, when possible, to all individuals with programmed surgeries and acute clinical events that do not need emergency transfusions. These measures will certainly help to reduce the rates of RBC alloimmunization and hemolytic transfusion reactions, with positive consequences on transfusion safety and the quality of Brazilian hemotherapy.
Acknowledgments
The authors would like to thank Marieta Queluz, biologist of the Immunohematology Laboratory of the Hemocentro Regional de Uberaba, for her help with laboratorial exams and RicardoAparecido Olivo, hematologist, hemotherapist and professor of the Department of Hematology and Hemotherapy at UFTM for his assistance in the interpretation of the results. Also thanks go to REUNI (Programa de Apoio ao Plano de Reestruturação e Expansão das Universidades Federais) for the Master's degree scholarship.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1. Novaretti MC.Investigação laboratorial em pacientes com anticorpos eritrocitários In: Bordin JO, Langhi Júnior DM, Covas DT.Hemoterapia: fundamentos e prática. São Paulo: Atheneu; 2007. p 186-89 [Google Scholar]
- 2. Baptista-González HA, Rosenfeld-Mann F, Pérez-Pérez JD, Quintanar-Garcia E.Anticuerpos irregulares antieritrocitarios fuera del sistema ABO en el periodo perínatal. Bol Med Hosp Infant Mex. 1991; 48(11): 814-9 [PubMed] [Google Scholar]
- 3. Lee CK, Ma ES, Tang M, Lam CC, Lin CK, Chan LC.Prevalence and specificity of clinically significant red cell alloantibodies in Chinese women during pregnancy - a review of cases from 1997 to 2001. Transfus Med. 2003; 13(4): 227-31 [DOI] [PubMed] [Google Scholar]
- 4. Serrano J.Incidencia y caracterización de anticuerpos eritrocitários en un banco de sangre hospitalario. Sangre (Barc). 1990; 35(5): 363-8 [PubMed] [Google Scholar]
- 5. Redman M, Regan F, Contreras M.A prospective study of the incidence of red cell alloimmunization following transfusion. Vox Sang. 1996; 71(4): 216-20 [DOI] [PubMed] [Google Scholar]
- 6. Santos FW, Magalhães SM, Mota RM, Pitombeira MH.Posttransfusion red alloimmunization in patients with acute disorders and medical emergencies. Rev Bras Hematol Hemoter. 2007; 29(4): 369-72 [Google Scholar]
- 7. Schonewille H, Van de Watering LM, Brand A.Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: is it time to take precautionary measures? Transfusion. 2006; 46(4): 630-5 [DOI] [PubMed] [Google Scholar]
- 8. Thakral B, Saluja K, Sharma RR, Marwaha N.Red cell alloimmunization in a transfused patient population: a study from a tertiary care hospital in north India. Hematology. 2008; 13(5): 313-8 [DOI] [PubMed] [Google Scholar]
- 9. Schonewille H, van de Watering LM, Loomans DS, Brand A.Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006; 46(2): 250-6 [DOI] [PubMed] [Google Scholar]
- 10. Langhi Júnior DM, Pereira JPM, Pereira CM.Reações transfusionais hemolíticas In: Bordin JO, Langhi Júnior DM, Covas DT.Hemoterapia: fundamentos e prática. São Paulo: Atheneu; 2007. p. 438-44 [Google Scholar]
- 11. Powers A, Chandrashekar S, Mohammed M, Uhl L.Identification and evaluation of false-negative antibody screens. Transfusion. 2010; 50(3): 617-21 [DOI] [PubMed] [Google Scholar]
- 12. Natukunda B, Schonewille H, Van de Watering L, Brand A.Prevalence and specificities of red blood cell alloantibodies in transfused Ugandans with different diseases. Vox Sang. 2010; 98(2): 167-71 [DOI] [PubMed] [Google Scholar]
- 13. Moore DS.A estatística básica e sua prática. Rio de Janeiro: LTC; 2005. [Google Scholar]
- 14. Martins PR, Alves VM, Pereira GA, Moraes-Souza H.[Frequency of irregular antibodies in multiple-transfused patients at the Regional Blood Bank of Uberaba, from 1997 to 2005]. Rev Bras Hematol Hemoter. 2008; 30(4): 272-6 Portuguese. [Google Scholar]
- 15. Cozac AP.Estudo do potencial antigênico relativo dos antígenos de grupos sanguíneos menores em pacientes sob esquema de transfusão [tese]. Ribeirão Preto: Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo; 2009. [Google Scholar]
- 16. Daniels G.Human Blood Groups. 2nd ed Oxford: Blackwell Science; 2002. [Google Scholar]
- 17. Reid ME, Rios M, Powell VI, Charles-Pierre D, Malavade V.DNA from blood samples can be used to genotype patients who have recently received a transfusion. Transfusion. 2000; 40(1): 48-53 [DOI] [PubMed] [Google Scholar]
- 18. Singleton BK, Green CA, Avent ND, Martin PG, Smart E, Daka A, et al. The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in Africans with the RhD-negative blood group phenotype. Blood. 2000; 95(1): 12-8 [PubMed] [Google Scholar]
- 19. Schonewille H, Haak HL, Van Zijl AM.RBC antibody persistence. Transfusion. 2000; 40(9): 1127-31 [DOI] [PubMed] [Google Scholar]