Abstract
Edited magnetic resonance spectroscopy makes possible non-invasive studies of the role of the inhibitory neurotransmitter GABA in the healthy brain and in disease processes. A major limitation of the methodology is co-editing of macromolecular signals. Although it has previously been shown that macromolecular signal can be suppressed using a symmetrical editing scheme, this approach is rarely applied at field strength of 3T as insufficiently selective pulses result in loss of GABA signal (in addition to the intended suppression of macromolecular signal). In this manuscript, we show that increasing the echo time to 80 ms lets more selective editing pulses to be used, allowing for symmetric editing-based suppression of co-edited macromolecular signal without loss of GABA signal. The method is applied to acquire MM-suppressed GABA-edited spectra in ten healthy participants.
Keywords: GABA, editing, 3T, MEGA-PRESS, MM-suppressed
Introduction
Detection of the inhibitory neurotransmitter GABA by magnetic resonance spectroscopy (MRS) provides an exciting opportunity to probe inhibitory function in healthy subjects e.g. (1–4) and in clinical populations (e.g.(5–9); for a review see (10)). Although J-difference editing of GABA (11) was originally proposed almost twenty years ago, uptake of these methods has accelerated more recently with implementation into a standard PRESS sequence (12) and more widespread dissemination of scanner sequences. A fundamental weakness of GABA-edited MRS as implemented at 3T with a standard echo time of 68 ms is the potential for contamination of edited GABA signal with co-edited macromolecular (MM) signals(11,13). Although two different approaches have been suggested for removing MM signal (the ‘pre-inversion’(11) and ‘symmetrical suppression’(13) methods), both have significant limitations(10). The most common approach in the literature currently is to accept MM-contamination as a methodological limitation and report total GABA+MM signal, sometimes referred to as GABA+. Given the extremely prominent role of GABAergic inhibition in cortical processing, it is a significant problem that a wide variety of studies that relate GABA+ to psychiatric disease or cognitive function have dual interpretations: the observed effect could either be driven by GABA or MM. Therefore, the purpose of this study was to develop a simple, widely-accessible sequence for the MM-suppressed detection of GABA.
Theory
J-difference editing relies upon the fact that frequency-selective editing pulses applied to GABA signals at 1.9 ppm can modulate the appearance in the spectrum of GABA signals at 3 ppm by refocusing the evolution of the scalar coupling. Thus by acquiring two experiments, with and without editing pulses applied to the 1.9 ppm GABA resonances (typically referred to as the ‘ON’ and ‘OFF’ experiments, see Figure 1a), a difference spectrum can be calculated that contains a GABA signal at 3 ppm, and does not contain signals from metabolites unaffected by the editing pulses (such as overlying Cr at 3 ppm). Co-edited MM signal arises because the editing pulses applied to GABA spins at 1.9 ppm also partially invert MM signals at 1.7 ppm, which are coupled to MM signals at 3 ppm, with the result that MM signals also appear in the difference spectrumat 3 ppm. MM signals can be separated from GABA on the basis of differences in T1 using pre-inversion (11), or on the differences in chemical shift using symmetric editing-based suppression (13).
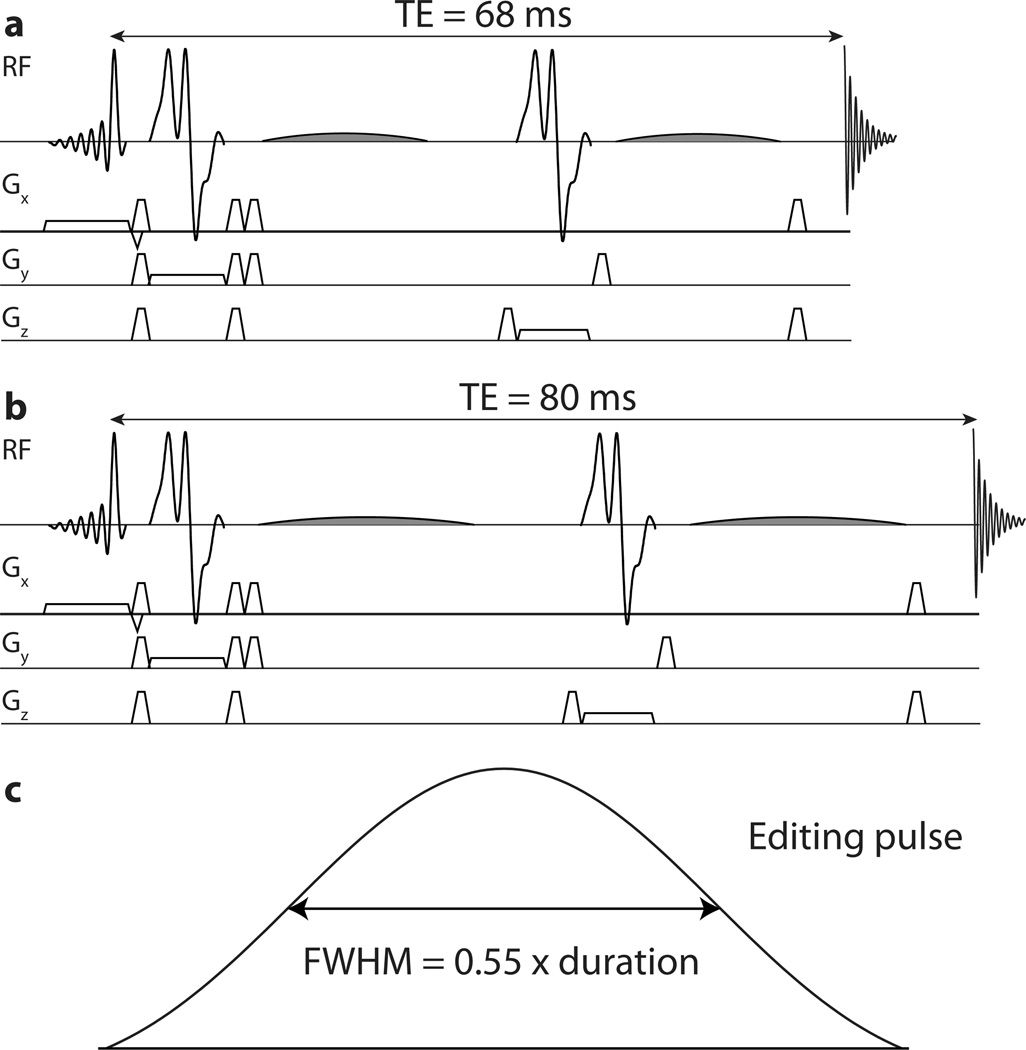
Figure 1.
Pulse sequences: a) MEGA-PRESS acquired at TE = 68 ms incorporating 14 ms editing pulses; b) MEGA-PRESS acquired at TE = 80 ms incorporating 20 ms editing pulses; c) editing pulse amplitude waveform (highlighted in gray in (a) and (b)) noting that the amplitude is above 50% of maximum for 55% of the pulse duration.
The pre-inversion method involves acquiring an additional MM-only acquisition acquired with pre-inversion followed by excitation at the null-point of metabolite T1 recovery. This method has three main limitations. Firstly, it assumes that the T1 of GABA is a known quantity (for accurate nulling) which is not the case. Secondly, it doubles experiment time for a net 30% reduction in GABA signal-to-noise (as the MM-only acquisition effectively adds noise to the GABA measurement). Thirdly, it involves subtraction of two scans of approximately ten-minute duration (which cannot be interleaved), requiring excellent subject compliance.
The symmetric-suppression method utilizes the fact that the MM signal co-edited at 3 ppm is coupled to spins at 1.7 ppm (13), by acquiring the GABA experiment so that editing pulses are applied at 1.9 ppm for the ON scans and 1.5 ppm for the OFF scans. Thus, editing pulses in both ON and OFF scans are applied 0.2 ppm away from the MM signals, which is inverted to an equal extent in both scans, and MM signals are suppressed from the difference spectrum. This elegant idea was first proposed at 4.7T (13) and is unfortunately not widely transferrable to 3T (14), because it has a strong requirement for editing pulse selectivity – editing pulses applied at 1.5 ppm in the OFF scans must not significantly affect GABA spins at 1.9 ppm or a loss of edited GABA signal will result. Due to a combination of limitations on peak B1 (e.g. ~14 uT on a typical 3T system using a body transmit coil) and echo time (to ~68 ms), editing pulses of ~14 ms duration are used (as seen in Figure 1a), which are insufficiently selective at 3T to avoid substantial suppression of GABA signal in addition to MM suppression (14).
Recently one solution to this problem was proposed: MEGA-SPECIAL (15). By incorporating J-difference editing within a SPECIAL localization sequence (16) rather than PRESS, it is possible to use the longer, more selective editing pulses required for editing-based MM suppression. The main drawbacks of this approach are that SPECIAL relies upon a subtraction for spatial localization, making MEGA-SPECIAL doubly sensitive to subject/scanner instability (using subtractions for both localization and editing), and that SPECIAL localization is not widely available on clinical platforms.
This paper proposes a different approach; while an echo time (TE) of 68 ms has long been used for the edited observation of GABA (11), the TE-modulation of the edited GABA signal is not particularly strong and therefore GABA can be measured over a fairly wide range of TE values without much loss of signal(17). Indeed close examination of Figure 2d in Reference (17) suggests that the underlying editing efficiency for this implementation of the MEGA-PRESS sequence is maximal at 80 ms. Therefore increasing the TE to 80ms allows an additional 6 ms for each editing pulse (as seen in Figure 1b), and with the corresponding gain in editing selectivity, symmetric suppression of co-edited MM signal can be used at 3T with the MEGA-PRESS sequence.
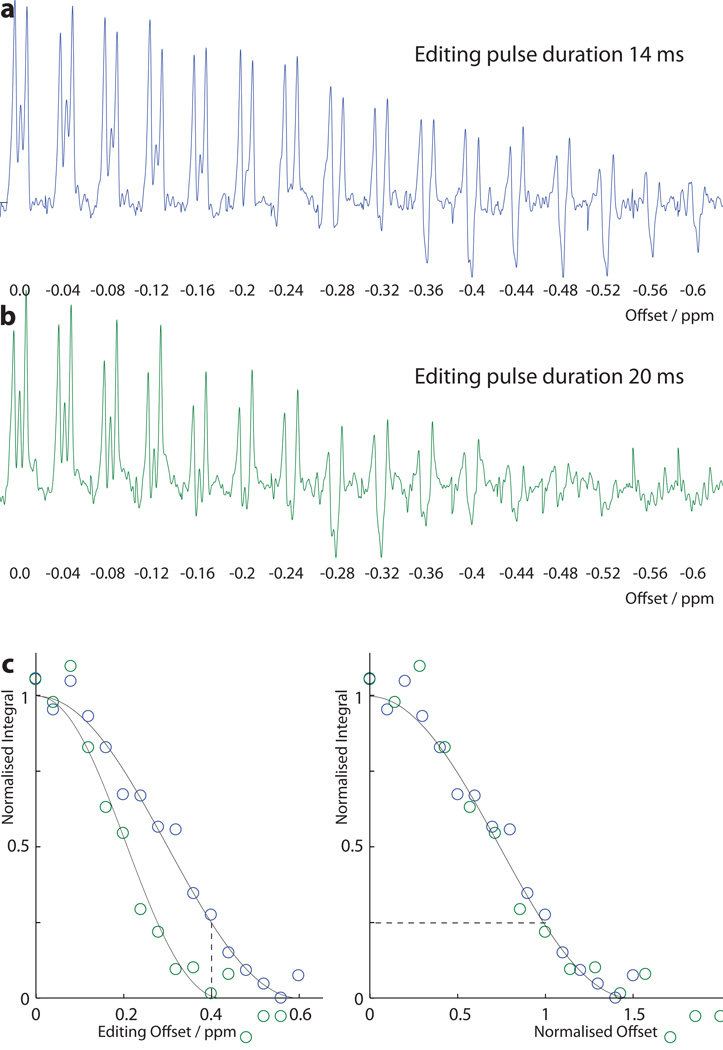
Figure 2.
Frequency-dependence of editing in a 10mM GABA phantom: a) 14 ms editing pulses; and b) 20 ms editing pulses. Editing pulse frequency during ‘ON’ scans is expressed as an offset in ppm from 1.9 ppm. Editing pulses are maintained at 7.5 ppm during all ‘OFF’ scans. The integrals of these scans are plotted in c) against offset (left) and against offset normalized to pulse length (right).
Methods
All data were acquired on a Philips Achieva 3T MRI scanner using an 8-channel phased array head coil for receive and the body coil for transmit. The scanner’s body RF coil can generate up to 14 uT and its gradients can generate up to 40mT/m with a 200 mT/m/ms slew rate or 80 mT/m with a 100 mT/m/ms slew rate.
Slice-selective refocusing was performed using amplitude-modulated refocusing pulses (‘GTST1203’) of bandwidth 1.4 kHz. The single-lobe sinc-Gaussian RF pulse shown in Figure 1c was used for editing inversion in all experiments. Coherence transfer pathway gradients of trapezoidal shape with slew rate 100 mT/m/ms, and amplitude 30 mT/m and duration 1.5 ms and 1.8 ms were used.
Phantom experiments
Phantom data were acquired in a one-liter bottle (Nalgene style 2125, Sigma Aldritch) containing a 10 mM solution of GABA (A2129, Sigma Aldritch) in phosphate-buffered saline (pH 7.4, P5368, Sigma Aldritch).
Edited spectra were acquired with the editing pulses applied at a range of frequency offsets in order to investigate the envelope of the editing pulses. A MEGA-PRESS sequence (12) was used for GABA editing, whose editing pulses have a single inversion lobe (i.e. without simultaneous water suppression).The frequency of the editing pulse in the ‘ON’ experiments was varied from 1.9 ppm to 1.3 ppm in increments of 0.04 ppm. The frequency of the editing pulse was kept at 7.5 ppm for all ‘OFF’ experiments. These experiments were acquired both at TE of 70 ms using 14 ms editing pulses and at TE 80 ms using 20 ms editing pulses. Each experiment consisted of 64 averages, acquired as 16 repeats of a 4-scan phase cycle with editing pulse frequencies switched for ‘ON’ and ‘OFF’ experiments on alternate phase cycles. Other experimental parameters include: TR 2000 ms; 2048 datapoints sampled at 2kHz spectral width; (2.5 cm)3 voxel; chemical shift selective pre-saturation of water signals. Data were processed using in-house softwarewith 3-Hz exponential line broadening, and the edited GABA signal was integrated from 2.83 ppm to 3.14 ppm.
Two further series of phantom experiments were acquired in order to quantify the underlying difference in edited signal at 68 ms and 80 ms. First, edited experiments were acquired with TE ranging from 70 ms to 220 ms in 10 ms increments. The ‘ON’ spectra from these data were used to calculate the T2 of the phantom, according to reference (17). A second series of edited experiments were acquired with TE ranging from 60 ms to 80 ms in 4 ms increments. In order to access short TE, the duration of the editing pulse in this second series was reduced to 10 ms. Other experimental parameters include: TR 2000 ms; 1024 datapoints sampled at 2kHz spectral width; (2.5 cm)3 voxel; chemical shift selective pre-saturation of water signals.
In vivo experiments
In vivo measurements were acquired in ten healthy volunteers (aged 35.4 ± 8.8 years; 5 male) who provided written informed consent under the approval of the institutional review board. In all subjects, a single measurement was acquired from a medial parietal voxel sized (3.5 cm)3. Editing pulses were applied at 1.9 ppm in ‘ON’ scans and 1.5 ppm in ‘OFF’ scans, applying the symmetrical-suppression method to suppress MM signal (13). Other experimental parameters include: TE 80 ms; TR 2000 ms; 256 averages; 1024 datapoints sampled at 2kHz spectral width; (3.5 cm)3 midline parietal volume; CHESS water suppression.
Results
Edited spectra acquired at a range of editing pulse offsets are shown in Figure 2a and b. It can clearly be seen that the signal drops off as the editing pulse is moved off-resonance relative to the 1.9 ppm GABA spins, and that the envelope of the frequency-selective editing is narrower for 20 ms editing pulses (b) than 14 ms pulses (a). These spectra are integrated in Figure 2c; on the right, the x-axis is plotted as offset normalized relative to editing pulse duration (i.e. offset in ppm divided by pulse duration in ms multiplied by 35). This demonstrates that the curves for 14 ms and 20 ms pulses are mutually consistent, as expected because the underlying shape of the frequency response is determined by the amplitude modulation of the editing pulses and the bandwidth is scaled as the duration changes.
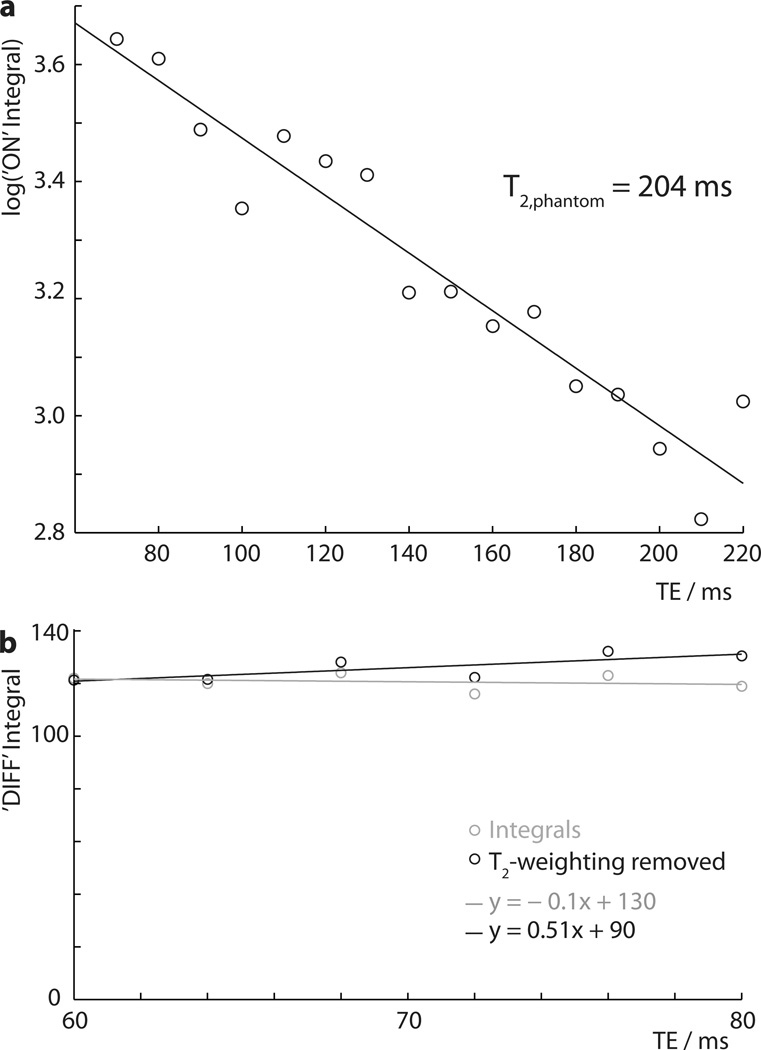
The T2 of GABA is required in order to separate T2 relaxation effects from underlying J-coupling modulation. The ‘ON’ spectra are acquired such that evolution of J-couplings during TE are refocused, and therefore, to a first approximation, the form of the acquired multiplet is not TE-dependent, and any change in intensity is driven solely by T2 relaxation (17). The phantom spectra recorded with TEs from 70 to 220 ms were integrated, and the log of these integrals plotted against TE in Figure 3a, showing a linear relationship corresponding to monoexponential decay with a time constant T2,phantom of 204 ms.
Figure 3.
Spectra recorded as a function of TE in a 10 mM GABA phantom: a) log integral plot of ‘ON’ spectra acquired at a range of TEs to determine the T2,phantom = 204 ms; b) integral plot of ‘DIFF’ (i.e. edited) spectra acquired at TE from 60 ms to 80 ms, showing signal integrals in gray (with T2 weighting) and with T2 relaxation removed in black.
The integrals of the DIFF spectra from the more finely sampled TE series (60 to 80 ms) are plotted in Figure 3b, both as raw integrals (in gray) and with the T2-weighting removed (in black). Linear models of best fit for each are also plotted. It can be seen that there is relatively little change in integral as TE increases from 60 to 80 ms, giving a small increase after accounting for T2 relaxation. The difference in slope of these two lines suggests that there is ~6% more signal at 68 ms.
Edited spectra with TE 80 ms were successfully acquired in all ten healthy subjects, and are plotted in Figure 4.
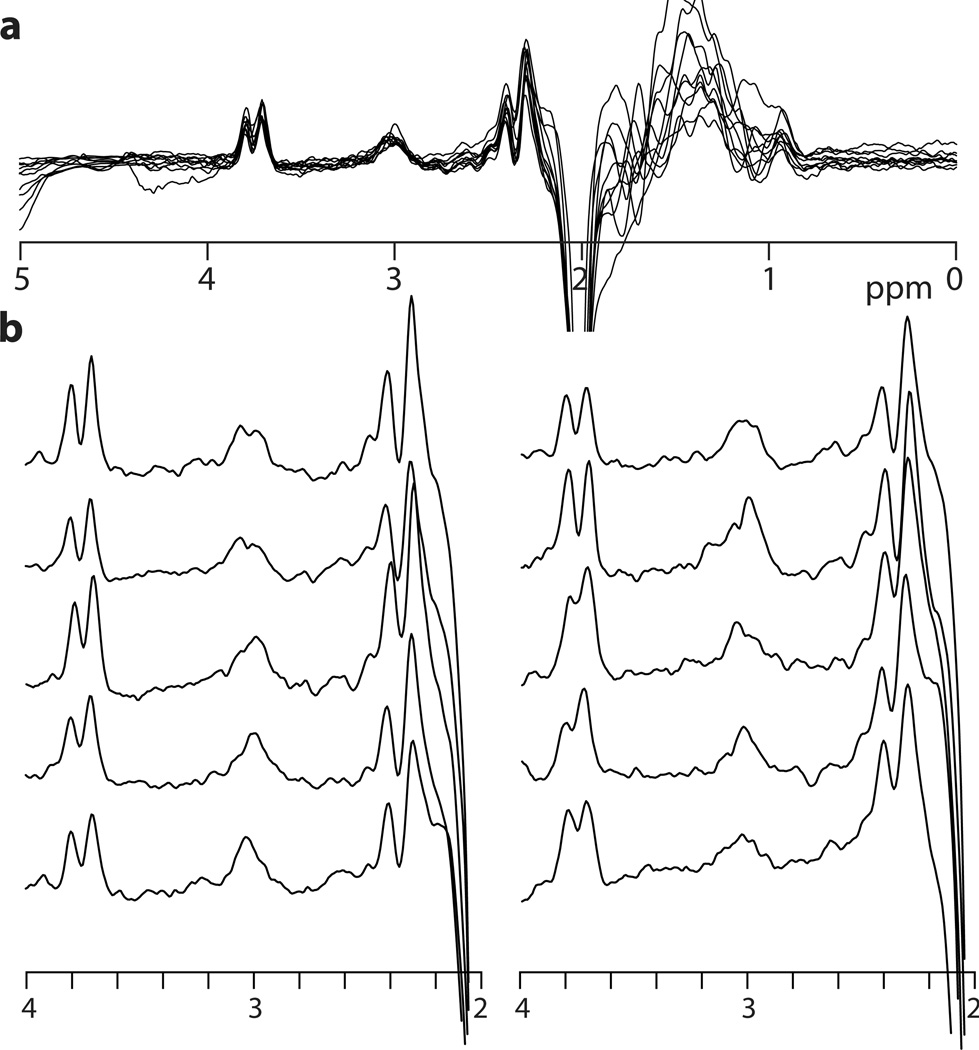
Figure 4.
In vivo GABA-edited spectra from the medial parietal lobe with TE = 80 ms and 20 ms editing pulses applied at 1.9 and 1.5 ppm ((3.5 cm)3, scan time = 8.5 min) from ten subjects a) overlaid, and b) plotted individually.
Discussion
The choice of 68 ms echo time in the original 1993 paper on GABA editing (11) is based upon the assumption that the 3-ppm GABA signal can be approximated as a triplet with coupling constant 7.3 Hz (i.e. TE = 1/2J). This is a useful simplification, but due to the magnetic non-equivalence of the GABA CH2 spins, the 3-ppm signal is more accurately characterized as two doublets of doublets at the same chemical shift with several different coupling constants as detailed in references(18,19). Thus, the echo time of maximum editing efficiency can only be determined either by careful phantom experiments, or by using density matrix simulations of the MEGA-PRESS experiment, incorporating full slice-selective pulses to account for regional differences in coupling evolution (20). Our own experiments suggest that there is a 6% improvement in editing efficiency at TE of 80 ms over 68 ms for the specific implementation of the sequence used here. Combining this with the effects of T2 relaxation in vivo (T2=88 ms (17)), the in vivo GABA signal would be expected to be reduced by 7.5% at 80 ms compared to 68 ms. Thus, this simple increase in TE allows MM to be suppressed in MEGA-PRESS experiments at 3T with minimal decrease in GABA SNR.
The extent of GABA signal loss that occurs when applying symmetric MM suppression depends strongly on the selectivity of editing pulses. This in turn depends on the length of the editing pulses, but also on their shape. Particularly, for Gaussian or sinc-Gaussian editing pulses, the FWHM duration of the amplitude waveform is more closely linked to pulse selectivity than the overall pulse duration. For the sinc-Gaussian pulse in the current implementation (shown in Figure 1c), the loss of edited GABA signal that would be expected with 14 ms editing pulses is 25%. Greater losses would be expected for less selective pulses of the same duration, such as pure Gaussian editing pulses. This is greater than the signal loss that occurs due to additional T2 relaxation at a TE of 80 ms. An additional benefit of more selective editing pulses is reduced B0-dependence of MM nulling. This arises because the editing efficiency function is the sum of a positive inversion lobe centered on 1.9 ppm and a negative lobe centered on 1.5 ppm with a zero crossing at 1.7 ppm as required to null MM. The gradient of this function at the 1.7 ppm MM null point is reduced for more selective editing pulses with the result that frequency instability results in less serious bleed-through of MM signal.
The in vivo spectra shown in Figure 4 are relatively good quality. Relative to GABA+ spectra commonly seen in the literature, the intensity of GABA signal is reduced relative to the co-edited Glx signal at 3.75 ppm. The subtraction artifacts arising from imperfect subtraction of creatine signals are relatively more prominent than seen in GABA+ spectra, since these artifacts are the same size but now superimposed on a smaller signal. However, all ten spectra clearly show edited GABA signal at 3 ppm.
In conclusion, a simple modification of the timing of the MEGA-PRESS sequence allows for the incorporation of symmetric suppression of co-edited MM signals in the edited detection of GABA at 3T. This involves a small increase in echo time (from 68 ms to 80 ms) allowing more selective editing pulses to be used. Although a small loss in GABA signal results (~7%), this development should greatly improve the specificity of future work using MRS of GABA to study the role of inhibition in both the healthy brain and in neurological or psychiatric diseases.
Acknowledgements
Grant Support: This project was supported by NIH grants P41 EB015909 and R21 NS077300. NAJP holds an Autism Speaks Translational Postdoctoral Fellowship.
References
- 1.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70(9):866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31(46):16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101(6):2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edden R, Crocetti D, Zhu H, Gilbert D, Mostofsky S. Reduced GABA Concentration in Attention-deficit Hyperactivity Disorder. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.2280. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64(2):579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112(1–3):192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Petroff OA, Rothman DL, Behar KL, Mattson RH. Low brain GABA level is associated with poor seizure control. Ann Neurol. 1996;40(6):908–911. doi: 10.1002/ana.410400613. [DOI] [PubMed] [Google Scholar]
- 9.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159(4):663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 10.Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog NMR Spect. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31(1):204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 15.Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR Biomed. 2011;24(10):1277–1285. doi: 10.1002/nbm.1688. [DOI] [PubMed] [Google Scholar]
- 16.Mlynarik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56(5):965–970. doi: 10.1002/mrm.21043. [DOI] [PubMed] [Google Scholar]
- 17.Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T(2) in vivo with J-difference editing: Application to GABA at 3 tesla. J Magn Reson Imaging. 2011;35(1):229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21(1):22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 20.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58(6):1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]