Introduction
Phenotypic characteristics of the eye in Sjögren's syndrome have been a key component in diagnosing the syndrome since its first description by Sjögren in 19331. Rose Bengal (the 4,5,6,7-tetrachloro 2′,4′,5′,7′-tetraiodo form of fluorescein) was used by Sjögren to demonstrate changes in the cornea and interpalpebral areas of the conjunctiva in patients with a form of dry eyes he named keratoconjunctivitis sicca (KCS) . Even though the use of vital dyes to stain the ocular surface was first reported by Pfluger2 in 1882, Sjögren was the first to recognize the diagnostic significance of the staining pattern by rose Bengal1.
In 1949, Holm3 attempted to categorize Sjögren's observations by dividing the pattern into grades A (clinically severe), B (moderate), and C (mild). This scheme was followed in 1969 by a semi-quantitative method reported by van Bijsterveld4 whose observations became the basis for subsequent grading systems. In 1973, Norn5 was the first to report the use of lissamine green, an acidic synthetically produced organic food dye, for vital staining of the cornea and conjunctiva. Norn and others noted that while lissamine green had staining properties very similar to rose Bengal, it did not cause the ocular irritation so prominent with rose Bengal6,7.
A 1995 report from the National Eye Institute workshop on clinical trials involving participants with dry eyes outlined a comprehensive new grading system for KCS8 using fluorescein dye to stain the cornea and rose Bengal to evaluate the conjunctiva. The underlying principle of using different stains for the cornea and conjunctiva provided a significant advance, but the system was not readily adopted because of its complexity. This was followed in 2003 by the “Oxford grading scheme” developed by Bron9 where fluorescein was used to stain the cornea and either rose Bengal or lissamine green to stain the conjunctiva. By counting the number of dots stained with each dye, Bron's grading scheme became the first quantitative method of accessing ocular changes in patients with KCS; however, the scoring system was never validated with patient data.
The Sjögren's International Collaborative Clinical Alliance (SICCA) is an NIH-funded international registry created to develop standardized classification criteria for Sjögren's syndrome, store clinical data and biospecimens collected from the SICCA cohort that ranges from those with possibly early Sjögren's syndrome to those with advanced disease, and disseminating those data and specimens for future Sjögren's syndrome research10. As part of SICCA, we have adapted from the previous grading systems cited above to develop a new, simplified, quantitative dry eye grading scheme that yields the SICCA Ocular Staining Score (OSS). The SICCA OSS is novel in that it uses lissamine green dye to grade the conjunctiva and fluorescein dye to grade the cornea, and is easily applied in clinical practice. The OSS gives equal numerical weight to corneal and conjunctival changes, is less time-consuming than previous grading systems, and emphasizes clinical relevance. It is the product of a collaborative effort between SICCA ophthalmologists and researchers in Argentina, China, Denmark, Japan, the United Kingdom and the United States and represents a new international standard for identifying and grading KCS in these patients.
The objectives of this article are to 1) describe the grading system that produces the OSS; and 2) analyze the distribution of the OSS among the current participants in the SICCA registry, and its association with other phenotypic characteristics of Sjögren's syndrome (e.g., other ocular, oral, and serologic measures).
Methods
SICCA Registry Participant Cohort
To be eligible for the SICCA registry, participants must be at least 21 years of age and have one of the following: 1) a complaint of dry eyes or dry mouth; 2) bilateral parotid enlargement; 3) a recent increase in dental caries; 4) a previous diagnosis of Sjögren's syndrome (SS); or 5) elevated titers of: antinuclear antibodies (ANA), rheumatoid factor (RF), anti-SS-A or anti-SS-B antibodies. Participants are recruited through local or national Sjögren's syndrome patient support groups, healthcare providers, public media, and populations served by all six SICCA locations.
Exclusion criteria include known diagnoses of: hepatitis C infection, HIV infection, sarcoidosis, amyloidosis, active tuberculosis; graft versus host disease, autoimmune connective tissue diseases other than rheumatoid arthritis or lupus; past head and neck radiation treatment; current treatment with daily eye drops for glaucoma; corneal surgery in the last 5 years to correct vision; cosmetic eyelid surgery in the last 5 years; or physical or mental condition interfering with successful participation in the study. Contact lens wearers are asked to discontinue wear for 7 days before the SICCA examination. We do not exclude participants who are taking prescription drugs that may affect salivary or lacrimal secretion, but do record their use and all other medications currently taken. Those taking cholinomimetic drugs (approximately 10% of the cohort) are asked to discontinue use one day before their SICCA clinical evaluation.
SICCA Registry Ocular Examination Sequence
The sequence and time intervals of these ocular tests are of critical importance to their accuracy and reproducibility. The application of a vital dye or any other substance to the ocular surface can alter the tear film and adversely affect subsequent results. For this reason, the Schirmer test I (without anesthesia) is done first, followed by instillation of fluorescein dye, determining the tear break up time, and grading the corneal fluorescein staining pattern. After fluorescein grading of the cornea, lissamine green dye is applied and the conjunctiva quickly examined and graded before the dye diffuses or the intensity of staining diminishes. The total time for the complete eye examination is approximately 20 minutes.
1) The Schirmer Test
The Schirmer test I (without anesthesia) is done before any drops are instilled in the eye. Standardized Schirmer strips are bent at the notch and placed carefully over the lower lid margin as far toward the temporal angle of the lids as possible. The patient is instructed to keep her/his eyelids closed during the test. Strips remain in place for five minutes, or until they are completely saturated with tears. After five minutes, wetting of the strips is measured using the millimeter scale on each strip (Eagle vision (Cat # 0039). It is generally agreed that a Schirmer I test of 5mm or less in 5 minutes is abnormal, but the variability of the test, even in normal eyes, invalidates any direct comparison between individual patients with KCS and normal controls.4,8 Immediately after removing the strips, one drop of 0.5% fluorescein (Leiter's Pharmacy San Jose, California) is applied to the conjunctival fornix of each eye, and the participant told to squeeze the eyelids tightly to remove excess dye. During this interval, the tear strips are processed by cutting them lengthwise and placing them in cryovials to be frozen and banked.
2) Tear Break Up Time (TBUT)
Two minutes after the application of fluorescein dye the TBUT is measured at the slit lamp. The oculars are set at 10× magnification and the illumination is set on “high”, using the cobalt blue filter over the light source. The patient is asked to blink once and then keep her/his eyes open. The TBUT, defined as the time in seconds between the patient's last blink and the first appearance of a random dry spot on the corneal surface, is measured three times and the mean value is recorded. It is generally accepted that a TBUT of less than 10 seconds is abnormal.8 Mengher, et al. confirmed this observation by comparing tear breakup time values in 33 KCS patients with 66 normal controls. The value of 10 seconds proved to be the critical level separating the two groups with a sensitivity of 82% and specificity of 86%.11 The presence of absence of mucus shreds and/or debris in the tear film is noted.
3) Corneal Fluorescein Staining Pattern (Step 1 of the Ocular SICCA grading)
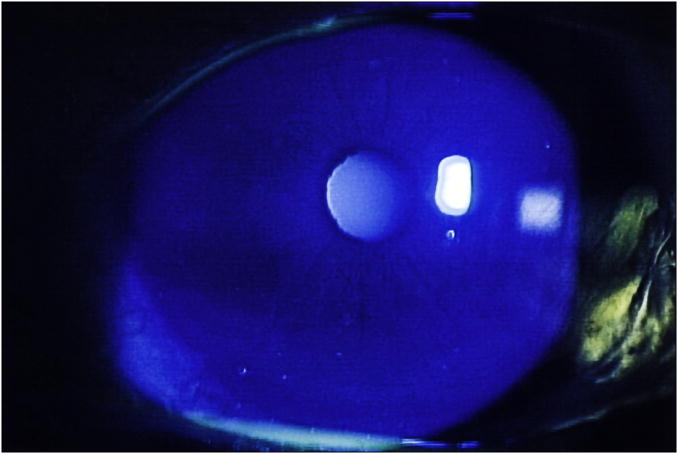
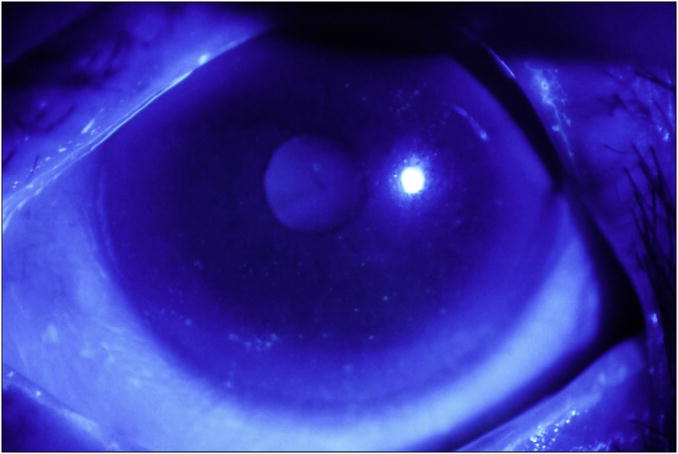
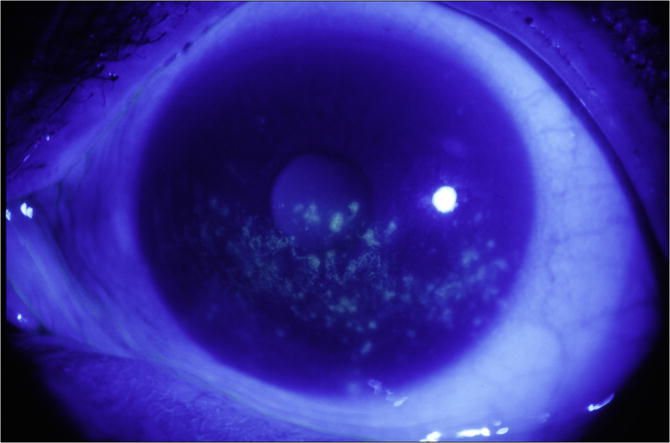
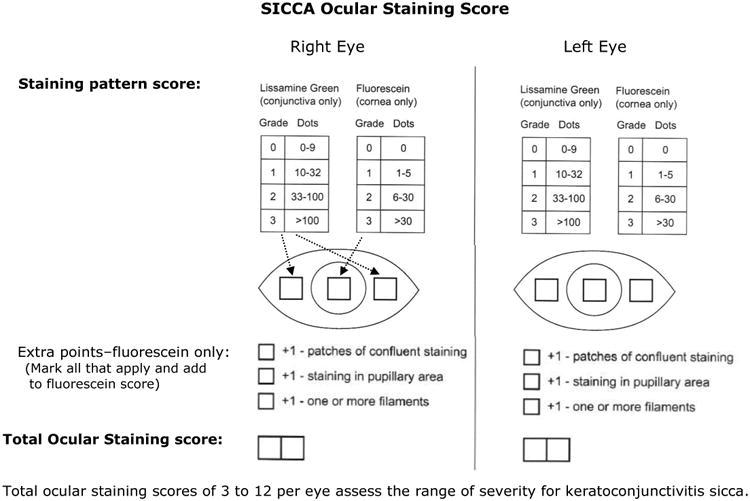
Each cornea is examined at the slit lamp using the cobalt blue filter. Corneal epithelial staining is a dynamic and time-sensitive process; therefore to insure reproducibility, grading of the fluorescein pattern is consistently initiated between four and eight minutes following instillation. Punctate epithelial erosions (PEE) that stain with fluorescein are counted, and scored. If there are no PEE the score is 0. If 1-5 PEE are seen, the corneal score is 1 (Figure 1a); 6-30 PEE are scored as 2; and >30 PEE are scored as 3. An additional point is added if: 1) PEE occurred in the central 4mm diameter portion of the cornea (Figure 1b); 2) one or more filaments is seen anywhere on the cornea; or 3) one or more patches of confluent staining, including linear stains, are found anywhere on the cornea, (Figure 1c).The total fluorescein score for the cornea (the PEE grade plus any extra points for modifiers) is noted in the central square of the SICCA ocular staining score form (Figure 2). The maximum possible score for each cornea is 6.
Figure 1.
Fluorescein staining of the cornea in keratoconjunctivitis sicca:
Figure 1a. Fluorescein staining of the cornea in keratoconjunctivitis sicca, Score 1 (1-5 very fine discrete punctate epithelial erosions seen inferiorly). Photograph courtesy of Dr. Kitagawa.
Figure 1b. Fluorescein staining of the cornea in keratoconjunctivitis sicca, Score 3 (6-30 punctate epithelial erosions seen inferiorly, score 2, plus one punctate epithelial erosion present in the papillary area, for a total of 3). Photograph courtesy of Dr. Kitagawa.
Figure 1c. Fluorescein staining of the cornea in keratoconjunctivitis sicca, Score 5 (>30 punctate epithelial erosions, many patches of confluent staining, and staining in the papillary area; no filaments). Photograph courtesy of Dr. Kitagawa.
Figure 2. Sjögren's International Collaborative Clinical Alliance (SICCA) ocular staining score form.
4) External Eye Examination at the Slit Lamp
Examination of the external eye is performed following fluorescein instillation but before the application of lissamine green. The slit lamp is used with the same magnification but with decreased illumination using a neutral density filter. Since this portion of the eye examination is not time dependent it is performed slowly and carefully, noting the presence or absence of abnormalities of the lids, conjunctiva, and cornea, as well as specific diseases that might affect the OSS, such as lagophthalmos, entropion, pterygium, and pingueculum.Special attention is directed toward recognizing clinical signs of blepharitis (ulceration around the base of the lashes, collarettes, misdirected lashes, absent lashes, poliosis, and tylosis) as well as evidence of meibomitis (inflammation of the meibomian glands, plugging of the orifices with inspissated secretions, expression of thick material from the glands, lid telangiectasia, and signs of rosacea).
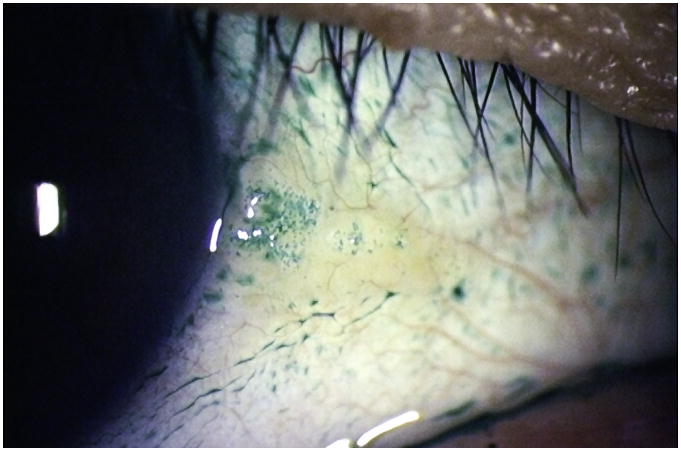
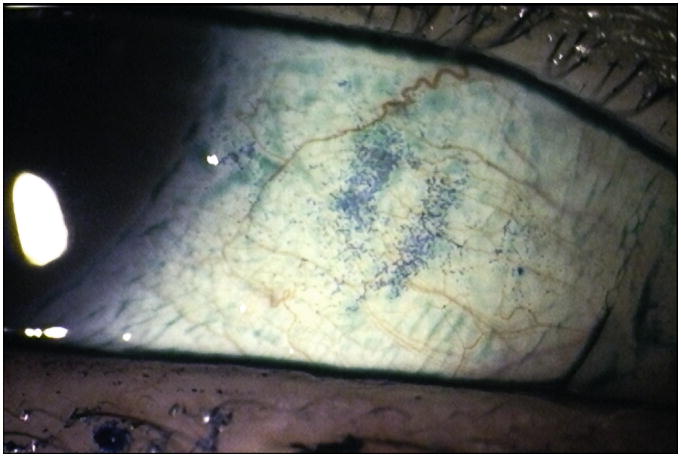
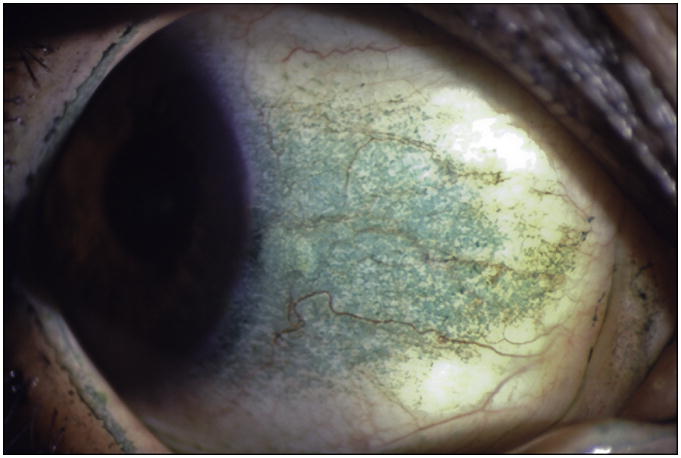
5) Conjunctival Lissamine Green Staining Pattern (Step 2 of the Ocular SICCA grading)
After the external examination, one drop of 1% lissamine green dye (Leiter's Pharmacy San Jose, California) is applied to the inferior conjunctival fornix of both eyes.The conjunctivae are examined with the slit lamp at 10× magnification, using a neutral density filter over the light source to avoid blanching of the conjunctiva. It is important to examine and grade the eyes immediately after instilling lissamine green dye because the intensity and extent of the ocular staining diminishes rapidly after the first two minutes. It is also important for the patient to blink several times to keep dye from pooling in the conjunctival folds, which can mimic conjunctival staining. If adequate dye is not instilled initially, a second drop can be given and the examination performed immediately thereafter. In the OSS, grade 0 (Figure 3a) is defined as 0 to 9 dots of lissamine green staining of the interpalpebral bulbar conjunctiva (nasal and temporal bulbar conjunctivae graded separately); grade 1 (Figure 3b) is defined by the presence of 10 to 32 dots; grade 2 (Figure 3c) by 33 to 100; and grade 3 (Figure 3d) >100 dots. Because of the difficulty of counting individual dots in a moving eye at the slit lamp, any area of confluent staining ≥4mm2 is considered to be >100 dots. A 4th grade consisting of totally confluent staining was initially considered but later determined not to add quantitatively to the diagnostic grading scheme, although it could be qualitatively meaningful for monitoring treatment in patients with KCS. Nasal and temporal areas of the conjunctiva are graded separately with a maximum score of three for each area or a total maximum score of 6 for each eye (nasal plus temporal). The total OSS for each eye is the summation of the fluorescein score for the cornea and the lissamine green scores for the nasal and temporal bulbar conjunctiva. Therefore, the maximum possible score for each eye is 12. The eyes are graded separately and the scores recorded on the SICCA ocular staining score form (Figure 2) at each patient visit. Staining of pinguecula, pterygia, and artifacts caused by Schirmer strips are not included in the score.
Figure 3.
Lissamine green staining of the bulbar conjunctiva in keratoconjunctivitis sicca
Figure 3a. Lissamine green background staining of the bulbar conjunctiva, Score 0. (Some pooling of dye is present; but <10 discrete individual green dots are seen in the interpalpebral area). Photograph courtesy of Dr. Kitagawa.
Figure 3b. Lissamine green staining of the bulbar conjunctiva in keratoconjunctivitis sicca, Score 1 with >10 and <33 green dots in the interpalpebral area. (Staining on the surface of the adjacent pingueculum is considered an artifact and is therefore ignored). Photograph courtesy of Dr. Kitagawa.
Figure 3c.. Lissamine green staining of the bulbar conjunctiva in keratoconjunctivitis sicca, Score 2 with 33 to100 green dots present in the interpalpebral area. (The two areas of partial confluent staining are each < 4 mm2 and are therefore scored as 33 to 100 dots – see text). Photograph courtesy of Dr. Kitagawa.
Figure 3d. Lissamine green staining of the bulbar conjunctiva in keratoconjunctivitis sicca, Score 3 with >100 individual green dots in the interpalpebral area associated with many areas of confluent staining. Photograph courtesy of Dr. Kitagawa.
Interpretation of the OSS
An OSS higher than 0 is considered to be abnormal and may be a sign of KCS. But scores of 1 or 2 can also represent a late staining artifact if interpretation of the fluorescein corneal staining pattern is delayed beyond 8 minutes. Because this could lead to a high level of misclassification, an abnormal OSS is defined as being a score of 3 or above.
Statistical Analyses
We computed standard summary statistics to describe the SICCA cohort characteristics with respect to eye symptoms, use of ophthalmic drops, use of systemic anticholinergic drugs, diseases affecting the bulbar conjunctiva or lids, results from Schirmer test, TBUT, and the OSS. We used a signed-rank test to evaluate the difference between right and left eye with respect to those tests performed on both eyes (i.e. Schirmer, TBUT, OSS) to determine if it would be appropriate to use either the mean or the maximum score between the right and left eye.
We used a proportional Venn diagram to visualize the interrelationships between an abnormal OSS (≥ 3) or KCS and the other two main phenotypic characteristics of Sjögren's syndrome (a LSG biopsy yielding a diagnosis of focal lymphocytic sialadenitis and a focus score > 1, and a positive serology for anti-SSA or anti-SSB antibodies). This also allowed visualization of the extent of overlap between cohort participants with one or more of these three characteristics.
We explored specific factors that may be associated with KCS (with or without the other two main phenotypic characteristics of Sjögren's syndrome). Factors such as lid and conjunctival diseases and anticholinergic medications may cause dry eyes in the absence of Sjögren's syndrome. It is thus important to explore their association with KCS (with or without the other two main phenotypic characteristics of Sjögren's syndrome). We used contingency table methods, or non-parametric tests when relevant, to explore these associations
Results
Eye-related characteristics among participants in the SICCA registry
Among the 1208 participants for whom data were available as of September 15, 2008, the vast majority were women (93%), with a median age of 55 years (range: 21 to 90 years). The cohort was predominantly Caucasian (43%) and Asian (38%). A large proportion (85%) reported symptoms of dry eyes and among those, 43% reported that they had had these symptoms for more than 5 years (Table 1). A quarter of the participants (24%) reported having eye redness half of the time or more, and the same proportion reported an inability to produce tears. There was a widespread use of artificial tears with 32% reporting use 1 to 3 times per day, and 29% reporting use 4 or more times per day. Only 8% reported using cyclosporine drops, and 5% corticosteroid drops. With respect to lid and conjunctival diseases, the most common condition was the presence of a pingueculum (28%) followed by meibomitis (15%) and blepharitis (11%), either unilaterally or bilaterally.
Table 1. Eye-related cohort characteristics among participants in the Sjögren's International Collaborative Clinical Alliance as of September 15, 2008 (N = 1208).
| Eye-related characteristics | n | (%)a |
|---|---|---|
| Symptoms | ||
| Reported symptom of dry-eyes | 1025 | (85) |
| Duration since onset of dry eyes (years)b | ||
| ≤ 1 | 156 | (16) |
| 1; ≤ 2 | 119 | (12) |
| > 2; ≤ 5 | 280 | (29) |
| > 5; ≤ 10 | 214 | (22) |
| > 10 | 207 | (21) |
| Reported eye redness | ||
| Never | 441 | (37) |
| Sometime | 470 | (39) |
| Half of the time or more | 289 | (24) |
| Inability to produce tears | 290 | (24) |
|
| ||
| Use of Ophthalmic Drops | ||
| Frequency of artificial tear use: | ||
| Never | 468 | (39) |
| 1 to 3 times/day | 381 | (32) |
| 4 times/day or more | 348 | (29) |
| Use of medicated drops c | ||
| None | 851 | (71) |
| Cyclosporine | 80 | (8) |
| Antibiotic | 99 | (10) |
| Steroid | 45 | (5) |
| Other | 99 | (10) |
|
| ||
| LID and Conjunctiva Diseases (unilateral or bilateral)d | ||
| Pingueculum | 334 | (28) |
| Meibomitis | 184 | (15) |
| Blepharitis | 134 | (11) |
| Punctal occlusion (lower) | 93 | (8) |
Column percent may not sum to 100% for some variables due to rounding
Median duration since onset of dry eyes: 51 months (6.7 years); range < 1 to 723 months
Column percent does not add up to 100% because participants may use more than one type of medicated drops
Other conditions affecting lid or conjunctiva such as lagophthalmos, entropion, ectropion, were diagnosed in < 2% of participants
As described earlier, the OSS may have a value ranging from 0 (no corneal or conjunctival staining detected) to 12 for each eye. We used the maximum OSS between the right and left eye because 80% of participants had a minimal difference of 0 or 1 between the two eyes. However, the TBUT and Schirmer test had skewed distributions and higher variability between right and left eyes; therefore, we used the mean TBUT and Schirmer test values between the two eyes for each participant.
Interrelationship between abnormal OSS, LSG focus score > 1, and presence of anti-SS-A or B antibodies
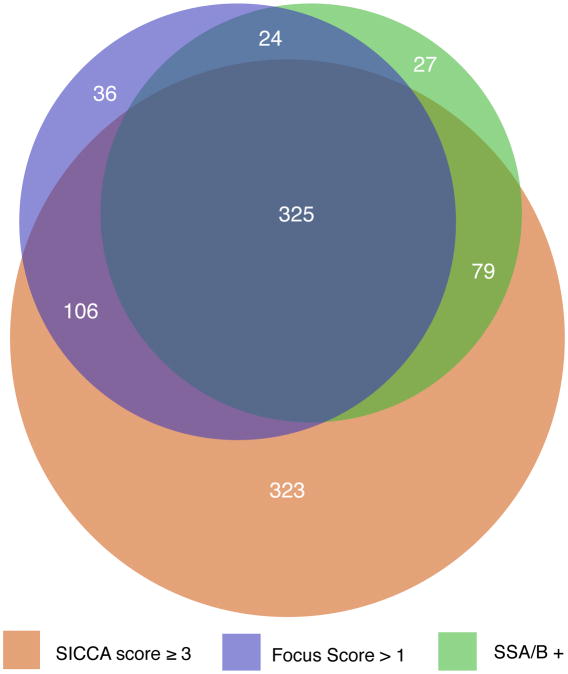
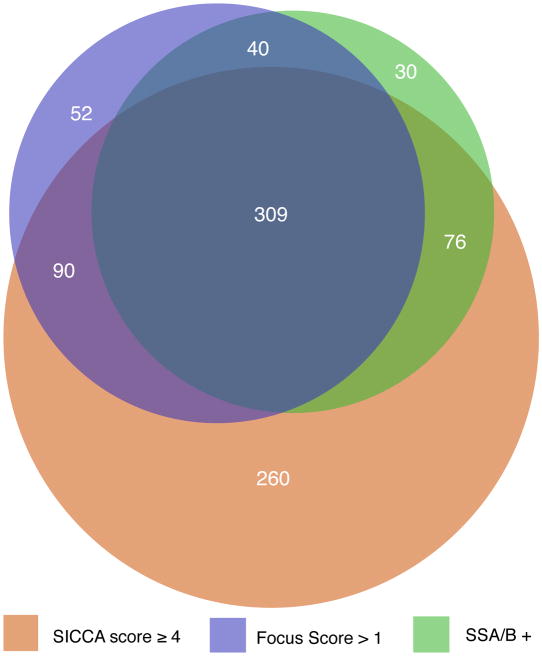
A total of 920 participants in the SICCA Registry had at least one of three phenotypic characteristics thought to be associated with Sjögren's syndrome: an abnormal OSS of 3 or greater; focal lymphocytic sialadenitis with a focus score > 1; and/or a positive serology to anti-SS-A or B antibodies. These participants were included in a proportional Venn diagram, which revealed that 28% had all three characteristics while 28% had an abnormal OSS only, 3% had an abnormal focus score only, and 2% had a positive serology to anti SS-A or B in the absence of the other two characteristics (Figure 4a). To explore whether or not the level at which we defined an abnormal OSS might explain the much larger proportion of participants with a positive OSS only (as compared to those with either FS>1, or with a positive serology to SS-A and/or B antibodies), we also constructed a Venn diagram with an abnormal OSS defined as 4 or greater (Figures 4b). Even though the overlap between groups shifted slightly, the large proportion of participants with abnormal OSS-only remained (at 22%).
Figure 4. Area-proportional Venn diagrams visualizing the interrelationships between abnormal Ocular Staining Score, labial salivary gland focus scores > 1, and positive anti-SSA and/or anti-SSB antibodies.
Figure 4a. Compares the three measures using an Ocular Staining Score cut-off value of ≥ 3
Figure 4b. Compares the three measures using an Ocular Staining Score cut-off value of ≥ 4
Because of the high percentage of participants who had an abnormal OSS in the absence of any other phenotypic features of Sjögren's syndrome we considered two subgroups of participants with an abnormal OSS in our analyses: those with a score of ≥3 but neither of the other two features of Sjögren's syndrome, and the other group with a score of ≥3 and at least one of the two other main phenotypic feature of Sjögren's syndrome (focal lymphocytic sialadenitis with a focus score > 1 and/or a positive serology to anti-SS-A or B antibodies). Because an abnormal OSS suggests a diagnosis of KCS we termed the two subgroups as follows: KCS-only (abnormal OSS but no focal lymphocytic sialadenitis / focus score > 1 and negative serology to anti-SS-A or B antibodies), and SS-KCS (abnormal OSS with focal lymphocytic sialadenitis / focus score > 1 and/or a positive serology to anti-SS-A or B antibodies)
Exploration of potential explanatory variables of SS-KCS versus KCS-only
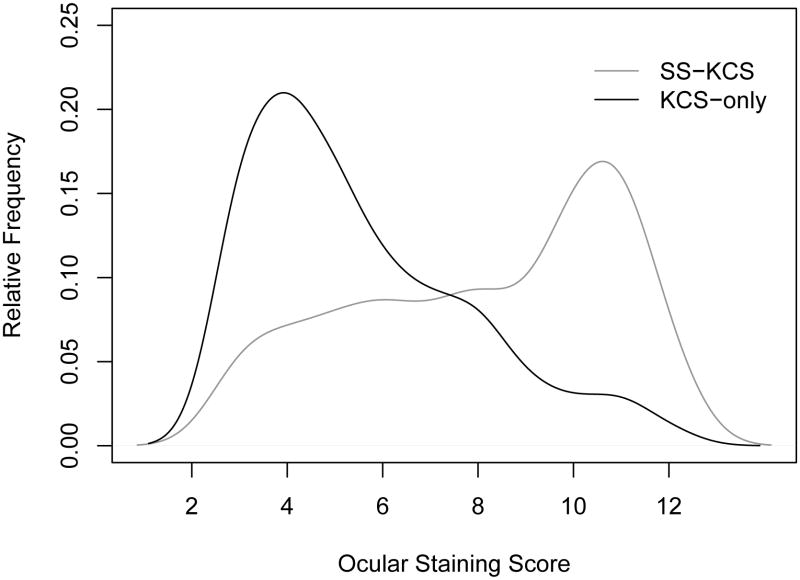
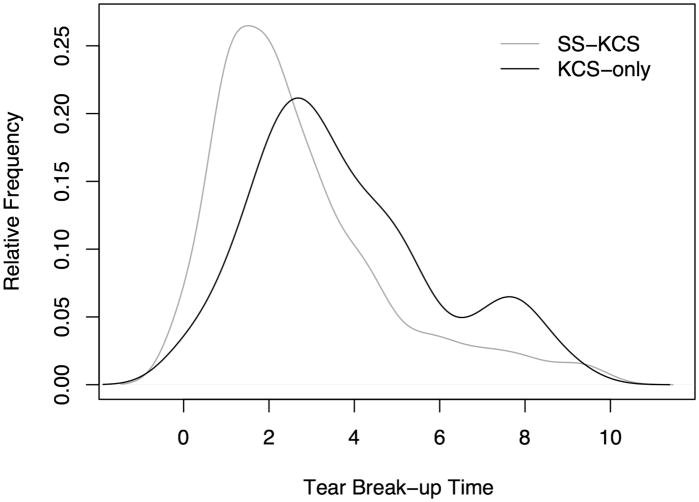
The distribution of the OSS differed in the two KCS subgroups (Figure 5). Among participants with KCS-only, the median OSS was 5 compared to 9 among those with SS-KCS, and a non-parametric test (Wilcoxon rank-sum test) revealed a statistically significant difference between the OSS in the two sub-groups (P < 0.0001). We also compared the distribution of the tear break-up time in the two subgroups, and also found a statistically significant difference, with a median tear break-up time of 3.5 among those with KCS-only compared to 2 among the SS-KCS group (P < 0.0001; Figure 6). However, the difference in distribution between the two subgroups was much more pronounced with respect to the OSS (Figure 5).
Figure 5.
Distribution of the Ocular Staining Score (OSS) among participants with Keratoconjunctivitis Sicca-Only and those with Sjögren's Syndrome-Keratoconjunctivitis Sicca. Keratoconjunctivitis Sicca-Only cases have abnormal OSS ≥ 3, but no focal lymphocytic sialadenitis or have focus scores <1 and have negative serology to anti-SS-A or B antibodies. Sjögren's Syndrome-Keratoconjunctivitis Sicca cases have OSS ≥ 3, focal lymphocytic sialadenitis with focus score >1 and/or positive anti-SS-A or B antibodies. The estimated relative frequency among the two groups extends slightly beyond the ranges of the OSS as a result of “smoothing” used in the estimation process.
Figure 6.
Distribution of the tear break-up time among participants with Keratoconjunctivitis Sicca-Only and those with Sjögren's Syndrome-Keratoconjunctivitis Sicca. Keratoconjunctivitis Sicca-Only cases have abnormal ocular staining score (OSS ≥ 3), but no focal lymphocytic sialadenitis or have focus scores <1 and have negative serology to anti-SS-A or B antibodies. Sjögren's Syndrome-Keratoconjunctivitis Sicca cases have OSS ≥ 3, focal lymphocytic sialadenitis with focus score >1 and/or positive anti-SS-A or B antibodies.
We compared other Sjögren's syndrome-related characteristics among SICCA cohort participants by KCS status, and found a statistically significant difference with respect to the Schirmer test, which was lower, reflecting a higher level of severity, in the SS-KCS than in the KCS-only group (Table 2). The rheumatoid factor, antinuclear antibody titer, and immunoglobulin G level also reflected a higher level of severity in the SS-KCS group as they were significantly higher than in the KCS-only group. The proportion of participants with an unstimulated whole salivary flow rate < 0.1 ml/minute was also significantly higher in the SS-KCS group as compared to the KCS-only group. However, there was no difference between the groups with respect to the prevalence of dry-eye or dry-mouth symptoms, nor with respect to the duration of dry-eye symptoms. The Comparison of socio-demographic and non-Sjögren's-related ocular characteristics among SICCA cohort participants by KCS status revealed no difference between the two groups with respect to gender or to the prevalence of blepharitis or meibomitis (Table 3). However, the two groups differed with respect to race and age: the median age of the KCS-only group was higher, and there was a significantly higher proportion of Asians in the SS-KCS group (52% versus 29%). The prevalence of anticholinergic drug use was also significantly higher in the KCS-only than in the SS-KCS group (39% versus 18%; P < 0.001).
Table 2. Comparison of phenotypic Sjögren's syndrome-related characteristics among participants in the Sjögren's International Collaborative Clinical Alliance cohort by keratoconjunctivitis sicca statusa.
| Sjögren's syndrome-related characteristics | KCS-onlya (N = 323) | SS-KCSa (N = 510) | |
|---|---|---|---|
| Continuous Variables | Median [range] | Median [range] | P-valueb |
| Schirmer test | 8.5 [0-35] | 5.5 [0-34] | < 0.001 |
| Tear break-up time | 3.5 [0-11] | 2.0 [0-11] | < 0.001 |
| Duration of dry-eye Symptom (years) | 4.2 [0.09-40] | 4.3 [0-36] | 0.9 |
| Rheumatoid Factor (IU/mL) | 8 [3-600] | 38 [3-888] | < 0.001 |
| Antinuclear antibody titer | 80 [40-1280] | 1280 [40-2560] | < 0.001 |
| Immunoglobulin G (mg/dL) | 1130 [435-3421] | 1755 [236-5940] | < 0.001 |
|
| |||
| Categorical Variables | n (%) | n (%) | P-valuec |
| Dry-eye symptoms | 285 (88) | 438 (86) | 0.3 |
| Dry-eye symptms | 290 (90) | 466 (91) | 0.5 |
| UWSd flow rate (< 0.1 mL/min) | 148 (46) | 351 (69) | < 0.001 |
The KCS-only group consists of participants with abnormal Ocular Staining Score (OSS) but no abnormal labial salivary gland focus score and negative serology to anti-SS-A and/or B antibodies. The SS-KCS group consists of those with OSS and at least one of the two other main phenotypic features of Sjögren's syndrome (focal lymphocytic sialadenitis with a focus score > 1 and/or a positive serology to anti-SS-A or B antibodies)
P-value for the Wilcoxon rank-sum test
P-value for the Fisher's exact test
Unstimulated whole saliva
Table 3. Comparison of socio-demographic and non-Sjögren's syndrome-related ocular characteristics among participants in the Sjögren's International Collaborative Clinical Alliance cohort by keratoconjunctivitis sicca statusa.
| Non-Sjögren's syndrome-related characteristics | KCS-onlya (N = 323) | SS-KCSa (N = 510) | |
|---|---|---|---|
| Categorical Variables | n (%)b | n (%)b | P-valuec |
| Gender | |||
| Female | 297 (92) | 485 (95) | |
| Male | 26 (8) | 25 (5) | 0.08 |
| Race | |||
| White | 134 (41) | 161 (32) | |
| Asian | 94 (29) | 263 (52) | |
| Other | 95 (29) | 84 (17) | < 0.001 |
| Current smoking | 53 (16) | 15 (3) | < 0.001 |
| Blepharitis | 47 (15) | 87 (17) | 0.4 |
| Meibomitis | 41 (13) | 60 (12) | 0.7 |
| Current use of anticholinergic drugs | 126 (39) | 89 (18) | < 0.001 |
|
| |||
| Continuous Variable | Median [range] | Median [range] | P-valued |
| Age (years) | 57 [21-89] | 54 [21-87] | 0.001 |
The KCS-only group consists of participants with abnormal Ocular Staining Score (OSS) but no abnormal labial salivary gland focus score and negative serology to anti-SS-A and/or B antibodies. The SS-KCS group consists of those with OSS and at least one of the two other main phenotypic features of Sjögren's syndrome (focal lymphocytic sialadenitis with a focus score > 1 and/or a positive serology to anti-SS-A or B antibodies)
Column percent may not sum to 100% for some variables due to rounding
P-value for the Fisher's exact test
Wilcoxon rank-sum test for the continuous variable
Discussion
In this paper, we describe the OSS, a new quantitative simplified method of grading KCS in patients with primary Sjögren's syndrome. Previous grading systems have been simplified, standardized, and combined to produce the OSS, which is then subjected to validation by non-ocular data collected from patients with primary Sjögren's syndrome or its partial phenotype. With 1208 participants as of September 15, 2008, the SICCA Registry is a large, and ethnically and geographically diverse, ongoing cohort of women and men with suspected Sjögren's syndrome. Most of the participants (85%) reported symptoms of dry eyes, and among those, nearly half reported having these symptoms for more than 5 years. An unexpected finding was the high number of participants (n = 323) with objective clinical signs of KCS in the absence of any other phenotypic features of Sjögren's syndrome while 510 participants (Figure 4a) had KCS together with at least one of the two other main phenotypic features of Sjögren's syndrome (focal lymphocytic sialadenitis with a focus score > 1 and/or a positive serology to anti-SS-A or B antibodies). The presence of clinical KCS in the absence of any other phenotypic features of Sjögren's syndrome (and without any associated lid or conjunctival disease) represents an interesting and unexpected finding that will require additional study.
One of the primary goals of the SICCA Registry is to establish standardized classification criteria for the diagnosis of Sjögren's syndrome. For the eye, this entails developing a KCS grading scheme that is simple, quantitative, reproducible, and straight forward enough to be universally adopted by eye care providers. A unique innovation of the OSS is the use of two different vital dyes to grade different areas of the ocular surface: fluorescein to grade the cornea and lissamine green to grade the bulbar conjunctiva. Previous grading schemes have used rose Bengal or lissamine green exclusively to grade both the cornea and the conjunctiva. While both of these dyes primarily stain goblet cells, keratinized epithelial cells, and devitalized cells, rose Bengal has been the dye of choice for grading conjunctival damage in patients with KCS. Interestingly, rose Bengal is not a true vital dye and is inherently toxic to epithelial cells. This is especially true in KCS where the protective function of the preocular tear film is disrupted12 and the dye becomes so irritating that many investigators recommend prior instillation of a topical anesthetic.9 This is in direct contrast to lissamine green; a true, non-irritating vital dye.13 Fluorescein is also nontoxic, nonirritating and is the most useful dye for staining the precorneal tear film and those areas of the cornea where the epithelium is absent or eroded.14 Under cobalt blue illumination, fluorescein is extraordinarily sensitive in delineating individual epithelial cell loss and PEE. We used a solution of 0.5% because it routinely produces the most reliable corneal staining pattern during the four to eight minute time interval following instillation.
To develop a quantitative, reproducible and reliable KCS grading system, we used a modification of the Oxford grading scheme proposed by Bron, et al in 2003.9 By quantitatively grading both the cornea and bulbar conjunctiva, and giving equal weight to clinical findings in both areas, the OSS provides a simplified, non-irritating, quantitative grading system that is easily applicable to clinical practice without the need for specialized equipment other than a slit lamp. Its reliability was confirmed by the observation that 80% of participants in the study had an OSS with a difference between the two eyes of 1 or less, while both the TBUT and Schirmer tests had skewed distributions with a higher variability between the two eyes. While Vesura, et al.15,16 promotes using a combination of tests to diagnose KCS, he also acknowledges that the ocular staining pattern with lissamine green is unquestionably of greatest importance. The SICCA study confirms this assertion and promotes the OSS as the key ocular diagnostic parameter for KCS.
Even with the development of the OSS, the diagnosis of Sjögren's syndrome remains problematic. Sjögren's syndrome is defined as an autoimmune exocrinopathy characterized by lymphoid infiltration and functional deterioration of exocrine glands, especially of lachrymal and salivary glands. Sjögren's syndrome may develop in the absence (primary SS) or in the presence (secondary SS) of another autoimmune disease.17 Although the true incidence is not known, the diagnosis of primary SS by primary care physicians in a defined population has been reported to be as infrequent as 4 per 100,000.18 This figure is undoubtedly low because it is based on a core of patients with primary SS in a community setting rather than on a case detection survey where there are well-established lines of referral. Higher physician awareness and simplified classification criteria for primary SS would lead to more frequent identification and less misclassification of cases in the population.19 Paradoxically, KCS is one of the most frequently made diagnoses in an ophthalmic practice. Studies indicate that up to 20% of adults aged 45 or older experience dry eye symptoms in varying degrees of severity.20 This disparity between the large number of symptomatic patients who are being diagnosed with KCS and the relatively small number of patients with primary SS has never been explained.21
With this in mind, an intriguing finding of the SICCA study to date is the documentation of two different forms of KCS. Over the course of study, a group of KCS patients emerged that have all of the ocular diagnostic parameters, but none of the non-ocular characteristics of Sjögren's syndrome (KCS-only). The surprising size of this group (323 of 920 patients or 35 %) is best appreciated in the proportional Venn diagram (Figure 4a), where overlapping areas are exactly proportional to the number of patients with the specified characteristics. The lower “bulge” in the diagram represents the large number of patients who have KCS as confirmed by a positive OSS and supported by decreased TBUT and low Schirmer values, but who do not have abnormal LSG focus scores and/or a positive serology for anti-SS-A and/or B antibodies. The “bulge” does not change substantially in size (260 of 857 patients or 30%) when the OSS grade is raised from 3 to 4 (Figure 4b). The other group, consisting of individuals with a positive OSS and at least one of the two other diagnostic characteristics for SS also remains stable when the OSS changes from 3 to 4. Further comparison of the two groups reveals that they have several different characteristics that are statistically significant (Tables 2 and 3). For example, those with KCS-only had significantly higher scores for other ocular tests commonly used in the evaluation of Sjögren's syndrome (Schirmer test and TBUT), as well as greater unstimulated whole saliva flow rates. They had significantly lower levels of specific markers of autoimmunity (e.g., rheumatoid factor, ANA, and IgG), were significantly older, more likely to be current smokers and more likely to be taking anti-cholinergic medications. Yet, there was no difference between the KCS-only and SS-KCS groups with respect to dry-eye or dry-mouth symptoms, duration of dry-eye symptoms, or presence of specific diseases of the lids and conjunctiva (e.g., blepharitis and meibomitis). These findings suggest that KCS-only and SS-KCS may represents separate clinical entities with clear characteristic differences between the two patient populations.
For decades ophthalmologists have acknowledged anecdotally that a form of KCS exists that is not related to Sjögren's syndrome and for which there is no obvious biological explanation. In a long-term follow up study, Kruize, et al17 alluded to this form of isolated KCS but did not fully characterize the small group of patients. It was noted, however, that none of the patients with isolated KCS or secondary SS developed lymphoproliferative disease over a 10 to 12 year period, while 10% of the primary SS patients died of lymphoma. This suggests that patients with isolated KCS represent a distinct clinical population that is much more common and milder than SS-KCS and likely accounts for the majority of dry eye patients seen in ophthalmic practices. The authors of the NEI-sponsored DEWS (Dry Eye Workshop) Reports22, 23 in 2007 also discussed this clinical entity, calling it non-Sjögren's syndrome (NSS) KCS, but data were not presented. The only way to prove that these “NSS-KCS” patients do not eventually develop SS-KCS is to follow them over time. The SICCA Registry provides the opportunity to follow these patients over time and to answer the following questions: 1) do patients with KCS-only eventually convert to SS-KCS on follow up? 2) do both forms become clinically worse over time? or 3) do two distinct disease processes exist that follow different courses and ultimately have different outcomes?
In the process of developing the OSS for the purpose of establishing new universal classification criteria for the diagnosis of SS, we have identified a large subset of dry eye patients, who have clinical characteristics that are distinctly different from those patients with SS-KCS. The underlying cause of this condition is at present unknown and represents a new and interesting area for future study that we hope to investigate with the large data base that is currently being collected as part of the SICCA Registry.
Acknowledgments
Funding/Support. This project has been and continues to be funded by NIH contract NO1-DE-32636, from 2003 through 2013.
Other acknowledgements. The authors are very grateful to the many patients who participated in this Registry. We also gratefully acknowledge the contributions of two consultants during development of the SICCA ocular evaluation process during 2003-05: Stephen Pflugfelder (Baylor College of Medicine) and Austin Mircheff (University of Southern California, School of Medicine).
In addition to the listed authors, other professional collaborators in the Sjögren's International Collaborative Clinical Alliance have been essential to the conduct of this project and their roles include the following:
University of California, San Francisco, USA. Rheumatology K Sack, D Lee, Ophthalmology E Strauss, M Chan, Oral Medicine A Wu, D Greenspan, Pathology D Cox, R Jordan, Operations Director Y DeSouza, Clinical Coordinator / Phlebotomy D Drury, Clinical Assistant L Scott.
University of Buenos Aires and German Hospitals, Buenos Aires, Argentina: Group Director H Lanfranchi, Rheumatology C Vollenweider, Stomatology I Adler, A Smith, LSG biopsies M Gandolfo, PM Bisio, Pathology A Keszler, A Chirife, Specimen processing S Daverio, Group Coordinator V Kambo
Peking Union Medical College Hospital, Beijing, China: Group Director Y Dong, Group Co-Director / Rheumatology Y Zhao, Rheumatology M Li, W Zhang, W Zheng, Y Jiang, D Xu, J Su, Ophthalmology J Zhao, Stomatology / Pathology D Du, Stomatology / LSG biopsies J Xiao, H Wang, Specimens / Rheumatology Q Wu, Phlebotomy C Zhang, W Meng.
Copenhagen University Hospital Glostrup, Denmark: Group Director M Schiødt, Initial Ophthalmologist S Johansen, Rheumatology P Helin, Stomatology / LSG biopsies J Schiødt, H Holm, Pathology P Ibsen, Group Coordinators/Specimen Handling AM Manniche, SP Kreutzmann.
Kanazawa Medical University, Ishikawa, Japan: Group Director H Umehara, Rheumatology Y Masaki, M Tanaka, Rheumatology / LSG biopsies T Sawaki, Ophthalmology K Hagiwara, Pathology T Nojima, N Kurose, Stomatology M Hondo, M Takahashi, Specimen processing T Kawanami, Group Coordinator K Fujimoto.
King's College London, UK: Group Director S Challacombe, Co-Director P Shirlaw, Oral Medicine B Jacobs, Rheumatology F Barone, Pathology E Odell, P Morgan, Specimen processing L Fernandes-Naglik.
Acronyms used in the text
- ANA
antinuclear antibody
- KCS
keratoconjunctivitis sicca
- LSG
labial salivary gland biopsy
- OSS
ocular staining score
- PEE
punctuate epithelial erosions
- RF
rheumatoid factor
- SICCA
Sjögren's International Collaborative Clinical Alliance
- SS
Sjögren's syndrome
- TBUT
tear breakup time
Footnotes
Financial Disclosures. None of the authors have financial disclosures regarding the conduct of this study, during its entirety.
Contributions to Authors. Conception and study design (JW, CS, JG, TD); Analysis and interpretation (JW, CS, SS, TD); Writing the article (JW, CS, NM, TD); Critical revision of article (JW, CS, SS, AH, NM, TD); Final approval of the article (all authors); Data collection (JW, AH, KK, SZ, SH, GL, NM); Statistical expertise (SS, CS); Literature search (JW, NM); Obtain funding (TD, JG).
Statement about Conformity with Author Information. The University of California San Francisco IRB (Committee on Human Research) has approved the entire study, of which this paper represents a part. In addition, IRBs at each of those institutions listed on the title page and below, have approved this study and all of those institutions have had US Department of Health and Human Services Federal-Wide Assurance (FWA) registration during its conduct. Informed consent was obtained from all participants and (in the US) in accordance with HIPAA regulations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sjögren H. Zur Kenntis der Keratoconjunctivitis Sicca (Keratitis filiformis bei Hypofunktion der Tranendrusen) Acta Ophthalmol. 1933;(2):1–151. [Google Scholar]
- 2.fluger P. Zur Ernahrung der cornea. Klin Monatsbl Augenheilkd. 1882;20:69–81. [Google Scholar]
- 3.Holm S. Keratoconjunctivitis sicca and the sicca syndrome. Acta Ophthamologica. 1949;33(Suppl):1–230. [Google Scholar]
- 4.van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthal. 1969;82:10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]
- 5.Norn MS. Lissamine green: Vital staining of cornea and conjunctiva. Acta Ophthalmologica. 1973;51:483–91. doi: 10.1111/j.1755-3768.1973.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 6.Norn MS. Vital Staining of Cornea and Conjunctiva: Fluorescein-rose Bengal Mixture and Tetrazolium-Alcian Blue Mixture. Acta Ophthamologica. 1972;113(Suppl):3–66. [PubMed] [Google Scholar]
- 7.Manning FJ, Wehrly SR, Foulks GN. Patient tolerance and ocular surface staining characteristics of lissamine green versus Rose Bengal. Ophthalmology. 1995;102:1953–7. doi: 10.1016/s0161-6420(95)30769-5. [DOI] [PubMed] [Google Scholar]
- 8.Lemp MA. Report of the National Eye Institute/Industry Workshop on clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 9.Bron AJ, Evans VE, Smith JA, et al. Grading of corneal and conjunctival staining in the context of other dry tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Daniels TE, Criswell LA, Shiboski C, et al. An early view of the International Sjögren's syndrome Registry. Arthritis Rheum. 2009;61:711–14. doi: 10.1002/art.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mengher LS, Pandher KS, Bron AJ. Non-invasive tear film break-up time: sensitivity and specificity. Acta Ophthalmol. 1986 Aug;64(4):441–4. doi: 10.1111/j.1755-3768.1986.tb06950.x. [DOI] [PubMed] [Google Scholar]
- 12.Feenstra RP, Tseng SC. What is actually stained by rose Bengal? Arch Ophthalmol. 1992;110:984–93. doi: 10.1001/archopht.1992.01080190090035. [DOI] [PubMed] [Google Scholar]
- 13.Chodosh J, Dix RD, Howell WG, et al. Staining characteristics and antiviral activity of sulforhodamine B and lissamine green B. Invest Ophthalmol Vis Sci. 1994;35:1046–58. [PubMed] [Google Scholar]
- 14.Feenstra RP, Tseng SC. Comparison of fluorescein and rose Bengal staining. Ophthalmology. 1992;99:605–17. doi: 10.1016/s0161-6420(92)31947-5. [DOI] [PubMed] [Google Scholar]
- 15.Versura P, Frigato M, Mule R, et al. A proposal of new ocular items in Sjögren's syndrome classification criteria. Clin Exp Rheumatol. 2006;24:567–72. [PubMed] [Google Scholar]
- 16.Versura P, Frigato M, Cellini M, et al. Diagnostic performance of tear function tests in Sjögren's syndrome. Eye. 2007;21:229–37. doi: 10.1038/sj.eye.6702204. [DOI] [PubMed] [Google Scholar]
- 17.Kruize AA, Hene R, van der Heide A, et al. Long-term follow-up of patients with Sjögren's syndrome. Arthritis Rheum. 1996;39:297–303. doi: 10.1002/art.1780390219. [DOI] [PubMed] [Google Scholar]
- 18.Pillemer SR, Matteson E, Jacobsson L, et al. Incidence of physician-diagnosed primary Sjögren syndrome in residents of Olmsted county, Minnesota. Mayo Clin Proc. 2001;76:593–9. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 19.Vitali C, Moutsopoulos H, Bombardieri S. The European community study group on diagnostic criteria for Sjögren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren's syndrome. Ann Rheum Dis. 1994;53:637–47. doi: 10.1136/ard.53.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45:S199–202. doi: 10.1016/s0039-6257(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 21.Whitcher JP, Gritz DC, Daniels TE. The Dry Eye: A Diagnostic Dilemma. Int Opthalmol Clin. 1998;38(4):23–37. doi: 10.1097/00004397-199803840-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dry Eye Workshop. Methodologies to diagnose and monitor dry eye disease: report of the diagnostic methodology subcommittee of the international dry eye workshop (2007) The Ocular Surface. 2007;5:108–52. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 23.Dry Eye Workshop. Research in dry eye: report of the research subcommittee of the international dry eye workshop (2007) The Ocular Surface. 2007;5:179–93. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]