Abstract
Heterotrimers composed of collagen type IV alpha 1 (COL4A1) and alpha 2 (COL4A2) constitute one of the most abundant components of nearly all basement membranes. Accordingly, mutations in COL4A1 or COL4A2 are pleiotropic and contribute to a broad spectrum of disorders, including myopathy, glaucoma and hemorrhagic stroke. Here, we summarize the contributions of COL4A1 and COL4A2 mutations in human disease, integrate knowledge gained from model organisms and evaluate the implications for pathogenic mechanisms and therapeutic approaches.
TYPE IV COLLAGENS
The type IV collagens are encoded by three pairs of paralogous genes [collagen type IV alpha 1 (COL4A1) through COL4A6]. COL4A1 and COL4A2 are highly conserved across species and their protein products are present in almost all basement membranes, whereas COL4A3 through COL4A6 are more spatially and temporally restricted (1). The proteins encoded by these six genes associate non-randomly into three distinct heterotrimers in vivo: α1α1α2, α3α4α5 and α5α5α6 (2–4). Mutations in COL4A3, COL4A4 and COL4A5 cause Alport Syndrome—a pleiotropic disease affecting the retina, cochlea and kidney that often results in end-stage renal disease (5). Large deletions involving the adjacent COL4A5 and COL4A6 genes are reported to cause diffuse leiomyomatosis (6). Here, we review emerging developments regarding the biology and pathogenic mechanisms underlying COL4A1- and COL4A2-associated diseases.
COL4A1 (NM_001845) and COL4A2 (NM_001846) comprise 52 and 48 exons, respectively, and are arranged head to head on opposite strands of human Chromosome 13 (13q34). The two genes are separated by 127 nucleotides containing a shared bi-directional promoter that requires additional elements to control tissue specificity and the level and ratio of expression (Fig. 1) (7). Murine Col4a1 (NM_009931) and Col4a2 (NM_009932) are located on chromosome 8 (5.0 cM) in a similar genomic organization (8,9). COL4A1 and COL4A2 mRNAs are subject to post-transcriptional control, including regulation by a family of microRNAs that down-regulate their expression (10–16) and other microRNAs that indirectly regulate collagen synthesis (17,18). The Caenorhabditis elegans COL4A2 ortholog has a developmentally regulated, alternatively spliced isoform (19). Alternatively spliced COL4A1 and COL4A2 isoforms are predicted in humans and mice. One in particular (ENST00000397198) omits amino acids 498–848 which encompass an angiogenesis regulatory domain, putative integrin-binding sites and a region containing an interesting class of mutations in human patients (20) (see below); however, there is currently little empirical evidence to support the existence of alternative splicing in vivo.
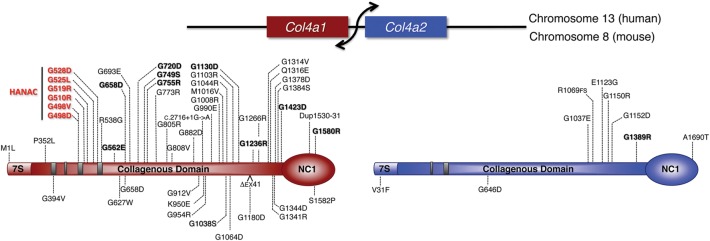
Figure 1.
Distribution of COL4A1 and COL4A2 mutations in schematics of human and mouse proteins. The Col4a1 and Col4a2 genes are transcribed from a shared, bidirectional promoter. Mature proteins are composed of three distinct domains: 7S, collagenous and non-collagenous (NC1). Mutations identified in humans and in mice are indicated above and below the schematics, respectively, with mutations causing HANAC Syndrome (hereditary angiopathy with nephropathy, aneurysms and muscle cramps) shown in red. Probable pathogenic human mutations, defined as displaying an unambiguous familial inheritance pattern, are in bold while other putative pathogenic human mutations are in plain text.
COL4A1 and COL4A2 proteins contain three major domains: an amino-terminal 7S domain, a central triple-helix-forming (collagenous) domain and a carboxy-terminal non-collagenous (NC1) domain (Fig. 1). The 7S domain participates in inter-molecular cross-linking and macromolecular organization. The collagenous domain constitutes the majority of the protein and consists of long stretches of (Gly-X-Y)n repeats where X and Y are variable amino acids, with proline often occupying the Y position. Unlike fibrillar collagens, the collagenous domains of type IV collagens have frequent interruptions of the Gly-X-Y repeats that are proposed to confer structural flexibility to the collagen IV network (21). Human and mouse COL4A1 have 21 positionally conserved repeat interruptions that divide the collagenous domain into 22 sub-domains. Similarly, human and mouse COL4A2 have 23 conserved repeat interruptions that align with those in COL4A1. All cysteine residues in the collagenous domain of COL4A1 and COL4A2 are present within repeat interruptions, suggesting that interruptions are also important sites for intermolecular cross-linking. The NC1 domains are globular domains responsible for the initiation of heterotrimers assembly (22).
BIOSYNTHESIS of α1α1α2 HETEROTRIMERS
COL4A1 and COL4A2 are translated at the rough endoplasmic reticulum (ER) where nascent peptides interact with ER resident proteins to ensure proper folding, post-translational modification and heterotrimer assembly (Fig. 2A). NC1 domains are folded and stabilized by intra-molecular cross-links formed by protein disulfide isomerase (PDI) before determining the register of the triple helix and initiating heterotrimer formation with one COL4A2 and two COL4A1 peptides (α1α1α2) (3,23). Prior to triple helix formation, the individual peptides of the trimeric complex undergo several post-translational modifications, including hydroxylation of prolyl and lysyl residues and N-linked and O-linked glycosylation.
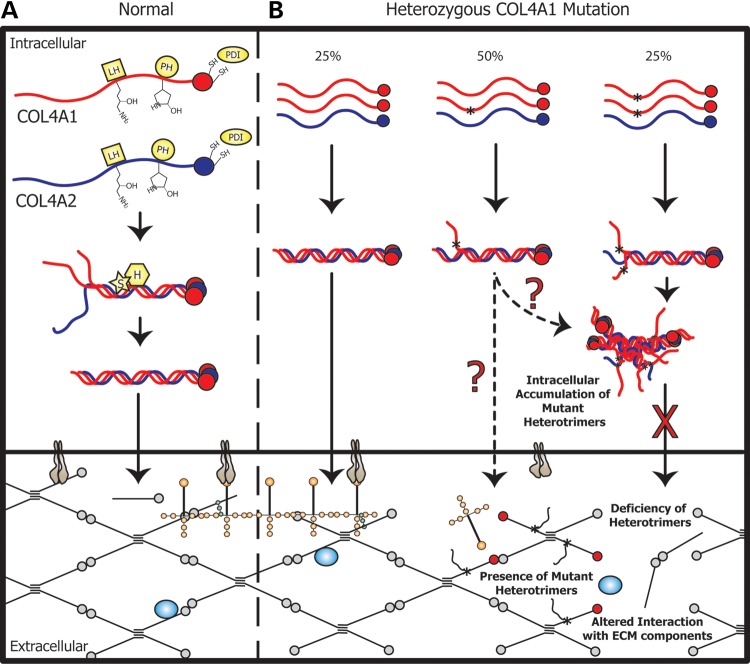
Figure 2.
Schematic representation of type IV collagen biosynthesis and potential sites for pathogenic insults. (A) Collagen proteins undergo extensive post-translational modifications and assemble into heterotrimers for secretion into the ECM where they polymerize into a network and interact with other extracellular and membrane bound molecules [LH, lysyl hydroxylase; PH, prolyl hydroxylase; PDI, protein disulphide isomerase; S, secreted protein, acidic, cysteine-rich (SPARC); H, heat shock protein 47 (HSP47)]. (B) Assuming random assembly within the ER of cells heterozygous for COL4A1 mutations, 25% of heterotrimers will be normal, 50% of heterotrimers will incorporate one mutant COL4A1 protein and 25% of heterotrimers will incorporate two mutant COL4A1 proteins. Normal heterotrimers (left) are presumed to be secreted, while heterotrimers containing two mutant proteins (right) are not. It is unknown if heterotrimers with one normal and one mutant COL4A1 are secreted. (Heterozygous mutations in COL4A2 would produce only the first two classes of heterotrimers at 50% each.) The primary pathogenic insult may be intracellular (cytotoxic accumulation of mutant heterotrimers) or extracellular (either the presence of mutant heterotrimers or the deficiency of heterotrimers in basement membranes). Either putative extracellular insult could directly or indirectly alter interactions with signaling molecules such as BMPs (represented as blue circles) or cell-surface receptors such as integrins (represented as grey structures), which can in turn lead to autocrine or paracrine intracellular signaling defects.
Hydroxylation of proline to hydroxyproline (Hyp) is critical for triple helix stabilization. Without Hyp, melting temperatures of triple helices are near physiological temperatures. Hydroxylation of Y-position prolines to 4-Hyp is catalyzed by prolyl 4-hydroxylase (P4H). In mammals, P4H is an α2β2 tetramer in which PDI is the β-subunit and the α-subunit posses the substrate recognition domain and the enzymatic active site. Vertebrates have three α-subunit isoforms, P4HA1-3, suggesting that there may be redundancy or substrate specificity. Caenorhabditis elegans lacking P4H activity or mice that are deficient for P4ha1 have basement membranes that lack type IV collagen and rupture easily, leading to lethality (24–26) (Table 1). Mice deficient for P4ha2 have no obvious phenotype (27) and mice deficient for P4ha3 have not been reported. Vertebrates also have three prolyl 3-hydroxylase (P3H) isoforms, P3H1-3, that catalyze the less common modification of X-position proline to 3-Hyp. Mutations in P3H1 (officially LEPRECAN 1: leucine proline-enriched proteoglycan) cause severe autosomal recessive osteogenesis imperfecta type VIII (28). The P3H2 gene (LEPREL1: leprecan-like 1) appears to encode the P3H responsible for modifying type IV collagens (29) and is expressed in the developing lens capsule (30). Mutation in P3H2 causes high myopia and variable early onset cataract and peripheral vitro-retinal degeneration (31), and mutation of P3H3 has not been described. P4H and P3H require ascorbate as a co-factor and insufficient dietary ascorbic acid renders both classes of enzymes inactive, leading to collagen instability and scurvy (32,33).
Table 1.
Consequences of mutations in proteins involved in type IV collagen biosynthesis
| Protein | Phenotypes in model organisms | Phenotypes in humans |
|---|---|---|
| P4HA1 | Lethality | Not reported |
| Basement membranes lack type IV collagen | ||
| P4HA2 | No obvious phenotype | Not reported |
| P4HA3 | Not reported | Not reported |
| P3H1 | Disruption in fibrillar collagen rich tissues | Osteogenesis imperfecta, type VIII |
| P3H2 | Not reported | High myopia |
| Early onset cataract | ||
| Peripheral vitro-retinal degeneration | ||
| Retinal detachment | ||
| P3H3 | Not reported | Not reported |
| PLOD1 | Abnormal collagen fibrils | Ehlers–Danlos syndrome, type VI |
| Hypotonia | ||
| Aortic rupture | ||
| PLOD2 | Not reported | Bruck syndrome, type 2 |
| PLOD3 | Lethality | Flat facial profile |
| Disrupted basement membranes | Deafness | |
| Accumulation of type IV collagen in ER | Myopia | |
| Cataract | ||
| Arterial rupture | ||
| Osteopenia | ||
| Joint contractures and fractures | ||
| Skin blistering | ||
| Nail abnormalities | ||
| HSP47 | Collagen misfolding and delayed secretion | Osteogenesis imperfecta, type X |
| SPARC | Disruption of collagen and laminin networks | Not reported |
| Early cataract formation | ||
| Osteopenia |
Hydroxylation of lysine to hydroxylysine is also vital to triple helix stabilization and provides O-linked glycosylation sites. Nearly 90% of the Y-position lysine residues of type IV collagens are hydroxylated and glycosylated by lysyl hydroxylases. Vertebrates have three lysyl hydroxylase isoforms, PLOD1-3. Mutations in PLOD1 (procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1) cause Ehlers–Danlos Syndrome, type VI (34,35), mutations in PLOD2 cause Bruck Syndrome, type 2 (36) and mutations in PLOD3 cause multi-system connective tissue disorder (37) (Table 1). PLOD3 is notable in that it can be secreted (38,39) and possesses hydroxylysyl galactosyltransferase (GT) and galactosylhydroxylysyl glucosyltransferase (GGT) activity (40). Mice completely deficient for Plod3 have disrupted basement membranes (41) and type IV collagen accumulates within dilated ER [similar to C. elegans with mutation of the lone lysyl hydroxylase ortholog (42)]. Elegant biochemical dissection of the functional domains revealed that pathology results from deficiency of GGT activity and not lysyl hydroxylase activity, demonstrating that glycosylation is necessary for intracellular trafficking (43,44).
Two additional proteins, HSP47 and SPARC, have important roles in collagen IV biosynthesis. HSP47 is an ER resident glycoprotein of the serpin family (official name is SERPINH1) (45) that acts as a collagen-specific molecular chaperone induced by heat shock but not ER stress. Whether HSP47 binds nascent peptides, monomers, unhydroxylated trimers or hydroxylated triple helices is debated (46–49); however, it appears to transiently associate with Y-position arginine motifs (i.e. Gly-X-Arg) (47,49,50) and dissociate in a pH-dependent manner after trafficking to the more acidic cis-Golgi (48). Although the precise role of HSP47 is unresolved, in its absence, collagen heterotrimers are misfolded and secretion is delayed (51–53) and HSP47 mutations cause severe, recessive Osteogenesis Imperfecta type X (54). SPARC (secreted protein, acidic, cysteine-rich) is a calcium-binding matricellular glycoprotein that recognizes non-denatured triple helical collagen (55). SPARC-deficient mice have early onset cataracts owing to disrupted collagen and laminin networks in the lens capsule (56–59) as well as decreased bone remodeling and severe osteopenia (60) (Table 1). SPARC has four predicted α1α1α2-binding sites and interacts with collagen in the ER and possibly in the extracellular matrix (ECM) (61). In Drosophila, cell-intrinsic SPARC is required and deficiency causes intracellular α1α1α2 accumulation (62). Collectively, these data suggest that SPARC influences collagen IV folding, assembly and/or modification in the ER.
Following post-translational modification, heterotrimers are transported from the ER to the Golgi where they are packaged into vesicles for secretion into the ECM (63). In the extracellular space, heterotrimers assemble into higher order macromolecular networks. NC1 domains of two heterotrimers are cross-linked via special S-hydroxylysyl-methionine bonds (64). At the N-terminus, 7S domains of four heterotrimers form lateral, anti-parallel interactions directed by hydroxylysine-linked disaccharides and stabilized by lysyl-derived cross-links (44,65).
Outside the cell, the α1α1α2 network interacts with other ECM components (laminins, perlecans, nidogens and proteoglycans), provides structural integrity and participates in cell–matrix and cell–cell communication. Collagens can signal directly to cells via integrins, discoidin domain receptors, mannose receptors and cell surface heparan sulfate proteoglycans (20,66). Type IV collagens can also influence intercellular communication and orchestrate developmental and homeostatic processes by modulating the distribution and bioavailability of diffusible morphogens (67–71). Additionally, cleaved fragments of type IV collagens such as arresten (the 229 amino acid NC1 domain of COL4A1) and canstatin (the 227 amino acid NC1 domain of COL4A2) can be biologically active and suppress angiogenesis and tumor growth (72–78). Thus, through their interactions with ECM components, cell surface receptors, and morphogens, or via proteolytic cleavage, type IV collagens can dynamically influence a broad range of biological processes. The extents to which each of these processes is perturbed in COL4A1- and COL4A2-related diseases constitute a growing area of research.
COL4A1 AND COL4A2 MUTATIONS CAUSE CLINICALLY DIVERSE DISEASES IN HUMANS
COL4A1 and COL4A2 mutations cause highly penetrant multi-system disorders (79–84) (Tables 2 and 3). A rapidly increasing number of COL4A1 mutations are being identified and the first COL4A2 mutations were recently reported (85–87) (Fig. 1). COL4A1 mutations were initially found to cause type I porencephaly, which is characterized by cystic cerebral cavities that communicate with the ventricles and are thought to arise from germinal matrix hemorrhages. Porencephaly commonly associates with infantile hemiparesis, hydrocephalus, seizures, poor or absent speech development, mental retardation and cerebral palsy. In addition to pre- and peri-natal hemorrhages, COL4A1 and COL4A2 mutations also cause sporadic and recurrent intracerebral hemorrhages (ICH) in young and old patients. Some hemorrhages occur spontaneously while others are induced by triggering events such as prenatal and birth trauma, head trauma, participation in sports, and anticoagulation use. This increased susceptibility to develop ICH is thought to result from systemic small-vessel disease, which has been confirmed by tissue biopsy. Skin and kidney biopsy samples from patients with COL4A1 and COL4A2 mutations were often normal on light microcopy evaluation but demonstrated significant ultrastructural abnormalities with focal interruptions or expanded and thickened, fragmented basement membrane capillaries on electromicroscopy. Even in the absence of overt porencephaly or hemorrhagic stroke, some individuals with COL4A1 mutations have clinically silent defects, such as diffuse or periventricular leukoencephalopathy and calcification, intracranial aneurysms and cerebral microbleeds, suggesting that COL4A1 and COL4A2 play an insidious and underappreciated role in a broad spectrum of cerebrovascular disease (CVD) (79–82,84–96). Still other patients with COL4A1 mutations have been identified without any evidence of CVD, which underscores the range in the severity of pathology from porencephaly and ICH that cause long-term disability or death to asymptomatic mutation carriers.
Table 2.
Cerebrovascular features associated with human COL4A1 and COL4A2 mutations
| Reference | Porencephaly | ICH/stroke | Leukoencephalopathy | Microbleeds | ICA | |
|---|---|---|---|---|---|---|
| COL4A1 mutations | ||||||
| p.M1L | Breedveld et al. (81) | 4/5 | 1/1 | 3/3 | 1/1 | |
| p.P352L | Weng et al. (84) | 1/1 | 1/1 | |||
| p.G498D | Plaisier et al. (94) | 0/3 | 1/3 | 1/2 | ||
| p.G498V | Plaisier et al. (90), Alamowitch et al. (91) | 0/8 | 3/6 | 5/6 | 2/6 | 3/6 |
| p.G510R | Plaisier et al. (94) | 0/3 | 0/2 | 0/2 | ||
| p.G519R | Plaisier et al. (90), Alamowitch et al. (91) | 0/4 | 1/2 | 1/2 | 1/2 | |
| p.G525L | Plaisier et al. (94) | 0/5 | 1/5 | 4/4 | 1/4 | |
| p.G528D | Plaisier et al. (90), Alamowitch et al. (91) | 0/2 | 0/1 | 1/1 | 1/1 | 1/1 |
| p.R538G | Weng et al. (84) | 1/1 | ||||
| p.G562E | Gould et al. (80), Vahedi et al. (89) | 2/6 | 2/6 | 6/6 | 3/4 | 0/1 |
| p.G658D | Livingston et al. (95) | 2/2 | ||||
| p.G693E | Livingston et al. (95) | 1/1 | 0/1 | |||
| p.G720D | Sibon et al. (88) | 1/5 | 2/5 | 5/5 | 1/5 | 1/1 |
| p.G749S | Gould et al. (79), Aguglia et al. (2004) (123), Vermeulen et al. (2011) (124) | 4/11 | 1/2 | 0/3 | ||
| p.G755R | Shah et al. (92) | 0/3 | 4/5 | 5/5 | 2/4 | 1/3 |
| Rouaud et al. (93) | ||||||
| Shah et al. (96) | ||||||
| p.G773R | Shah et al. (96) | 1/2 | 1/2 | 2/2 | ||
| p.G805R | Vahedi et al. (82) | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 |
| p.G808V | Meuwissen et al. (2011) (125) | 1/1 | 1/1 | |||
| p.G882D | Shah et al. (96) | 1/2 | ||||
| c.2716+1 G>A | Meuwissen et al. (2011) (125) | 1/1 | 1/1 | |||
| p.G990E | Livingston et al. (95) | 1/1 | 1/1 | |||
| p.G1008R | Meuwissen et al. (2011) (125) | 1/1 | 1/1 | |||
| p.M1016V | Labelle-Dumais et al. (97) | |||||
| p.G1044R | Meuwissen et al. (2011) (125) | 1/1 | 1/1 | |||
| p.G1103R | Lichtenbelt et al. (83) | 1/1 | 1/1 | |||
| p.G1130D | Breedveld et al. (81) | 3/3 | 2/2 | |||
| p.G1236R | Gould et al. (79) | 3/3 | 1/3 | 3/3 | 1/3 | 0/3 |
| van der Knaap et al. (2006) (126) | ||||||
| p.G1266R | Shah et al. (96) | 2/2 | 2/2 | 1/1 | ||
| p.G1314V | Livingston et al. (95) | 1/1 | 1/1 | |||
| p.Q1316E | Labelle-Dumais et al. (97) | |||||
| p.G1378D | Livingston et al. (95) | 1/1 | 1/1 | |||
| p.G1384S | Vermeulen et al. (2011) (124) | 1/1 | 1/1 | |||
| p.G1423R | Breedveld et al. (81) | 2/2 | 2/2 | |||
| p.P1530_M1531dup | Bilguvar et al. (2009) (127) | 2/4 | ||||
| p.G1580R | de Vries et al. (2009) (128) | 4/4 | 3/4 | 4/4 | 0/1 | 0/3 |
| COL4A2 mutations | ||||||
| p.G1037E | Yoneda et al. (87) | 1/1 | 1/1 | 0/1 | ||
| p.R1069fs | Verbeek et al. (86) | 2/3 | 0/2 | 0/2 | ||
| p. E1123G | Jeanne et al. (85) | 2/2 | ||||
| p.Q1150K | Jeanne et al. (85) | 1/1 | ||||
| p.G1152D | Yoneda et al. (87) | 3/4 | 0/1 | |||
| p.G1389R | Verbeek et al. (86) | 1/3 | 2/3 | 1/3 | ||
| p.A1690T | Jeanne et al. (85) | 1/1 | ||||
ICH, intracerebral hemorrhage; ICA, intracranial aneurysm.
Table 3.
Developmental, ocular, cardiac, renal, and musculoskeletal features associated with human COL4A1 and COL4A2 mutations
| Reference | MCD | RAT | Cataract | ASD | ONH | Cardiac abnormalities | Nephropathy | Urinary retention | Muscle cramps/ elevated CK | |
|---|---|---|---|---|---|---|---|---|---|---|
| COL4A1 mutations | ||||||||||
| p.M1L | Breedveld et al. (81) | |||||||||
| p.P352L | Weng et al. (84) | 1/1 (aortic valve replacement) | ||||||||
| p.G498D | Plaisier et al. (94) | 3/3 | 0/3 | 1/3 (SVA) | 1/3 (H) | 0/3 | ||||
| p.G498V | Plaisier et al (90), Alamowitch et al. (91) | 8/8 | 3/8 (SVA) | 8/8 (H, C) | 8/8 | |||||
| p.G510R | Plaisier et al. (94) | 3/3 | 0/3 | 1/2 (SVA) | 1/3 (mild RI) | 3/3 | ||||
| p.G519R | Plaisier et al (90), Alamowitch et al. (91) | 4/4 | 1/2 (mild RI, C) | 2/2 | ||||||
| p.G525L | Plaisier et al. (94) | 5/5 | 0/5 | 2/4 (SVA) | 2/4 (mild RI, C) | 5/5 | ||||
| p.G528D | Plaisier et al (90), Alamowitch et al. (91) | 2/2 | 1/1 (paroxysmal SVA) | 1/1 (mild RI, C) | 1/1 | |||||
| p.R538G | Weng et al. (84) | |||||||||
| p.G562E | Gould et al. (80), Vahedi et al (89) | 6/6 | 2/6 (H, mild RI) | |||||||
| p.G658D | Livingston et al. (95) | 0/1 | 1/2 | 0/1 | 0/1 | 1/1 (H) | 1/1 | |||
| p.G693E | Livingston et al. (95) | 1/1 | 1/1 | |||||||
| p.G720D | Sibon et al. (88) | 0/5 | 5/5 | 5/5 (GLC) | 0/2 | 0/1 | ||||
| p.G749S | Gould et al. (79), Aguglia et al. (2004) (123), Vermeulen et al. (2011) (124) | 4/6 (mitral valve prolapse) | ||||||||
| p.G755R | Shah et al. (92), Rouaud et al. (93), Shah et al. (96) | 0/5 | 5/5 | 0/2 | 0/2 | 1/1 | 0/1 | |||
| p.G773R | Shah et al. (96) | 1/1 | 0/2 | 2/2 | ||||||
| p.G805R | Vahedi et al. (82) | 1/1 | 1/1 | 0/1 | 0/1 | |||||
| p.G808V | Meuwissen et al. (2011) (125) | 1/1 (agenesis, C) | ||||||||
| p.G882D | Shah et al. (96) | 1/1 | 0/2 | 2/2 | 1/1 | 0/1 | 1/1 | |||
| c.2716 + 1 G>A | Meuwissen et al. (2011) (125) | |||||||||
| p.G990E | Livingston et al. (95) | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | ||||
| p.G1008R | Meuwissen et al. (2011) (125) | 1/1 | ||||||||
| p.M1016V | Labelle-Dumais et al. (97) | 1/1 | 1/1 | 0/1 | 1/1 | |||||
| p.G1044R | Meuwissen et al. (2011) (125) | 1/1 | ||||||||
| p.G1103R | Lichtenbelt et al. (83) | 1/1 | ||||||||
| p.G1130D | Breedveld et al. (81) | |||||||||
| p.G1236R | Gould et al. (79), van der Knaap et al. (2006) (126) | 1/3 | 3/3 | 0/3 | ||||||
| p.G1266R | Shah et al. (96) | 1/1 | 0/1 | 0/1 | ||||||
| p.G1314V | Livingston et al. (95) | 1/1 | 0/1 | 0/1 | 1/1 | |||||
| p.Q1316E | Labelle-Dumais et al. (97) | 1/1 | 1/1 | 1/1 | ||||||
| p.G1378D | Livingston et al. (95) | 1/1 | 1/1 | |||||||
| p.G1384S | Vermeulen et al. (2011) (124) | |||||||||
| p.G1423R | Breedveld et al. (81) | |||||||||
| p.P1530_M1531dup | Bilguvar et al. (2009) (127) | |||||||||
| p.G1580R | de Vries et al. (2009) (128) | 0/3 | 0/3 | 0/3 | ||||||
| COL4A2 mutations | ||||||||||
| p.G1037E | Yoneda et al. (87) | 0/1 | 0/1 | 1/1 | ||||||
| p.R1069fs | Verbeek et al. (86) | 1/2 | 1/3 | 0/1 | ||||||
| p. E1123G | Jeanne et al. (85) | |||||||||
| p.Q1150K | Jeanne et al. (85) | |||||||||
| p.G1152D | Yoneda et al. (87) | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| p.G1389R | Verbeek et al. (86) | 0/2 | ||||||||
| p.A1690T | Jeanne et al. (85) | |||||||||
MCD, malformations of cortical development; RAT, retinal artery tortuosity or hemorrhage; ASD, anterior segment dysgenesis; GLC, congenital glaucoma; ONH, optic nerve hypoplasia; SVA, supraventricular arrhythmia; H, hematuria; C, renal cysts; RI, renal insufficiency.
In addition to CVD, COL4A1 or COL4A2 mutations are confirmed to cause ocular, cerebral, renal and muscular defects (Table 3). Ophthalmologic examination is recommended in evaluating for COL4A1- and COL4A2-associated diseases as retinal vascular tortuosity is highly penetrant in patients with COL4A1 mutations, and may have predictive value for more serious CVD. Multiple patients have also been described with cataracts, ocular anterior segment dysgenesis (ASD), including Axenfeld Rieger Syndrome, and juvenile-onset glaucoma. Nephropathy manifests diversely, ranging from gross and microscopic hematuria to renal cysts to agenesis of the kidney. Functional renal defects have been reported in patients with small-vessel disease, leukoencephalopathy and congenital cataracts (95), and a multi-system syndrome referred to as HANAC: hereditary angiopathy with nephropathy, aneurysms and muscle cramps (90,94). Nearly, all families with HANAC syndrome have muscle cramps or elevated serum creatine kinase. Consistent with mutations causing myopathy, we identified two putative COL4A1 mutations in patients with congenital muscular dystrophy and cerebral cortical malformations, including lissencephaly, that are consistent with diagnoses of Walker–Warburg Syndrome or muscle–eye–brain disease (97).
To date, the clinical manifestations of COL4A1 mutations in patients represent only a subset of the phenotypes observed in mice with Col4a1 mutations. The high prevalence of CVD reported in patients with COL4A1 or COL4A2 mutations might reflect ascertainment bias since the original reports of mouse Col4a1 mutations described CVD. We predict that COL4A1 and COL4A2 mutations will be identified in diverse diseases and contribute to multiple, clinically distinct, developmental or acquired disorders as demonstrated in mouse models. The first COL4A2 mutations were only recently identified in patients with ICH and porencephaly (85–87). Evidence from model organisms suggests that COL4A2 mutations will phenocopy COL4A1 mutations and contribute to equally diverse disorders. However, for stochiometric reasons, it is possible that COL4A2 mutations may be less severe or even sub-clinical and thus, although equally abundant, may contribute less frequently to human diseases than COL4A1 mutations.
COL4A1 AND COL4A2 MUTATIONS CAUSE PLEIOTROPIC PHENOTYPES IN MICE
Mice completely deficient for both Col4a1 and Col4a2 (Col4a1−/−; Col4a2−/−) die at mid-gestation and exhibit various defects, including neuronal ectopias, disorganization of the capillary network during angiogenesis and impaired placental development (98) (Tables 4 and 5). Interestingly, mice double heterozygous for the Col4a1 and Col4a2 null alleles (Col4a1+/−; Col4a2+/−) are viable and without any overt phenotype. In contrast, mice heterozygous for missense or splicing mutations in Col4a1 or Col4a2 develop genetically complex, pleiotropic phenotypes. As such, mutagenesis screens in mice have been invaluable for understanding the roles of COL4A1 and COL4A2 in development and disease. To date, 13 Col4a1 mutations and 2 Col4a2 mutations have been identified in mice (79,99,100). The majority of these mutations are missense mutations of glycine residues occurring in the collagenous domain (Fig. 1). We identified a splice acceptor site mutation that leads to skipping of exon 41 during mRNA processing, which results in a 17-amino-acid deletion (p.G1169_K1185del) that contains a Gly-X-Y repeat interruption (79). (Note: The affected exon had been annotated as exon 40 and therefore this mutation was named Δex40 in previous publications. Subsequent builds of the mouse genome identify the exon as exon 41 and hereafter we refer to the mutation as Col4a1Δex41.)
Table 4.
Developmental, neurologic and vascular features associated with mouse Col4a1 and Col4a2 mutations
| COL4A1 mutations | Reference | Embryonic lethality (homozygous) | Growth retardation | Porencephaly/porencephalic lesions | Intracerebral hemorrhage | Malformations of cortical development | Vascular defects/systemic hemorrhages |
|---|---|---|---|---|---|---|---|
| COL4A1/COL4A2 double null | Pöschl et al. (2004) (98) | + | + (embryonic) | + | + (capillary organization abnormalities) | ||
| Δex41 | Gould et al. (79, 80, 101), Labelle-Dumais et al. (97) | + | + (reduced body size) | + | + | + | + (cerebral and systemic vasculature abnormalities) |
| p.G394V | Favor et al. (100) | + | + | + | + | + | |
| p.G627W | Van Agtmael et al. (99) | + | + (reduced body size) | + (bruising at birth) | |||
| p.G658D | Favor et al. (100) | + | + | + | + | + | |
| p.G912V | Favor et al. (100) | + | + | + | + | + | |
| p.K950E | Van Agtmael et al. (99,102) | + | + | ||||
| p.G954A | Favor et al. (100) | + | + | + | + | + | |
| p.G1038S | Favor et al. (100) | + | + | + | + | + | |
| p.G1064D | Van Agtmael et al. (99) | + | + (reduced body size) | + (bruising at birth) | |||
| p.G1180D | Favor et al. (100) | + | + | + | + | + | |
| p.G1341A | Favor et al. (100) | + | + | + | + | + | |
| p.G1344D | Favor et al. (100) | + | + | + | + | + | |
| p.S1582P | Favor et al. (100) | + | + | + | + | + | |
| COL4A2 Mutations | Reference | RAT | ASD | ONH | Other ocular defects | Nephropathy | Muscle defects/elevated CK |
| p.V31F | Favor et al. (100) | + | + | + | + | + | |
| p.G646D | Favor et al. (100), Jeanne et al. (85) | + | + | + | + | + |
Table 5.
Ocular, renal, muscular, pulmonary, and reproductive features associated with mouse Col4a1 and Col4a2 mutations
| Reference | RAT | ASD | ONH | Other ocular defects | Nephropathy | Muscle defects/ elevated CK | Pulmonary defects | Reduced reproductive functions | |
|---|---|---|---|---|---|---|---|---|---|
| COL4A1 mutations | |||||||||
| COL4A1/ COL4A2 double null | Pöschl et al. (2004) (98) | ||||||||
| ▵ex41 | Gould et al. (79, 80, 101), Labelle-Dumais et al. (97), unpublished dataa | + | + | + | + (hyphema) | + (glomerular BM abnormalities, MA, H) | + | + (perinatal) | + (males) |
| p.G394V | Favor et al. (100), unpublished data | + | + | + | +(M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.G627W | Van Agtmael et al. (99) | + | + (RD, RGC degeneration, B, optic nerve cupping, C) | + (Bowman's capsule abnormalities) | |||||
| p.G658D | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.G912V | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.K950E | Van Agtmael et al. (99) | + (retinal arteriolar silvering) | – | ||||||
| p.G954A | Favor et al. (100) | + | + (M, B, NV, LO, C) | + (females) | |||||
| p.G1038S | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.G1064D | Van Agtmael et al. (99) | + | + (RD, RGC degeneration, C) | ||||||
| p.G1180D | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.G1341A | Favor et al. (100) | + | + (M, B, NV, LO, C) | + (females) | |||||
| p.G1344D | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
| p.S1582P | Favor et al. (100), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (H) | + (females) | ||
| COL4A2 mutations | |||||||||
| p.V31F | Favor et al. (100) | + | + (M, B, NV, LO, C) | – | + (females) | ||||
| p.G646D | Favor et al. (100), Jeanne et al. (85), unpublished dataa | + | + | + | + (M, B, NV, LO, C) | + (MA, H) | + | + (females) | |
RAT, retinal arteriolar tortuosity; ASD, anterior segment dysgenesis; ONH, optic nerve hypoplasia; M, microphthalmia; B, buphthalmos; NV, preretinal neovascularization; LO, lens opacity; C, cataracts; RD, retinal detachment; RGC, retinal ganglion cell layer; MA, microalbuminuria; H, hematuria.
aUnpublished data provided by Dr Mao Mao and Dr Marion Jeanne.
Mice heterozygous for semi-dominant Col4a1 or Col4a2 mutations have multi-system disorders including ocular, renal, pulmonary, muscular, vascular, reproductive and central nervous pathology (Tables 4 and 5). Col4a1+/Δex41 mutant mice have pre- and peri-natal ICH, including porencephalic lesions. Independent of porencephaly, mutant mice also have highly penetrant tortuosity of the retinal vasculature, and fully penetrant multi-focal and recurrent ICH as adults (79,80).
Col4a1 and Col4a2 mutations were originally identified because of severe, highly penetrant cataracts and ocular ASD. Subsequent ocular analysis revealed optic nerve hypoplasia, retinal vascular tortuosity and progressive defects including retinal degeneration and elevated intraocular pressure that may advance to glaucoma (79,80,99–102). The relatively high penetrance of ocular dysgenesis in mice compared with humans may reflect selection bias due to the ease with which this pathology is detected in phenotype-driven mutagenesis screens. Col4a1 mutant mice also have thinning and splitting of the glomerular basement membrane and functional renal pathology, including microalbuminurea and hematuria (80). In addition to glomerular defects, abnormalities in Bowman's capsule are also reported (99).
The NC1 domains of α1α1α2 have a role in synaptogenesis of the neuromuscular junction (103) and, more recently, we detected myopathy including muscle weakness, elevated creatine kinase, split muscle fibers and increased non-peripheral nuclei in Col4a1+/Δex41 mice (97). This observation along with ocular dysgenesis and focal lamination defects in the cerebral cortex, resembling lissencephaly observed in human patients (97), established Col4a1 mutant mice as a novel model for a spectrum of congenital muscular dystrophy disorders that includes Walker–Warburg Syndrome and muscle–eye–brain disease. Pathology has been detected in every organ examined to date and the collection of pleiotropic phenotypes in Col4a1 and Col4a2 mutant mice reflects the nearly ubiquitous distribution of COL4A1 and COL4A2 in basement membranes. Additional disease-related defects are likely to be revealed as further detailed characterizations are conducted.
Genetic modifiers almost certainly contribute to variable expressivity of disease in patients. In mice, the penetrance and severity of pathology is genetic-context dependent. Although not all phenotypes have been evaluated, pathology resulting from the Col4a1Δex41 mutation is more severe in C57BL/6J mice compared with mice that have been crossed to either 129SvEvTac or CAST/EiJ (97,101). In a pilot experiment, a locus on CAST/EiJ Chromosome 1 was identified that rescued ocular dysgenesis (101). Identifying modifier genes and determining how they prevent pathology will reveal cellular pathways that may be amenable to therapeutic interventions that prevent or delay progressive diseases, such as glaucoma, myopathy and hemorrhagic stroke.
GENERAL PATHOGENIC MECHANISMS
There are several plausible, non-mutually exclusive, mechanisms by which COL4A1 and COL4A2 mutations might be pathogenic (Fig. 2B). Moreover, the mechanisms might be tissue-specific or even heterogeneous whereby different mechanisms play greater or lesser roles in different diseases. Thus, dissecting the primary cellular mechanisms underlying various COL4A1- and COL4A2-related diseases are active and important areas of research.
Mice heterozygous for null alleles of Col4a1 and Col4a2 had no detectable pathology, and supplemental transgenic expression of normal Col4a1 was insufficient to fully complement Col4a1 mutations in Drosophila (104). Together, these data argue against amorphic or hypo-morphic insults and support that pathogenicity occurs, at least in part, via anti-morphic or neo-morphic effects where there is a requirement for the presence of a mutant protein. Broadly, the pathogenic insult(s) could be intracellular and/or extracellular. Many mutations result in accumulation of α1α1α2 heterotrimers within cells and, in some cases, lead to activation of an ER stress response (79,85,101,105) which may produce acute or chronic cytotoxic effects. Alternatively, intracellular sequestration of mutant heterotrimers could lead to extracellular deficiency of α1α1α2 heterotrimers. In cells heterozygous for COL4A1 mutations, 75% of heterotrimers are expected to be abnormal, which could reduce the levels of extracellular heterotrimers below a critical threshold. However, for COL4A2 mutations, 50% of heterotrimers should be normal and secreted, and yet, COL4A2 mutations phenocopy COL4A1 mutations. This observation does not reconcile easily with the absence of a phenotype in heterozygous null mice (although a reduction in extracellular α1α1α2 has never been demonstrated in these mice). A simple model of extracellular deficiency also cannot explain the inability for supplemental COL4A1 expression to fully rescue pathology in mutant Drosophila (104). A third possibility is a dominant negative effect of extracellular mutant proteins. Although intracellular retention suggests that mutant heterotrimers might not reach the ECM, these observations are based upon relatively insensitive techniques and mutant α1α1α2 may indeed be secreted. Therefore, understanding the fate of mutant α1α1α2 heterotrimers is an unresolved issue. More complex factors might also influence outcomes, including feedback mechanisms and transcriptional control that might alter the overall biology of mutant cells. Perhaps, cells heterozygous for null alleles simply produce more protein from the normal allele to compensate, whereas cells expressing pathogenic mutations are instructed to stop this compensation. To date, the quantity or quality of heterotrimers reaching basement membranes have not been determined.
There are multiple pathways by which extracellular insults could cause disease. Either deficiency of normal heterotrimers or presence of mutant heterotrimers could lead to compromised basement membranes that perturb cell–matrix signaling via cell surface receptors or growth factor-mediated cell–cell signaling. Importantly, these deleterious effects could also be indirect via other matrix molecules if mutant basement membranes are altered in structure or composition. Observations from a subset of patients with COL4A1 mutations diagnosed with HANAC syndrome raise the intriguing possibility that some mutations might involve integrin signaling (see below). Alternatively, in Drosophila, type IV collagens directly bind to bone morphogenetic proteins (BMPs), a class of TGFβ signaling molecules, and participate in morphogen gradients and modulate their signaling (67,68). These data are interesting given that TGFβ signaling is necessary for angiogenesis and mutations in genes encoding proteins involved in this pathway cause hereditary hemorrhagic telangiectasia in which defects in angiogenesis predispose to ICH. Such a mechanism may be analogous to in Marfan Syndrome whereby mutations in the gene for another structural ECM protein, fibrillin-1, result in perturbed TGFβ signaling which causes some of the major clinical features of the disease (106–108). Finally, the reduction in proteolytically processed, biologically active NC1 domains of type IV collagens themselves may also be involved.
Thus, current data support that the pathogenic insult(s) of COL4A1 and COL4A2 mutations are anti-morphic or neo-morphic, may be intracellular or extracellular and, if extracellular, could be caused by deficiency or presence of mutant proteins. None of these potential mechanisms is mutually exclusive and may be differentially involved depending on the specific mutation. Understanding the extents to which mutant collagens induce intracellular stresses, affect signaling via cell surface receptors or perturb intercellular communication via morphogens are unresolved and important questions.
ALLELIC HETEROGENEITY TO EXPLAIN VARIABLE EXPRESSIVITY AND UNDERSTAND PATHOGENIC MECHANISM(S)
Emerging evidence from human patients and mouse models supports that allelic heterogeneity contributes to phenotypic variability. Understanding the functional differences between mutations can also be exploited to determine cellular mechanisms of disease. Glycine residues are required at every third position of the collagenous domain because the absence of a side chain allows glycine to fit into the core of the triple helix (109). However, both the position of the mutation and the residue replacing glycine can influence the biosynthetic consequence of the mutation (110). Non-glycine missense mutations are potentially less disruptive to triple helix formation and may lead to milder phenotypes (99) or act via different mechanisms such as disruption of post-translational modification or impacting functional sub-domains that interact with other matrix molecules, cell surface receptors or growth factors (20). For instance, the COL4A1Δex41 mutant protein, which is missing 17 amino acids from the collagenous domain, including a repeat interruption, has a very strong effect on intracellular retention of COL4A1 and COL4A2 and mice with this mutation tend to have more severe phenotypes (79,101,105). However, it is somewhat premature to compare the effects of different murine mutations because, to date, the mutations have not been systematically evaluated on a uniform genetic context. It is even more perilous to compare the clinical consequences of mutation in patients because they are usually identified in one or few people and the genetic context is even less defined. Despite this caution, emerging evidence supports allelic heterogeneity in human patients. Six families with COL4A1 mutations have a diagnosis of HANAC syndrome, which is suggested to be clinically distinguishable. Interestingly, all six mutations cluster within a 31-amino-acid region of the COL4A1 protein (located in exons 24 and 25) that contains multiple putative integrin-binding sites as well as other potential protein–protein interaction domains (20,94) (Fig. 1), suggesting that pathology might involve interactions with integrins or other extracellular proteins. Further studies to understand how allelic heterogeneity contributes to phenotypic heterogeneity and the extent to which it might reflect mechanistic heterogeneity are needed. Such insights could hold significant value as prognostic tools and for developing targeted treatments for different disease processes.
THERAPEUTIC STRATEGIES FOR COL4A1- AND COL4A2-ASSOCIATED DISEASES
There is evidence for external factors playing a role in the expressivity of COL4A1- and COL4A2-associated diseases. Environmental factors that contribute to disease often represent modifiable risk factors. For example, surgical delivery of Col4a1 mutant pups greatly reduced the incidence of perinatal ICH, suggesting that Cesarean delivery could improve clinical outcomes in patients with COL4A1 mutations (80). We have also observed an exercise-dependent difference in serum creatine kinase activity in Col4a1Δex41/+ mice (97). Moreover, head trauma, sports activity and use of anticoagulants have all been reported as factors contributing to ICH in patients with COL4A1 mutations. These reports suggest that management of modifiable risk factors may improve quality of life in patients with COL4A1 or COL4A2 mutations.
The next frontier for therapeutic strategies will capitalize on specific disease mechanism(s). Thus, the impetus for understanding the molecular mechanisms of COL4A1 and COL4A2 pathogenesis is to ultimately develop targeted therapeutic approaches to prevent, delay or diminish disease. For example, if heterotrimer deficiency in basement membranes is pathogenic, then efforts to up-regulate expression might be beneficial. However, if misfolded proteins are cytotoxic, then increasing production of COL4A1 and COL4A2 might exacerbate the insult and efforts to promote degradation of intracellular accumulations might be beneficial. Misfolded proteins within the ER are generally eliminated via the proteasomal (ER-associated degradation) or the lysosomal pathway (autophagy) (111,112). Misfolded COL1A1 heterotrimers are degraded via autophagy, and inducing autophagy with rapamycin decreased detergent-insoluble protein aggregates (113). There are several FDA-approved autophagy-inducing drugs (114,115) whose efficacy in preventing or treating COL4A1- and COL4A2-related diseases can be tested.
Other therapeutic approaches include promoting protein folding to shift the fate of mutant heterotrimers and alleviate intracellular and extracellular insults. Proof of principle for this approach comes from key experiments in C. elegans where conditions that promote protein folding decreased intracellular accumulation, restored secretion and rescued the viability of mutant animals (116). Similar results could be achieved using chemical chaperones (117) which are currently being explored as treatment options in a variety of diseases associated with protein misfolding and are worthy of exploration for their efficacy in treating COL4A1- and COL4A2-related disorders (118–122).
FUTURE DIRECTIONS
In a relatively brief period of time, COL4A1 and COL4A2 mutations have been discovered to cause a broad spectrum of debilitating or fatal diseases. Pathology varies in penetrance and severity among tissues and can be modulated by genetic and environmental factors. Model organisms will be key for understanding the molecular mechanisms associated with specific mutations. Mouse models can be used to understand genotype–phenotype correlations, provide insight into the contribution of allelic heterogeneity and identify genetic modifiers that could lead to the discovery of novel molecular pathways involved in the etiology of COL4A1- and COL4A2-associated disorders. The use of conditional mutants will be valuable to genetically dissect the tissue-specific effects of different mutations. Understanding the biological consequences of COL4A1 and COL4A2 mutations is critical to the development of personalized therapeutic strategies. As the molecular mechanisms of disease are discovered, we can better predict and address the effects of particular mutations, and potentially provide targeted therapeutic options to patients with COL4A1 and COL4A2 mutations.
FUNDING
D.S.K. was supported by a Howard Hughes Medical Institute Medical Research Fellowship. Support was provided by the Muscular Dystrophy Association, the American Heart Association, Research to Prevent Blindness (Unrestricted Grant to the Ophthalmology Department and a Career Development Award to D.B.G.) and the NIH (R01EY019514 and R01EY019887) all to D.B.G. Funding to pay the Open Access publication charges for this article was provided by That Man May See.
Acknowledgements
The authors would like to thank Drs Marion Jeanne and Mao Mao for their valuable comments and for sharing unpublished data.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Sado Y., Kagawa M., Kishiro Y., Sugihara K., Naito I., Seyer J.M., Sugimoto M., Oohashi T., Ninomiya Y. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem. Cell Biol. 1995;104:267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- 2.Borza D.B., Bondar O., Ninomiya Y., Sado Y., Naito I., Todd P., Hudson B.G. The NC1 domain of collagen IV encodes a novel network composed of the alpha 1, alpha 2, alpha 5, and alpha 6 chains in smooth muscle basement membranes. J. Biol. Chem. 2001;276:28532–28540. doi: 10.1074/jbc.M103690200. [DOI] [PubMed] [Google Scholar]
- 3.Boutaud A., Borza D.B., Bondar O., Gunwar S., Netzer K.O., Singh N., Ninomiya Y., Sado Y., Noelken M.E., Hudson B.G. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J. Biol. Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 4.Gunwar S., Ballester F., Noelken M.E., Sado Y., Ninomiya Y., Hudson B.G. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 1998;273:8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 5.Hudson B.G., Tryggvason K., Sundaramoorthy M., Neilson E.G. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N. Engl. J. Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J., Mochizuki T., Smeets H., Antignac C., Laurila P., De Paepe A., Tryggvason K., Reeders S.T. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science. 1993;261:1167–1169. doi: 10.1126/science.8356449. [DOI] [PubMed] [Google Scholar]
- 7.Haniel A., Welge-Lüssen U., Kühn K., Poschl E. Identification and characterization of a novel transcriptional silencer in the human collagen type IV gene COL4A2. J. Biol. Chem. 1995;270:11209–11215. doi: 10.1074/jbc.270.19.11209. [DOI] [PubMed] [Google Scholar]
- 8.Burbelo P.D., Martin G.R., Yamada Y. Alpha 1(IV) and alpha 2(IV) collagen genes are regulated by a bidirectional promoter and a shared enhancer. Proc. Natl Acad. Sci. USA. 1988;85:9679–9682. doi: 10.1073/pnas.85.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killen P.D., Burbelo P.D., Martin G.R., Yamada Y. Characterization of the promoter for the alpha 1 (IV) collagen gene. DNA sequences within the first intron enhance transcription. J. Biol. Chem. 1988;263:12310–12314. [PubMed] [Google Scholar]
- 10.Du B., Ma L.-M., Huang M.-B., Zhou H., Huang H.-L., Shao P., Chen Y.-Q., Qu L.-H. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010;584:811–816. doi: 10.1016/j.febslet.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Hassan M.Q., Jafferji M., Aqeilan R.I., Garzon R., Croce C.M., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Taylor N.E., Lu L., Usa K., Cowley A.W., Ferreri N.R., Yeo N.C., Liang M. Renal medullary micrornas in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luna C., Li G., Qiu J., Epstein D.L., Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer B., Stanczyk J., Jüngel A., Akhmetshina A., Trenkmann M., Brock M., Kowal-Bielecka O., Gay R.E., Michel B.A., Distler J.H.W., et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta S., den Boon J.A., Chen I.-H., Newton M.A., Stanhope S.A., Cheng Y.-J., Chen C.-J., Hildesheim A., Sugden B., Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl Acad. Sci. USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele R., Mott J.L., Ray R.B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1:381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M., Wang L., Putta S., Wang M., Yuan H., Sun G., Lanting L., Todorov I., Rossi J.J., Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TFG-β-induced collagen expression in kidney cells. J. Biol. Chem. 2010;285:34004–34015. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung A.C.K., Huang X.R., Meng X., Lan H.Y. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibley M.H., Johnson J.J., Mello C.C., Kramer J.M. Genetic identification, sequence, and alternative splicing of the Caenorhabditis elegans alpha 2(IV) collagen gene. J. Cell Biol. 1993;123:255–264. doi: 10.1083/jcb.123.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkin J.D., San Antonio J.D., Pedchenko V., Hudson B., Jensen S.T., Savige J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum. Mutat. 2011;32:127–143. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann H., Voss T., Kühn K., Engel J. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J. Mol. Biol. 1984;172:325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- 22.Trüeb B., Gröbli B., Spiess M., Odermatt B.F., Winterhalter K.H. Basement membrane (type IV) collagen is a heteropolymer. J. Biol. Chem. 1982;257:5239–5245. [PubMed] [Google Scholar]
- 23.Khoshnoodi J., Cartailler J.-P., Alvares K., Veis A., Hudson B.G. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 24.Winter A.D., Page A.P. Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol. Cell Biol. 2000;20:4084–4093. doi: 10.1128/mcb.20.11.4084-4093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman L., Higgin J.J., Moulder G., Barstead R., Raines R.T., Kimble J. Prolyl 4-hydroxylase is required for viability and morphogenesis in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2000;97:4736–4741. doi: 10.1073/pnas.97.9.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holster T., Pakkanen O., Soininen R., Sormunen R., Nokelainen M., Kivirikko K.I., Myllyharju J. Loss of assembly of the main basement membrane collagen, type IV, but not fibril-forming collagens and embryonic death in collagen prolyl 4-hydroxylase I null mice. J. Biol. Chem. 2007;282:2512–2519. doi: 10.1074/jbc.M606608200. [DOI] [PubMed] [Google Scholar]
- 27.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann. Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- 28.Vranka J.A., Pokidysheva E., Hayashi L., Zientek K., Mizuno K., Ishikawa Y., Maddox K., Tufa S., Keene D.R., Klein R., et al. Prolyl 3-hydroxylase 1 null mice display abnormalities in fibrillar collagen-rich tissues such as tendons, skin, and bones. J. Biol. Chem. 2010;285:17253–17262. doi: 10.1074/jbc.M110.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiainen P., Pasanen A., Sormunen R., Myllyharju J. Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV. J. Biol. Chem. 2008;283:19432–19439. doi: 10.1074/jbc.M802973200. [DOI] [PubMed] [Google Scholar]
- 30.Vranka J., Stadler H.S., Bächinger H.P. Expression of prolyl 3-hydroxylase genes in embryonic and adult mouse tissues. Cell Struct. Funct. 2009;34:97–104. doi: 10.1247/csf.09002. [DOI] [PubMed] [Google Scholar]
- 31.Mordechai S., Gradstein L., Pasanen A., Ofir R., El Amour K., Levy J., Belfair N., Lifshitz T., Joshua S., Narkis G., et al. High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. Am. J. Hum. Genet. 2011;89:438–445. doi: 10.1016/j.ajhg.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorres K.L., Raines R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vranka J.A., Sakai L.Y., Bächinger H.P. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J. Biol. Chem. 2004;279:23615–23621. doi: 10.1074/jbc.M312807200. [DOI] [PubMed] [Google Scholar]
- 34.Hautala T., Heikkinen J., Kivirikko K. A large duplication in the gene for lysyl hydroxylase accounts for the type VI variant of Ehlers-Danlos syndrome in two siblings. Genomics. 1993;15:399–404. doi: 10.1006/geno.1993.1074. [DOI] [PubMed] [Google Scholar]
- 35.Pinnell S.R., Krane S.M., Kenzora J.E., Glimcher M.J. A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N. Engl. J. Med. 1972;286:1013–1020. doi: 10.1056/NEJM197205112861901. [DOI] [PubMed] [Google Scholar]
- 36.van der Slot A.J., Zuurmond A.M., Bardoel A.F.J., Wijmenga C., Pruijs H.E.H., Sillence D.O., Brinckmann J., Abraham D.J., Black C.M., Verzijl N., et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J. Biol. Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 37.Salo A.M., Cox H., Farndon P., Moss C., Grindulis H., Risteli M., Robins S.P., Myllylä R. A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene. Am. J. Hum. Genet. 2008;83:495–503. doi: 10.1016/j.ajhg.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salo A.M., Wang C., Sipilä L., Sormunen R., Vapola M., Kervinen P., Ruotsalainen H., Heikkinen J., Myllylä R. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J. Cell. Physiol. 2006;207:644–653. doi: 10.1002/jcp.20596. [DOI] [PubMed] [Google Scholar]
- 39.Wang C., Ristiluoma M.-M., Salo A.M., Eskelinen S., Myllylä R. Lysyl hydroxylase 3 is secreted from cells by two pathways. J. Cell Physiol. 2012;227:668–675. doi: 10.1002/jcp.22774. [DOI] [PubMed] [Google Scholar]
- 40.Heikkinen J., Risteli M., Wang C., Latvala J., Rossi M., Valtavaara M., Myllylä R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J. Biol. Chem. 2000;275:36158–36163. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- 41.Rautavuoma K., Takaluoma K., Sormunen R., Myllyharju J., Kivirikko K.I., Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc. Natl Acad. Sci. USA. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norman K.R., Moerman D.G. The Let-268 locus of Caenorhabditis elegans encodes a procollagen lysyl hydroxylase that is essential for type IV collagen secretion. Dev. Biol. 2000;227:690–705. doi: 10.1006/dbio.2000.9897. [DOI] [PubMed] [Google Scholar]
- 43.Ruotsalainen H., Sipilä L., Vapola M., Sormunen R., Salo A.M., Uitto L., Mercer D.K., Robins S.P., Risteli M., Aszodi A., et al. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J. Cell Sci. 2006;119:625–635. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- 44.Sipilä L., Ruotsalainen H., Sormunen R., Baker N.L., Lamandé S.R., Vapola M., Wang C., Sado Y., Aszodi A., Myllylä R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J. Biol. Chem. 2007;282:33381–33388. doi: 10.1074/jbc.M704198200. [DOI] [PubMed] [Google Scholar]
- 45.Ishida Y., Nagata K. Hsp47 as a collagen-specific molecular chaperone. Meth. Enzymol. 2011;499:167–182. doi: 10.1016/B978-0-12-386471-0.00009-2. [DOI] [PubMed] [Google Scholar]
- 46.Tasab M., Batten M.R., Bulleid N.J. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000;19:2204–2211. doi: 10.1093/emboj/19.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tasab M., Jenkinson L., Bulleid N.J. Sequence-specific recognition of collagen triple helices by the collagen-specific molecular chaperone HSP47. J. Biol. Chem. 2002;277:35007–35012. doi: 10.1074/jbc.M202782200. [DOI] [PubMed] [Google Scholar]
- 48.Dafforn T.R., Della M., Miller A.D. The molecular interactions of heat shock protein 47 (Hsp47) and their implications for collagen biosynthesis. J. Biol. Chem. 2001;276:49310–49319. doi: 10.1074/jbc.M108896200. [DOI] [PubMed] [Google Scholar]
- 49.Koide T., Nishikawa Y., Asada S., Yamazaki C.M., Takahara Y., Homma D.L., Otaka A., Ohtani K., Wakamiya N., Nagata K., et al. Specific recognition of the collagen triple helix by chaperone Hsp47. II. The Hsp47-binding structural motif in collagens and related proteins. J. Biol. Chem. 2006;281:11177–11185. doi: 10.1074/jbc.M601369200. [DOI] [PubMed] [Google Scholar]
- 50.Koide T., Takahara Y., Asada S., Nagata K. Xaa-Arg-Gly triplets in the collagen triple helix are dominant binding sites for the molecular chaperone HSP47. J. Biol. Chem. 2002;277:6178–6182. doi: 10.1074/jbc.M106497200. [DOI] [PubMed] [Google Scholar]
- 51.Nagai N., Hosokawa M., Itohara S., Adachi E., Matsushita T., Hosokawa N., Nagata K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 2000;150:1499–1506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuoka Y., Kubota H., Adachi E., Nagai N., Marutani T., Hosokawa N., Nagata K. Insufficient folding of type IV collagen and formation of abnormal basement membrane-like structure in embryoid bodies derived from Hsp47-null embryonic stem cells. Mol. Biol. Cell. 2004;15:4467–4475. doi: 10.1091/mbc.E04-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marutani T., Yamamoto A., Nagai N., Kubota H., Nagata K. Accumulation of type IV collagen in dilated ER leads to apoptosis in Hsp47-knockout mouse embryos via induction of CHOP. J. Cell Sci. 2004;117:5913–5922. doi: 10.1242/jcs.01514. [DOI] [PubMed] [Google Scholar]
- 54.Christiansen H.E., Schwarze U., Pyott S.M., AlSwaid A., Al Balwi M., Alrasheed S., Pepin M.G., Weis M.A., Eyre D.R., Byers P.H. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive Osteogenesis imperfecta. Am. J. Hum. Genet. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki T., Hohenester E., Göhring W., Timpl R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J. 1998;17:1625–1634. doi: 10.1093/emboj/17.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan Q., Perdue N., Blake D., Sage E.H. Absence of SPARC in murine lens epithelium leads to increased deposition of laminin-1 in lens capsule. Invest. Ophthalmol. Vis. Sci. 2005;46:4652–4660. doi: 10.1167/iovs.05-0460. [DOI] [PubMed] [Google Scholar]
- 57.Yan Q., Clark J.I., Wight T.N., Sage E.H. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. J. Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- 58.Norose K., Lo W.K., Clark J.I., Sage E.H., Howe C.C. Lenses of SPARC-null mice exhibit an abnormal cell surface-basement membrane interface. Exp. Eye Res. 2000;71:295–307. doi: 10.1006/exer.2000.0884. [DOI] [PubMed] [Google Scholar]
- 59.Gilmour D.T., Lyon G.J., Carlton M.B., Sanes J.R., Cunningham J.M., Anderson J.R., Hogan B.L., Evans M.J., Colledge W.H. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delany A.M., Amling M., Priemel M., Howe C., Baron R., Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Invest. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hohenester E., Sasaki T., Giudici C., Farndale R.W., Bächinger H.P. Structural basis of sequence-specific collagen recognition by SPARC. Proc. Natl Acad. Sci. USA. 2008;105:18273–18277. doi: 10.1073/pnas.0808452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinek N., Shahab J., Saathoff M., Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J. Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- 63.Fatemi S.H. The role of secretory granules in the transport of basement membrane components: radioautographic studies of rat parietal yolk sac employing 3H-proline as a precursor of type IV collagen. Connect. Tissue Res. 1987;16:1–14. doi: 10.3109/03008208709001990. [DOI] [PubMed] [Google Scholar]
- 64.Than M.E., Henrich S., Huber R., Ries A., Mann K., Kühn K., Timpl R., Bourenkov G.P., Bartunik H.D., Bode W. The 1.9-A crystal structure of the noncollagenous (NC1) domain of human placenta collagen IV shows stabilization via a novel type of covalent Met-Lys cross-link. Proc. Natl Acad. Sci. USA. 2002;99:6607–6612. doi: 10.1073/pnas.062183499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bignon M., Pichol-Thievend C., Hardouin J., Malbouyres M., Bréchot N., Nasciutti L., Barret A., Teillon J., Guillon E., Etienne E., et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118:3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 66.Leitinger B., Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Bunt S., Hooley C., Hu N., Scahill C., Weavers H., Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Harris R.E., Bayston L.J., Ashe H.L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 69.Paralkar V.M., Weeks B.S., Yu Y.M., Kleinman H.K., Reddi A.H. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. J. Cell Biol. 1992;119:1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paralkar V.M., Vukicevic S., Reddi A.H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 71.Madri J.A., Pratt B.M., Tucker A.M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hynes R.O. Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 2007;5(Suppl. 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 73.Colorado P.C., Torre A., Kamphaus G., Maeshima Y., Hopfer H., Takahashi K., Volk R., Zamborsky E.D., Herman S., Sarkar P.K., et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 74.Kamphaus G.D., Colorado P.C., Panka D.J., Hopfer H., Ramchandran R., Torre A., Maeshima Y., Mier J.W., Sukhatme V.P., Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg P., Xie L., Sugimoto H., Colorado P., Sund M., Holthaus K., Sudhakar A., Salo T., Kalluri R. Characterization of the anti-angiogenic properties of arresten, an α1β1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 2008;314:3292–3305. doi: 10.1016/j.yexcr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sudhakar A., Boosani C.S. Signaling mechanisms of endogenous angiogenesis inhibitors derived from type IV collagen. Gene Regul. Syst. Biol. 2007;1:217–226. doi: 10.4137/grsb.s345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudhakar A., Nyberg P., Keshamouni V.G., Mannam A.P., Li J., Sugimoto H., Cosgrove D., Kalluri R. Human α1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by α1β1 integrin. J. Clin. Invest. 2005;115:2801–2810. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Sund M., Hamano Y., Sugimoto H., Sudhakar A., Soubasakos M., Yerramalla U., Benjamin L.E., Lawler J., Kieran M., Shah A., et al. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc. Natl Acad. Sci. USA. 2005;102:2934–2939. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gould D.B., Phalan F.C., Breedveld G.J., van Mil S.E., Smith R.S., Schimenti J.C., Aguglia U., van der Knaap M.S., Heutink P., John S.W.M. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 80.Gould D.B., Phalan F.C., van Mil S.E., Sundberg J.P., Vahedi K., Massin P., Bousser M.G., Heutink P., Miner J.H., Tournier-Lasserve E., et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 81.Breedveld G., de Coo I.F., Lequin M.H., Arts W.F.M., Heutink P., Gould D.B., John S.W.M., Oostra B., Mancini G.M.S. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J. Med. Genet. 2006;43:490–495. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vahedi K., Kubis N., Boukobza M., Arnoult M., Massin P., Tournier-Lasserve E., Bousser M.G. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–1464. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- 83.Lichtenbelt K.D., Pistorius L.R., de Tollenaer S.M., Mancini G.M., de Vries L.S. Prenatal genetic confirmation of a COL4A1 mutation presenting with sonographic fetal intracranial hemorrhage. Ultrasound Obstet. Gynecol. 2012;39:726–727. doi: 10.1002/uog.11070. [DOI] [PubMed] [Google Scholar]
- 84.Weng Y.-C., Sonni A., Labelle-Dumais C., De Leau M., Kauffman W.B., Jeanne M., Biffi A., Greenberg S.M., Rosand J., Gould D.B. COL4A1 mutations in patients with sporadic late-onset intracerebral hemorrhage. Ann. Neurol. 2012;71:470–477. doi: 10.1002/ana.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeanne M., Labelle-Dumais C., Jorgensen J., Kauffman W.B., Mancini G.M., Favor J., Valant V., Greenberg S.M., Rosand J., Gould D.B. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am. J. Hum. Genet. 2012;90:91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbeek E., Meuwissen M.E., Verheijen F.W., Govaert P.P., Licht D.J., Kuo D.S., Poulton C.J., Schot R., Lequin M.H., Dudink J., et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur. J. Hum. Genet. 2012;20:844–851. doi: 10.1038/ejhg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoneda Y., Haginoya K., Arai H., Yamaoka S., Tsurusaki Y., Doi H., Miyake N., Yokochi K., Osaka H., Kato M., et al. De novo and inherited mutations in COL4A2, encoding the type IV collagen α2 chain cause porencephaly. Am. J. Hum. Genet. 2012;90:86–90. doi: 10.1016/j.ajhg.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sibon I., Coupry I., Menegon P., Bouchet J.-P., Gorry P., Burgelin I., Calvas P., Orignac I., Dousset V., Lacombe D., et al. COL4A1 mutation in Axenfeld-Rieger anomaly with leukoencephalopathy and stroke. Ann. Neurol. 2007;62:177–184. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- 89.Vahedi K., Boukobza M., Massin P., Gould D.B., Tournier-Lasserve E., Bousser M.-G. Clinical and brain MRI follow-up study of a family with COL4A1 mutation. Neurology. 2007;69:1564–1568. doi: 10.1212/01.wnl.0000295994.46586.e7. [DOI] [PubMed] [Google Scholar]
- 90.Plaisier E., Gribouval O., Alamowitch S., Mougenot B., Prost C., Verpont M.C., Marro B., Desmettre T., Cohen S.Y., Roullet E., et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N. Engl. J. Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 91.Alamowitch S., Plaisier E., Favrole P., Prost C., Chen Z., Van Agtmael T., Marro B., Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–1882. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah S., Kumar Y., McLean B., Churchill A., Stoodley N., Rankin J., Rizzu P., van der Knaap M., Jardine P. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur. J. Paediatr. Neurol. 2010;14:182–187. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Rouaud T., Labauge P., Tournier Lasserve E., Mine M., Coustans M., Deburghgraeve V., Edan G. Acute urinary retention due to a novel collagen COL4A1 mutation. Neurology. 2010;75:747–749. doi: 10.1212/WNL.0b013e3181eee440. [DOI] [PubMed] [Google Scholar]
- 94.Plaisier E., Chen Z., Gekeler F., Benhassine S., Dahan K., Marro B., Alamowitch S., Paques M., Ronco P. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am. J. Med. Genet. A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 95.Livingston J., Doherty D., Orcesi S., Tonduti D., Piechiecchio A., La Piana R., Tournier-Lasserve E., Majumdar A., Tomkins S., Rice G., et al. COL4A1 mutations associated with a characteristic pattern of intracranial calcification. Neuropediatrics. 2011;42:227–233. doi: 10.1055/s-0031-1295493. [DOI] [PubMed] [Google Scholar]
- 96.Shah S., Ellard S., Kneen R., Lim M., Osborne N., Rankin J., Stoodley N., van der Knaap M., Whitney A., Jardine P. Childhood presentation of COL4A1 mutations. Dev. Med. Child Neurol. 2012;54:569–574. doi: 10.1111/j.1469-8749.2011.04198.x. [DOI] [PubMed] [Google Scholar]
- 97.Labelle-Dumais C., Dilworth D.J., Harrington E.P., De Leau M., Lyons D., Kabaeva Z., Manzini M.C., Dobyns W.B., Walsh C.A., Michele D.E., et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011;7:e1002062. doi: 10.1371/journal.pgen.1002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 99.Van Agtmael T., Schlötzer-Schrehardt U., McKie L., Brownstein D.G., Lee A.W., Cross S.H., Sado Y., Mullins J.J., Pöschl E., Jackson I.J. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum. Mol. Genet. 2005;14:3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- 100.Favor J., Gloeckner C.J., Janik D., Klempt M., Neuhäuser-Klaus A., Pretsch W., Schmahl W., Quintanilla-Fend L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175:725–736. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gould D.B., Marchant J.K., Savinova O.V., Smith R.S., John S.W.M. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum. Mol. Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- 102.Van Agtmael T., Bailey M.A., Schlötzer-Schrehardt U., Craigie E., Jackson I.J., Brownstein D.G., Megson I.L., Mullins J.J. Col4a1 mutation in mice causes defects in vascular function and low blood pressure associated with reduced red blood cell volume. Hum. Mol. Genet. 2010;19:1119–1128. doi: 10.1093/hmg/ddp584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fox M.A., Sanes J.R., Borza D.-B., Eswarakumar V.P., Fässler R., Hudson B.G., John S.W.M., Ninomiya Y., Pedchenko V., Pfaff S.L., et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 104.Kelemen-Valkony I., Kiss M., Csiha J., Kiss A., Bircher U., Szidonya J., Maróy P., Juhász G., Komonyi O., Csiszár K., et al. Drosophila basement membrane collagen Col4a1 mutations cause severe myopathy. Matrix Biol. 2012;31:29–37. doi: 10.1016/j.matbio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Firtina Z., Danysh B.P., Bai X., Gould D.B., Kobayashi T., Duncan M.K. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J. Biol. Chem. 2009;284:35872–35884. doi: 10.1074/jbc.M109.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goumans M.-J., Liu Z., ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 107.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C., et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neptune E.R., Frischmeyer P.A., Arking D.E., Myers L., Bunton T.E., Gayraud B., Ramirez F., Sakai L.Y., Dietz H.C. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 109.Prockop D.J., Kivirikko K.I. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 110.Baker N.L., Mörgelin M., Pace R.A., Peat R.A., Adams N.E., Gardner R.J.M., Rowland L.P., Miller G., De Jonghe P., Ceulemans B., et al. Molecular consequences of dominant Bethlem myopathy collagen VI mutations. Ann. Neurol. 2007;62:390–405. doi: 10.1002/ana.21213. [DOI] [PubMed] [Google Scholar]
- 111.Gelman M.S., Kannegaard E.S., Kopito R.R. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2002;277:11709–11714. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- 112.Ishida Y., Kubota H., Yamamoto A., Kitamura A., Bächinger H.P., Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol. Biol. Cell. 2006;17:2346–2355. doi: 10.1091/mbc.E05-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishida Y., Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy. 2009;5:1217–1219. doi: 10.4161/auto.5.8.10168. [DOI] [PubMed] [Google Scholar]
- 114.Pan T., Kondo S., Le W., Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L., Yu J., Pan H., Hu P., Hao Y., Cai W., Zhu H., Yu A.D., Xie X., Ma D., et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl Acad. Sci. USA. 2007;104:19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta M.C., Graham P.L., Kramer J.M. Characterization of α1(IV) collagen mutations in Caenorhabditis elegans and the effects of α1 and α2(IV) mutations on type IV collagen distribution. J. Cell Biol. 1997;137:1185–1196. doi: 10.1083/jcb.137.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Welch W.J., Brown C.R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperon. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Almeida S.F., Picarote G., Fleming J.V., Carmo-Fonseca M., Azevedo J.E., de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J. Biol. Chem. 2007;282:27905–27912. doi: 10.1074/jbc.M702672200. [DOI] [PubMed] [Google Scholar]
- 119.Bonapace G., Waheed A., Shah G.N., Sly W.S. Chemical chaperones protect from effects of apoptosis-inducing mutation in carbonic anhydrase IV identified in retinitis pigmentosa 17. Proc. Natl Acad. Sci. USA. 2004;101:12300–12305. doi: 10.1073/pnas.0404764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown C.R., Hong-Brown L.Q., Biwersi J., Verkman A.S., Welch W.J. Chemical chaperones correct the mutant phenotype of the Delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperon. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yam G.H.-F., Gaplovska-Kysela K., Zuber C., Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest. Ophthalmol. Vis. Sci. 2007;48:1683–1690. doi: 10.1167/iovs.06-0943. [DOI] [PubMed] [Google Scholar]
- 123.Aguglia U., Gambardella A., Breedveld G.J., Oliveri R.L., Le Paine E., Messina D., Quattrone A., Heutink P. Suggestive evidence for linkage to chromosome 13qter for autosomal dominant type 1 porencephaly. Neurology. 2004;62:1613–1615. doi: 10.1212/01.wnl.0000123113.46672.68. [DOI] [PubMed] [Google Scholar]
- 124.Vermeulen R.J., Peeters-Scholte C., Van Vugt J.J.M., Van Vught J.J.M.G., Barkhof F., Rizzu P., van der Schoor S.R.D., van der Knaap M.S. Fetal origin of brain damage in 2 infants with a Col4a1 mutation: fetal and neonatal MRI. Neuropediatrics. 2011;42:1–3. doi: 10.1055/s-0031-1275343. [DOI] [PubMed] [Google Scholar]
- 125.Meuwissen M.E.C., de Vries L.S., Verbeek H.A., Lequin M.H., Govaert P.P., Schot R., Cowan F.M., Hennekam R., Rizzu P., Verheijen F.W., et al. Sporadic Col4a1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. 2011;76:844–846. doi: 10.1212/WNL.0b013e31820e7751. [DOI] [PubMed] [Google Scholar]
- 126.van der Knaap M.S., Smit L.M.E., Barkhof F., Pijnenburg Y.A.L., Zweegman S., Niessen H.W.M., Imhof S., Heutink P. Neonatal porencephaly and adult stroke related to mutations in Collagen IV A1. Ann. Neurol. 2006;59:504–511. doi: 10.1002/ana.20715. [DOI] [PubMed] [Google Scholar]
- 127.Bilguvar K., DiLuna M.L., Biazzaro M.J., Bayri Y., Schneider K.C., Lifton R.P., Ment L.R. Pacifier, Breastfeeding Trial Group. COL4A1 mutation in preterm intraventricular hemorrhage. J. Pediatr. 2009;155:743–745. doi: 10.1016/j.jpeds.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Vries L.S., Koopman C., Groenendaal F., Van Schooneveld M., Verheijen F.W., Verbeek E., Witkamp T.D., van der Worp H.B., Mancini G. Col4a1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann. Neurol. 2009;65:12–18. doi: 10.1002/ana.21525. [DOI] [PubMed] [Google Scholar]