Abstract
The morphological characteristics of the pigeon lagena were examined using histology, scanning electron microscopy, and biotinylated dextran amine (BDA) neural tracers. The lagena epithelium was observed to lie partially in a parasagittal plane, but was also U-shaped with orthogonal (lateral) directed tips. Hair cell planar polarities were oriented away from a central reversal line that ran nearly the length of the epithelium. Similar to the vertebrate utricle and saccule, three afferent classes were observed based upon their terminal innervation pattern, which include calyx, dimorph, and bouton fibers. Calyx and dimorph afferents innervated the striola region of the lagena, while bouton afferents innervated the extrastriola and a small region of the central striola known as the type II band. Calyx units had large calyceal terminal structures that innervated only type I hair cells. Dimorph afferents innervated both type I and II hair cells, with calyx and bouton terminals. Bouton afferents had the largest most complex innervation patterns and the greatest terminal areas contacting many hair cells.
Keywords: Vestibular, motion detection, spatial orientation, otolith
Introduction
In all terrestrial vertebrates, the utricle and saccule vestibular otolith receptors are known to function as linear accelerometers (Blanks and Precht, 1976; Fernandez and Goldberg, 1976; Loe et al., 1973; Si et al., 1997). However, in many non-mammalian vertebrates and monotremes, a third otolith organ, the lagena, also exists (Retzius, 1884; Ladhams and Pickles, 1996; Rosenhall, 1970). Relatively little work has examined lagena function, since it was first described as a vestibular receptor responsive to linear motion (MacNaughtan and McNally, 1946). In frogs, lagena afferents respond to either head tilts relative to gravity, to vertical high frequency vibrations, or both (Baird and Lewis, 1986; Caston et al., 1977; Cortopassi and Lewis, 1996). In fish, all otolith organs appear to serve dual roles as both auditory and motion sensors (Fay and Edds-Walton, 1997), including the lagena (Fay and Olsho, 1979; Lu et al., 2003). In addition to motion detection, it has been suggested that the lagena also may function as a magnetic field detector due to the reported presence of ferromagnetic iron particles (Harada, 2001). Indeed, lagena ablation was shown to disrupt the homing ability of pigeons (Harada, 2002). More recently, c-Fos, a marker for neural activation, was used to demonstrate that lagena ablation eliminates responses to changes in magnetic field inclination in the central nervous system, suggesting that magnetoreception in homing pigeons is in large part mediated through the lagena receptor (Wu and Dickman, 2011).
What do we know about lagena morphology? In pigeons, the lagena is located at the distal end of the basilar papilla, with a hair cell population and neural innervation that are distant from the auditory receptor cells (Harada, 2002, Jørgensen, 1970; Platt et al., 2004). Morphometric examinations have shown that both type I and type II hair cells are present in the sensory epithelium of the avian lagena (Jørgensen 1970; Rosenhall 1970; Ricci et al., 1997). Type I hair cells and their exclusive calyx terminal nerve endings lie in the central lagena and define an area known as the striola (Werner, 1933). Coursing through the lagena striola is a distinct strip of Type II cells, known as the Type II band (Rosenhall, 1970). Both the avian utricle and lagena exhibit a type II band, while the saccule does not (Si et al., 2003, Warchol and Speck, 2007; Zakir et al, 2003). The morphological polarizations of avian lagenar hair cells are similar to those of the saccule, being generally directed away from a central reversal line (Rosenhall, 1970) that courses through the central lagena epithelium. More complex polarization patterns have been described in fish (Lu and Popper, 1998). Much less is known regarding lagena afferent innervation patterns. In frogs, afferent location in the epithelium was found to be correlated with differences in response sensitivity (Baird and Lewis, 1986). In fish, lagena afferent innervations were described as larger, with more complex arborizations, and more numerous terminals than those of the saccule (Edds-Walton and Popper, 2000). Detailed examination of the innervation patterns for lagena afferents in other species remains lacking.
Here, we examined the general morphology and afferent innervations patterns of the lagena in homing pigeons. The size of the receptor epithelium and morphological polarization of hair cells were measured throughout the endorgan and the afferent innervation patterns for calyx, dimorph and bouton fiber types were reconstructed and quantified. Our findings compliment earlier works on the utricular and saccular maculae and together represent a complete a body of work regarding the morphology of pigeon otolith receptor organs (Si et al., 2003; Zakir et al., 2003).
Methods
Animals
The experiments were conducted in adult homing pigeons (Columbia livia) that ranged in age from 1 to 3 years. All animal procedures were performed in compliance with the Guide for Care and Use of Laboratory Animals (National Institute of Health publication 86–23) and approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis.
Lagena afferent innervation
Afferent innervation patterns were examined in five lagena receptor epithelia using retrograde tracing of biotinylated dextran amine (BDA; 10,000 MW; Molecular Probes) injected into the vestibular nuclei. Each animal was anesthetized with Isoflurane (3% in O2) and placed in a 12° nose down position in a stereotaxic device (Kopf Instruments, model 1430), which approximately aligned the horizontal semicircular canals in the stereotaxic horizontal plane (Dickman and Fang, 1996). A small flap in the parietal bone was retracted, and a glass micropipette (1.0mm OD, 0.8 mm ID) filled with BDA (10% in saline) was lowered into the brain (0° angle) using stereotaxic coordinates for the vestibular nuclei (Dickman and Fang, 1996). BDA was injected with positive current (8 μA, 50% duty cycle, Midgard Current Source) for 20 min, then a small bucking negative current (−0.04 uA) was applied (to eliminate additional tracer release during removal) and the micropipette retracted. The injection site was purposely varied between 0.5 – 3.5 mm posterior to AP0 and 1 – 3.5 mm lateral to the midline among animals to provide a more complete sampling of lagenar afferents due to regional variations in their central projections (Si et al., 2003; Zakir and Dickman, 2006; Zakir et al., 2003). Gelfoam was then placed over the brain surface and the bone flap replaced. The skin was sutured, butorphanol (10 mg/kg im) was given for postoperative pain, and the animal was returned to its cage.
Histology
Following a post-injection survival period of 10–14 days (Si et al., 2003; Haque et al., 2006; Zakir and Dickman, 2006; Zakir et al., 2003), each pigeon was re-anesthetized, the mastoid bone was opened, and an intralabyrinthine perfusion was performed using a 2% glutaraldehyde, 1% paraformaldehyde, and 1% acrolein solution (Anna Lysakowski, personal communication). The bird was subsequently perfused transcardially with sodium nitrite (1%) in saline (250 ml), followed by 750 ml of 2% glutaraldehyde and 1.25% paraformaldehyde fixative in 0.1M phosphate buffer (PB). The head was then placed in the aldehyde fixative for 24 h at 4°C. The next day, the membranous labyrinth was excised and the lagenar maculae were dissected free. The otoconial membrane and otoconia were removed using a fluid jet. Next, the lagenar maculae were processed for BDA using a modified diaminobenzidine (DAB) procedure (Brandt and Apkarian 1992). Briefly, the tissue was incubated for 12 h in a solution of 0.1M PB, 1% Triton-X100, and 0.25% avidin d-HRP (vector A-2004). The tissue was then reacted using the chromogen DAB with 1% nickel ammonium sulphate-cobalt chloride solution and 0.3% H2O2 until a dense reaction product was visualized. Next, the tissue was thoroughly rinsed in phosphate buffer. The tissue was next dehydrated using a series of graded alcohols, cleared with xylenes, embedded in plastic (Durcupan), then serially sectioned (10 μm thickness) using a rotary microtome. The tissue sections were mounted on glass slides and counterstained (Richardson et al. 1960).
SEM Preparation
In four additional animals, the lagenar maculae were prepared for scanning electron microscopy (SEM). The tissue was rinsed in distilled water and then dehydrated using a series of graded acetone washes. Next, two final washes in 100% acetone were performed, followed by rinses in increasing concentrations of tetramethylsilane (TMS). The tissue was placed in 100% TMS for 15 min and allowed to desiccate at 60° C in an open container. The dried maculae were mounted on aluminum studs and coated in gold. The lagenae were scanned using a Hitachi SM200 scanning electron microscope (20KV).
Reconstruction of lagenar afferents
The lagenae and their afferent innervation patterns were examined using video microscopy (Nikon E600) and an image based reconstruction program (Neurolucida, MicroBrightfield Inc.). There was no correction for tissue shrinkage, which has generally been shown to be between 5 and 10% for aldehyde fixation and plastic embedding (Kushida 1962). Several parameters for each section of the lagenae were measured, including the macular width, location of the type II band, and location of the hair cell morphological polarization reversal line.
For reconstruction of labeled afferents, only fibers that were darkly stained and sufficiently isolated from other afferents were traced in an effort to reproduce the complete innervation pattern for each unit. Afferents with partial staining (ghost fills), or those that overlapped other afferents and could not be assuredly distinguished were not quantitatively analyzed. The regional location of each reconstructed afferent within the lagenar macula was made using relative distance to the epithelial borders and the reversal line. The three-dimensional reconstructions of identified afferents were performed using an x60 objective (dry lens, 0.95 NA). Measured morphological parameters for each of the reconstructed afferents included:
Fiber length. Summation of all branching segments.
Parent axon length. Length of the initial fiber segment, from epithelium penetration to the first branch point.
Fiber volume. Calculated by modeling each branch as a frustum (a circular cone that has been truncated) along the total fiber length using the standard equation for volume, , where R1 and R2are the radii at the two ends of the current axonal section of interest.
Parent axon volume. Calculated volume for the parent fiber only.
Axon diameter. Average diameter of the first five microns of fiber length before epithelial penetration.
Innervation area. After reconstruction, each tracing was rotated in 3D space such that an observer’s view was obtained orthogonal to the apical surface of the receptor epithelium. An area contour was then traced around the entire terminal field, including all calyx and bouton terminals, for each fiber such that the boundaries were that of a minimum convex polygon (Brichta and Peterson 1994).
Fiber branches. Total number of branch segments in the afferent tree.
Branch order. Number of branching levels past the initial fiber segment, which was assigned a branch order of 1.
Number of bouton terminals. Number of terminal or en passant bouton endings noted.
Number of type I hair cells. Each constricted neck region observed in a calyx terminal was counted and used as evidence for the presence of a type I hair cell.
Total number of terminals. Sum of the number of bouton terminals and the number of type I hair cells observed for each fiber.
Calyx volume. The calyceal volume was calculated as the product of the areas for all contour outlines (focal plane every 1μm) by total calyx depth (thickness).
Terminal density. Number of terminals (type I hair cell, bouton, or total number of terminals) per unit area.
Statistical analyses
Calyx, dimorph, and bouton afferents were divided into three groups. Statistical comparisons were performed using multifactor ANOVA (Statistica; Statsoft, Tulsa, OK), with follow-up comparisons made using the Scheffé test.
Results
General morphology of the Lagena
We first characterized the general anatomical structure of the receptor organ and the nerve location. As illustrated in Figure 1, the lagenar macula was located just beyond the apex of the basilar papilla. However, the lagenar epithelium was found to be U-shaped, with a flat central surface and shorter lateral turned edges. The central region comprises approximately 60% of the total epithelium and is principally aligned along a parasagittal vertical plane. The lateral projected edges (one anterior and one posterior) comprise the remaining 40% of the epithelial surface (20% on each side) and are approximately aligned halfway between the planes of the anterior and posterior semicircular canals (Dickman, 1996). This orientation places the lagena principal macula roughly orthogonal to the utricle.
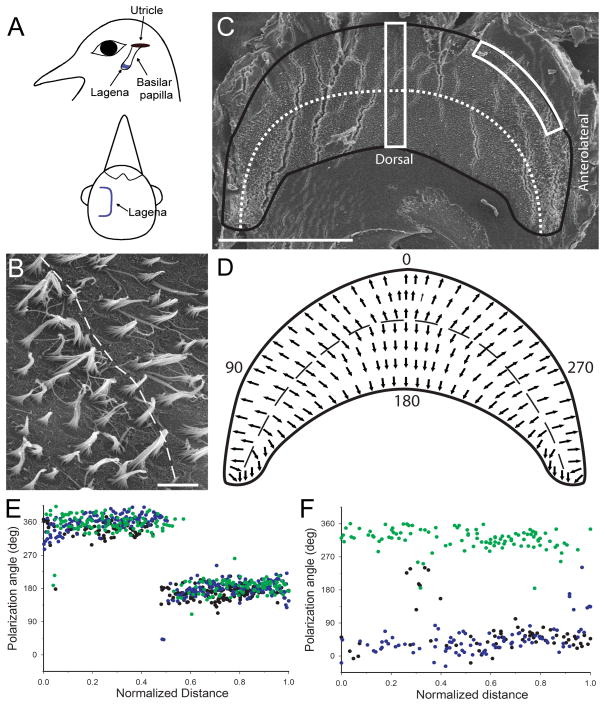
Figure 1.
Lagena orientation and receptor morphological polarization. A) Schematic illustration of lagena location and orientation in homing pigeons. The lagena lies vertical in the head at the apical end of the basilar papilla. The parasagittal central surface bends into two equal regions on either side that are directed laterally. B) Central lagenar hair cell stereocilia have opposite polarities directed away from the reversal line (dashed). C) Flattened left lagenar receptor surface (produced stress cracks in the epithelium) with border (black line) and reversal line (dotted) illustrated. Two counting frames (white boxes) were used for quantification of hair cell polarization. D) Schematic diagram of lagenar receptor epithelium showing morphological polarizations (arrows) and reversal line (dashed). Polarization directions were quantified, with the quadrant directions of 0° (ventral), 90° (postero-lateral), 180° (dorsal), and 270° (antero-lateral). E) Hair cell polarizations for all cells quantified in the central counting frame for 3 lagenas (green, black, blue dots) plotted as a function of normalized distance across the epithelium. F) Polarizations from the same 3 lagenas for all cells contained in the antero-lateral edge counting frame (green) or postero-lateral edge (black and blue). Scale bars: 500μm in A, C; 5μm in B.
Scanning electron microscopy (SEM) was used to determine the epithelial size and morphological polarizations (planar polarity) of hair cells across the lagenar surface. Seven lagenar maculae were measured across the central median, with an average length (anterior-posterior) of 1182 μm (SD±47) and width (dorsal-ventral) of 534 μm (SD±46). In the approximate center of the epithelium, a reversal line of morphological polarization was observed where hair cells exhibited planar polarities that were directed away from the reversal line (Fig. 1B). In order to accurately map the hair cell polarizations across the whole receptor epithelium, we flattened the surface into a unitary plane (Figure 1C). Two measures were then used. First, an average vector (visual observation only) for the polarization directions of a contiguous group of neighboring cells (15–20) was plotted throughout the epithelium, as shown in Figure 1D. The resultant vector map indicates that lagenar receptor cell polarizations follow a progressive orientation pattern along the circumference of the epithelium that spans 360° in directional space. In order to quantify hair cell planar polarity, adjacent SEM images were taken through two different 50μm wide counting frames; one that extended dorso-ventrally across the central epithelium and another that extended along the curved edge of either the anterior or posterior laterally directed edges (Fig. 1C). For each hair cell observed in the counting frames, a polarization vector was drawn from the shortest stereocilia to the lone kinocilium through the center of the stereocilia bundle (Huss et al., 2010; Rowe and Peterson, 2004). The vector direction for each cell was quantified, using a compass rosette laid across the epithelium with the form 0° = ventral pole, 90° = posterolateral, 180° = dorsal pole, 270° = anterolateral (Fig. 1D). Next, the polarity vectors for the measured cells were plotted as a function of normalized distance across the epithelium, as shown in Figures 1E, F. In the central lagena, hair cells in the ventral half (0 – 0.5 normalized distance) had polarity vectors directed toward the ventral lagenar pole (i.e., 0 or 360°). Opposite polarities were observed for cells in the dorsal half, across the reversal line (Fig. 1E). In the lateral edges of the lagena, hair cells exhibited polarities that followed a progressive pattern from ventral-to-lateral on the ventral half or dorsal-to-lateral on the dorsal half of the receptor epithelium (Fig. 1F). In the periphery, we observed a higher variance in hair cell polarizations than in the central lagena.
Afferent types and regional location
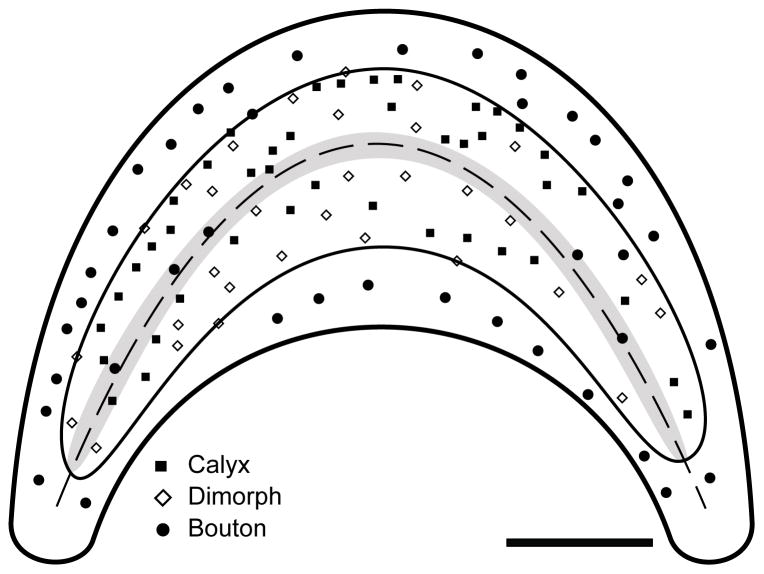
One hundred fourteen afferents from five lagenar organs were sufficiently labeled and isolated to perform anatomical reconstructions. Of these, there were 41 calyx, 33 dimorph, and 40 bouton type fibers, with examples of each shown in Figure 2. The three different afferent types were not distributed homogeneously throughout the epithelium, but were instead regionally localized. Afferents that contained calyx terminals were more centrally located in the lagenar macula. There were no calyx-bearing fibers observed in the peripheral macular edges. We used that distinction between the location of calyx-bearing and bouton type fibers to define the striola and extrastriola regions (Fig. 2A). The striola region comprised approximately 60% – 65% of the epithelial area. Within the striola, the calyx and dimorph afferent types were intermixed, with no clear zonal organization, as shown by the locations of all reconstructed fibers in Figure 3. Through the approximate center of the striola ran the reversal line (Fig. 2A) that was flanked on both sides by a narrow band of 6 – 8 type II hair cells (Fig. 3). Outside the striola and running along the peripheral circumference of the macula, lay the extrastriola, which consisted of the remaining ~35% of the receptor epithelium (Fig. 2A, 3).
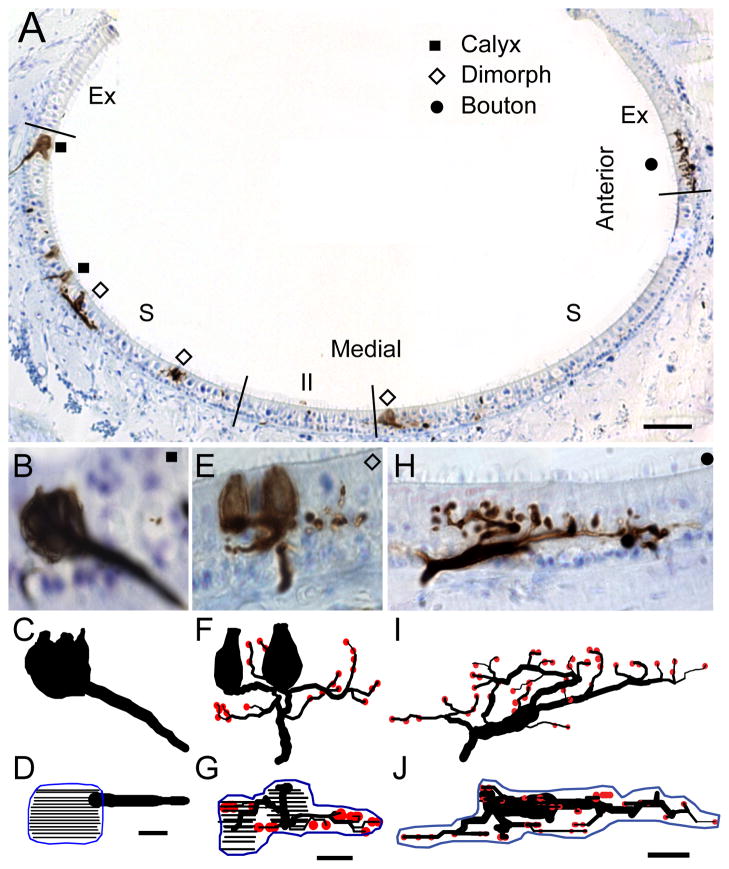
Figure 2.
Lagena receptor epithelium and afferent type. A) Cross section of the lagena along the dorsal-ventral axis of the epithelium, with striola (S), extrastriola (Ex), and type II band (II) regions indicated. Calyx (squares), dimorph (open diamonds), and bouton (circles) afferents filled with BDA neural tracer are shown. B – J) Photomicrographs and anatomical reconstructions of representative calyx (B–D), dimorph (E–G), and bouton (H–J) afferents. Below each photo image lies an anatomical reconstruction drawn in the same plane to scale (calyx terminals = closed contours; bouton terminals = red circles). The second reconstruction represents an apical view of the entire innervation pattern, with contours drawn for the calyx terminals at 1μm focal planes (black lines). Innervation area measurements were obtained from planimeter contours (blue) drawn around the terminal profile. Scale bar = 100μm in A; 5μm in B – J.
Figure 3.
Schematic illustration of lagenar apical surface with the locations of all 114 reconstructed afferents. The striola (thin black outline) was defined by the area containing calyx terminals. The extrastriola exclusively contained bouton afferents (circles). The reversal line (italics) ran through the central epithelium and was flanked by the Type II band (gray shade). Scale bar = 200μm.
Lagena afferents innervations
Figure 2 also shows photomicrographs and anatomical reconstructions of the terminal innervation patterns for representative calyx, dimorph, and bouton afferents. For each fiber, the photomicrograph shows a coronal section at a single plane of focus (Fig. 2B, E, H). Beneath the images are two views of the anatomical reconstruction for each fiber, drawn to scale. The transverse view reconstruction shows a similar plane to the photomicrograph section, however the entire fiber is illustrated (Fig.2C, F, I). The lower apical view shows a 90° rotation of the reconstruction, with an innervation area contour surrounding the terminal field (Fig 2D, G, J). For each afferent, the terminal innervation pattern could be characterized by two general classifications. First, the terminal pattern could be arranged in either a “flower” or a linear profile (Si et al., 2003; Zakir et al., 2003; Haque et al., 2006). Flower profiles consisted of terminals arranged in a concentric rounded or petal formation (Fig. 2B–D). Linear profiles consisted of terminals extended along a single polar trajectory (Fig. 2E–G, H–J). Second, each afferent innervation pattern could be characterized according to the complexity of the terminal profile. Fibers with simple innervation patterns had few branches off the parent axon, few terminals (calyceal or bouton), and innervated few hair cells. Fibers with complex innervation patterns exhibited large complicated arborization profiles that innervated many hair cells.
Calyx afferents
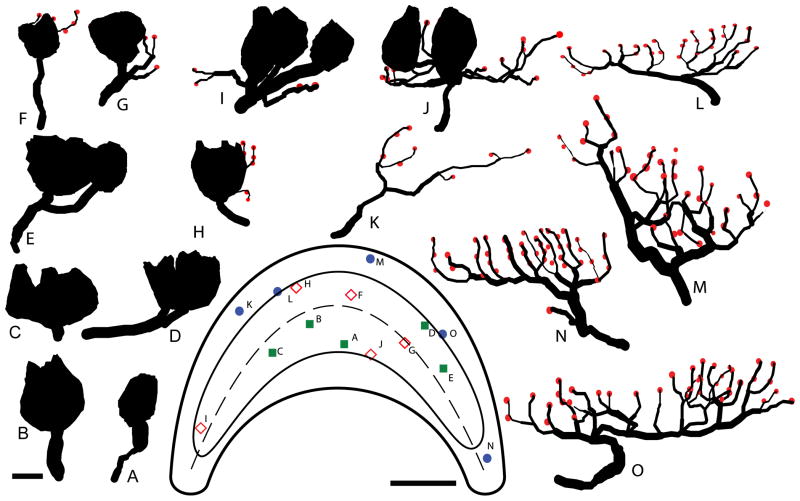
The calyx afferents were characterized by their large calyx terminal exclusively innervating type I hair cells. The calyx afferent shown in Fig. 2B had a single floral profile calyx terminal that contained three type I hair cells and covered an epithelial area of 243μm2. Figure 4 shows how the calyx units varied in complexity. Most calyx afferents (37/42) had a single large unbranched axon (Fig. 4A–C) with a mean diameter of 3.2μm for all cells (Table 1). Only a few (12%) of the units exhibited a second branch segment (Fig. 4D–E) after entering the epithelium with an additional calyx terminal. Calyx units ranged from the simplest having only two hair cells/calyx terminal (Fig. 4A) to the most complex containing 9 cells, with a mean value of 4.3 hair cells/calyx terminal (Table 1). The few branched calyx afferents also innervated larger numbers of hair cells, for example the unit shown in Fig. 4E had 5 type I cells in one terminal and 2 in the other. Most calyx afferents had floral shaped terminals (Fig. 4A, B, D, E), with linear profiles being less common (Fig. 4C).
Figure 4.
Innervation patterns of calyx, dimorph, and bouton afferents. Reconstructions of 5 calyx (A–E), 5 dimorph (F – J), and 5 bouton (K – 0) afferents are arranged in order of increasing complexity. Calyx terminals (closed contours) and bouton terminals (red circles) are shown, with all reconstructions drawn to scale. Each afferent’s corresponding location in the lagena (inset) is indicated on the illustrated apical surface. Scale bar = 5μm for the reconstructions; 200μm for the surface.
Table 1.
Terminal innervation parameters for calyx, bouton, and dimorph afferents
| Calyx (n=42) | Dimorph (n=34) | Bouton (n=43) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | |
| Axon diameter (μm) | 1 – 5.8 | 3.2 (0.8) | 1.7 – 5.3 | 2.9 (0.8) | 1.6 – 3.9 | 2.6 (0.6) |
| Fiber length (μm) | 5 – 56 | 24.0 (14.4) | 31 – 242 | 97 (49.3) | 115 – 474 | 260 (90.2) |
| Fiber volume (μm3) | 25 – 423 | 189 (118) | 52 – 657 | 273 (154) | 146 – 1054 | 401 (200) |
| Branch order | 1 – 2 | 1.1 (0.35) | 1 – 6 | 3.7 (1.2) | 3 – 9 | 5.9 (1.5) |
| Branches | 1 – 3 | 1.3 (0.8) | 2 – 25 | 9.4 (5.1) | 8 – 50 | 24.7 (9.8) |
| Innervation area (μm2) | 150 – 751 | 349 (138) | 155 – 895 | 452 (151) | 167 – 1078 | 561 (211) |
| Type I HC | 2 – 9 | 4.3 (1.8) | 1 – 7 | 3.2 (1.3) | ||
| Boutons | 1 – 23 | 6.7 (4.8) | 8 – 48 | 26.3 (10.5) | ||
| Terminals | 12 – 9 | 4.3 (1.8) | 1 – 26 | 9.8 (5.0) | 8 – 48 | 26.3 (10.5) |
| Terminal density (/μm2) | .005 – .022 | .013 (.004) | .007 – .044 | .022 (.010) | .024 – .104 | .049 (.019) |
SD – standard deviation.
Dimorph afferents
The dimorph afferent shown in Fig. 2E–G was located in the dorsal striolar region. After piercing the neuroepithelium, several branch segments were observed, two of which terminated in individual calyceal terminals, each with a single hair cell. This dimorph had 18 fiber branches, 23 bouton terminals, and coursed through the epithelium in a linear profile to innervate an area of 476μm2. Figure 4 shows the range in complexity for the lagenar dimorph innervations. The simplest dimorph afferents exhibited small calyx terminals that contained a single hair cell and 1–3 fiber branches with few (1–4) bouton terminals (Fig. 4F–G). The majority of the dimorph units observed (28/34) had a single calyx terminal with multiple type I hair cells (2–5), multiple branch segments (5 – 12), and a moderate number of bouton terminals (4 – 13), similar to the reconstructions shown in Fig. 4H–I. On average, the calyx terminals for dimorphs contained 3.2 (±1.3) type I hair cells (Table 1). Similar to the calyx afferents, there was no apparent topologic pattern for size of calyx terminal and epithelial location. Only 4 dimorphs were observed with 2 or 3 separate calyx terminals, similar to the unit of Fig. 4J, which innervated 4 type I hair cells in two calyceal terminals and had 12 bouton terminals.
Bouton afferents
Figure 3 shows that most of the bouton afferents were located in the periphery (37/43), except for those that innervated the type II band (6/43) in the striola (flanking the reversal line). Figure 2H–J shows a representative bouton afferent with a complex linear profile located in the lateral edges of the lagena. After piercing the neuroepithelium, the axon segment divided, with the major branch running parallel to the apical surface of the neuroepithelium for nearly 38μm from which numerous branches emerged. This fiber had 28 branches, a branch order of 8, and 42 bouton terminals. Across the epithelium, bouton afferents ranged from simple patterns with as few as 8 bouton terminals (Fig. 4K) to the more complex innervation patterns (Fig. 4N–O) that contained numerous branches and many bouton terminals. The majority of bouton afferents (32/43) exhibited a floral profile (Fig. 4M), with linear innervations being less numerous (Fig. 4N). In fact, most of the linear profile bouton afferents innervated the type II band (Fig. 4L).
Comparisons of afferent innervation
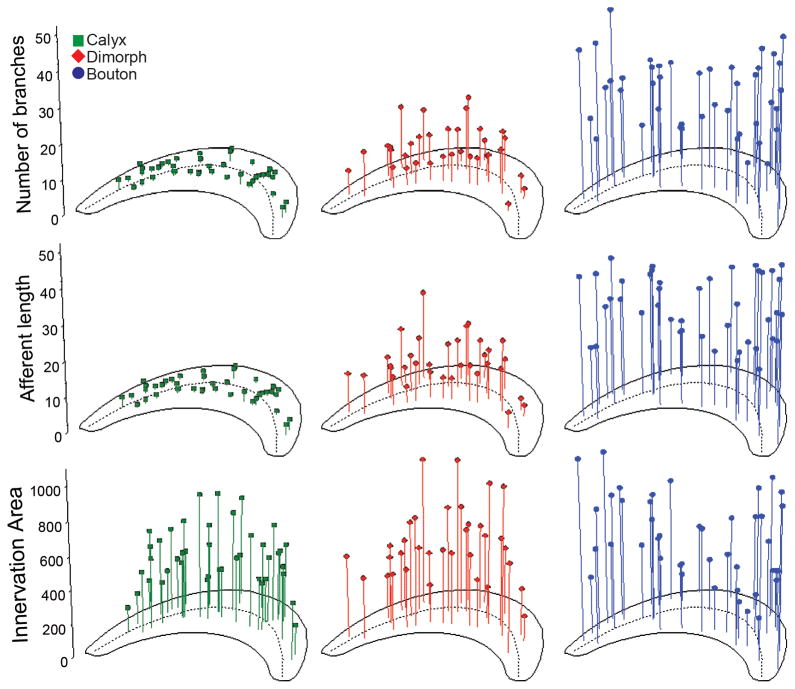
In order to quantitatively compare the differences in afferent terminal morphology, statistical comparisons were made between the three different afferent types for each of the morphometric parameters measured (Tables 1 & 2). As shown in Figure 6, the afferent innervation patterns varied systematically between the calyx, dimorph, and bouton afferents. Calyx bearing afferents had the largest axonal fiber diameters, but only the calyx units were significantly larger than bouton afferents (Table 2). Calyx units also exhibited the shortest fiber lengths with the smallest fiber volumes, the fewest number of branches with the lowest branch order, and the smallest innervation areas of all the fiber types (Table 1, Fig. 5). The calyx afferents had, on average, one more type I hair cell per calyceal terminal than did dimorph afferents, a significant finding (Table 1). Dimorph afferents were significantly longer with more ramified branches as compared to calyx afferents (Fig. 5), but were smaller and less complex on each of these measures as compared to bouton afferents (Table 2). Bouton afferents exhibited the longest fibers, with more branches and a higher branch order than any other fiber type (Fig. 5, Tables 1 & 2). Bouton afferents also had more bouton terminals per fiber than did dimorph afferents. To further delineate the size of the afferent terminal fields, innervation areas were measured. Figure 6 shows that similar to other measures of fiber pattern, there was a significant incremental increase in the innervation areas for calyx, dimorph, and bouton afferents (Tables 1 & 2). Dividing the innervation area by the total number of terminals produced innervation density measures. Again, the bouton afferents again exhibited the largest values (Table 2). In order to examine the distribution of innervation patterns across the lagenar surface, morphometric measure for all afferents were plotted as a function of epithelial location, as shown in Figure 6. Several general features emerged. As noted by the average value comparisons, calyx afferents exhibited the shortest lengths, the fewest number of branches, and the smallest innervation areas of all fibers. Second, dimorph afferents exhibited longer, more branched patterns that covered larger areas in the central lagena. Dimorphs located in the lateral edges of the lagena were smaller and less complex. The reverse was true for bouton afferents, where the longest and most branched fibers with the largest innervation areas were observed in the lateral wings of the lagena (Fig. 6).
Table 2.
Statistical comparisons for all afferents
| Comparison | Mean | Main Effect | Scheffé Post Hoc | ||||
|---|---|---|---|---|---|---|---|
| Calyx (n=42) | Dimorph (n=34) | Bouton (n=43) | Value | Cal vs Dim | Dim vs Bout | Cal vs Bout | |
| Axon diameter | 3.2 | 2.9 | 2.6 | F(2,116)=7.3 | NS | NS | p <0.001 |
| Fiber length | 24 | 97 | 260 | F (2,116)=167.0 | p<0.001 | p<0.001 | p <0.001 |
| Fiber volume | 189 | 273 | 401 | F (2,116)=17.6 | p<0.05 | p<0.01 | p<0.001 |
| Branch order | 1.1 | 3.7 | 5.9 | F (2,116)=197.5 | p <0.001 | p <0.001 | p <0.001 |
| Branches | 1.3 | 9.4 | 24.7 | F (2,116)=142.2 | p <0.001 | p <0.001 | p <0.001 |
| Innervation area | 349 | 452 | 561 | F (2,116)=16.4 | p<0.05 | p<0.05 | p <0.001 |
| Type I HC | 4.3 | 3.2 | F (1,74)=9.6 | p<0.01 | — | — | |
| Boutons | 6.4 | 26.3 | F (1,75)=108.4 | — | p <0.001 | — | |
| Terminals | 4.3 | 9.8 | 26.3 | F (2,116)=144.6 | p <0.001 | p <0.001 | p <0.001 |
| Terminal density | .013 | .022 | .049 | F(2,116)=97.9 | p<0.01 | p <0.001 | p <0.001 |
NS - not significant. Units of measurement are the same as shown in Table 1.
Figure 6.
Number of branches, afferent length and innervation area for fiber types. The locations of calyx (green squares; left panels), dimorph (red diamonds; middle panels) and bouton (blue circles; right panels) afferents are shown as a function of number of branches (tip), afferent fiber length (middle, μm), and innervation area (bottom, μm2), respectively. Dashed line represents reversal line.
Figure 5.
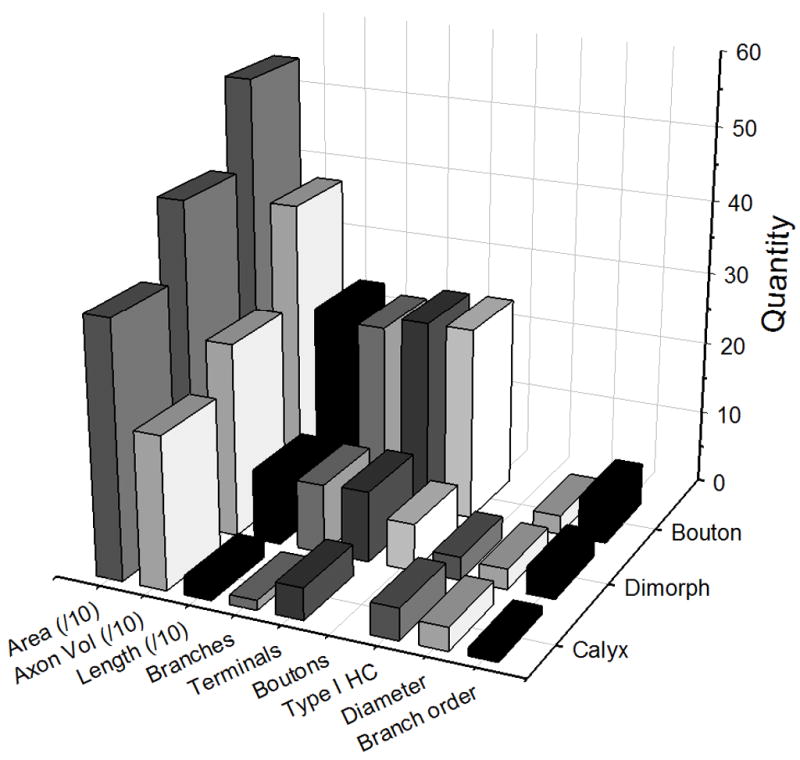
Comparison of afferent morphometric parameters by type. Area = innervation area (μm2, divided by a factor of 10); Axon Vol = total fiber volume (μm3, divided by a factor of 10); Length = total fiber length (μm, divided by a factor of 10); Branches, Terminals, Boutons, = total number of fiber branches, terminals, and boutons, respectively; Type I HC = number of type I hair cells per fiber; Diameter = average diameter (μm) of parent axon; Branch order = level of bifurcations.
Discussion
Here, we characterized the general morphometry and afferent innervation patterns of the pigeon lagena. Combined with our previous studies of the utricle (Si et al., 2003) and the saccule (Zakir et al., 2003), we have now quantified the organized structure of terminal innervations for all vestibular otolith organs in a single species. A number of thorough morphological investigations of the vestibular semicircular canals and their innervation patterns have been provided in several animal classes (Brichta and Peterson, 1994; Fernandez et al., 1988; Haque et al., 2006; Lysakowski et al., 1995). However, the otolith organs have received far less attention. Most previous works have concentrated upon the structure of terminal innervations in the utricle, as described for rodents (Desai et al., 2005; Fernández et al., 1990), amphibians (Baird and Lewis 1986, Baird and Schuff, 1994) and birds (Huss et al., 2010; Si et al., 2003). The saccule has only been examined for afferent innervations in fish (Lu and Popper, 2001) and pigeons (Zakir et al., 2003). Other than a few qualitative observations of lagena afferent innervations for fish (Edds-Walton and Popper, 2000) frogs (Baird and Lewis, 1986) and chickens (Manley et al., 1991), no other comprehensive lagena studies have been performed leaving comparative descriptions limited.
Morphology of lagenar macula
We observed that the lagena lies principally in a vertical head plane. The size of the lagena neuroepithelium, 1.2 mm length × 0.5 mm width, is on average smaller than the utricle (1.5 by 1.2 mm), but larger than the saccule (0.9 × 0.5 mm). When flattened, the lagenar neuroepithelium is sickle shaped, with a larger central region and narrow ends. The planar polarities of lagenar hair cells were directed away from the reversal line, similar to those observed in the saccule (Jørgensen, 1970; Rosenhall 1970; Zakir et al. 2003). The central lagenar region has hair cell morphological polarizations that should elicit responses to vertical head translations and roll tilts relative to gravity. Due to the U-shaped curvature of the lagena, the two laterally directed edges exhibit hair cell polarizations that gradually shift from cells responding to vertical translations to cells responding to lateral head translations. Thus, the lagenar epithelium has polarity orientations spanning parasagittal and horizontal planes. Together, the three otolith organs in pigeons would provide sensitivity to linear accelerations for all spatial directions, with partial redundancy from each.
Based upon the distribution of type I and type II receptor cells, the pigeon lagenar macula can be divided into two major organizational regions including the striola and extrastriola, similar to both the utricle and saccule in pigeons (Rosenhall, 1970; Si et al., 2003; Zakir et al., 2003). In fact, in birds type I hair cells appear to be exclusively located in the striola and using that morphological feature to define the region is consistent with previous studies. In contrast, mammalian otolith organs exhibit type I hair cells throughout the maculae, but the highest density is contained in the striola (Desai et al., 2005; Fernandez et al., 1990). In pigeons, we observed a fairly large striolar region in the lagena, more similar to the pigeon saccule; in contrast to the observations of Rosenhall (1970) who suggested that two narrow strips of type I hair cells define the lagenar striola region. However, our findings are consistent with Rosenhall (1970) in observing that a narrow region of type II hair cells, the type II band, runs through the central striola zone and encompasses the reversal line (Jørgensen, 1970; Rosenhall, 1970). There is no type II band observed in the avian saccular macula (Jørgensen and Andersen, 1973; Rosenhall, 1970; Zakir et al., 2003), nor in any maculae of mammals or turtles (Desai et al., 2005; Fernández et al., 1990; Moravec and Peterson, 2004) and the function of this special morphological feature remains unknown. For all avian maculae, only type II hair cells populate the extrastriola. Previously, we suggested that type I hair cells and calyceal terminals appear to be increasing through selective adaptation for terrestrial behavioral niches (Zakir et al., 2003). For example, type I hair cells are not present in fish or amphibians (Baird and Schuff, 1994; Chang et al., 1992), but first appear in reptiles and birds (Si et al., 2003; Moravec and Peterson, 2004; Zakir et al., 2003), then reach their highest levels in mammals (Desai et al., 2005; Fernandez et al., 1990). Within birds, the utricle, lagena, and saccule, exhibit increasing numbers of type I hair cells, respectively (Huss et al., 2010; Si et al., 2003; Zakir et al., 2003).
Afferent innervation patterns
Common to all amniote species studied to date, distinct calyx, dimorph, and bouton vestibular afferent innervation patterns were observed in the pigeon lagena (Brichta and Peterson, 1994; Fernandez et al., 1990; 1995; Lysakowski and Goldberg, 2008; Schessel et al. 1991; Si et al., 2003; Zakir et al., 2003). The different lagenar afferent types were distributed according to an organized regional topography. Calyx and dimorph afferents were restricted to the striola, while bouton afferents were exclusively located in either the type II band or the extrastriola, a pattern observed in all maculae of both birds (Si et al., 2003; Zakir et al., 2003) and turtles (Moravec and Peterson, 2004; Xue and Peterson, 2006). In rodents, the calyx afferents are restricted to the striola, however, dimorph afferents are observed throughout the macula (Desai et al., 2005; Fernandez et al., 1990; Li et al., 2008, Leonard and Kevetter, 2002). Ninety percent of the calyx units had a single calyceal terminal; all containing multiple hair cells. Thirty-eight percent contained 2 – 3 type I hair cells, 40% had 4 – 5 hair cells, and the remaining 22% innervated between 6 – 9 hair cells. Calyx afferents had a larger quantity of type I hair cells per fiber, as compared to dimorphs. Birds appear to be unique in having extremely large calyx terminals containing many type I hair cells, both in the semicircular canals and in the otolith maculae (Haque et al., 2006; Huss et al., 2010; Si et al. 2003; Zakir et al. 2003). However, both the range and the mean number of hair cells/fiber for lagenar calyx afferents were lower than those in pigeon utricular (range 2 – 15, mean of 7.1) and saccular (range 2 – 18, mean of 6.4) afferents. In contrast, calyx structures in mammals and reptiles rarely contained more than a few type I hair cells (Brichta and Peterson 1994; Brichta and Goldberg 2000; Desai et al., 2005; Fernandez et al. 1988; 1995; Schessel et al. 1991). How the number of hair cells contained in a single calyceal terminal relates to afferent physiological response sensitivity remains unknown. Of the three afferent types, calyx afferents had the shortest axonal lengths, the smallest axonal volumes, the least branch orders, fewest total number of branches, and smallest innervation areas.
Lagenar dimorph afferents exhibited more complex innervation patterns than calyx fibers, with an increased number of longer branches that innervated a larger epithelial area. Simple dimorph afferents were similar in structure to those reported for chinchillas (Fernandez et al., 1990) and contained only one calyx with a single hair cell and a few bouton terminals. More complex dimorph afferents had several calyx terminals that innervated multiple hair cells. Still, on average, the dimorph afferents innervated fewer type I hair cells than did calyx afferents, similar to the patterns observed for other bird otolith organs. Pigeon lagenar dimorph afferents had an average of 7 bouton terminals, smaller than either the mean utricular (17) or saccular (13) fibers, and less than those observed in the chinchilla (Fernandez et al., 1990).
Lagena bouton afferents were densely concentrated in the extrastriola. The bouton afferents had the smallest diameter axons, yet exhibited the most complex arborization profiles and the largest innervation areas of all three fiber types, a characteristic common to all avian otolith receptors. Lagenar bouton afferents had the highest terminal density, with nearly four times the number of boutons on average than dimorph fibers. However, lagenar bouton fibers had fewer bouton terminals, than did pigeon utricular or saccular bouton afferents, or even quail utricular fibers (Huss et al., 2010; Si et al., 2003; Zakir et al., 2003). By comparison, pigeon lagenar bouton afferents had an average of 26 bouton terminals, less than that of 60 for the pigeon utricle, 35 for the chinchilla utricle, and 150 for frog utricular fibers (Baird and Schuff, 1994; Fernandez et al., 1990; Si et al., 2003). These results suggest that pigeon lagenar bouton afferents have smaller innervation densities than either chinchilla, quail, or frog otolith afferents.
Although the morphological structure has been described for the otolith receptors in pigeons, little is known regarding their differences in function. In fact, there remains a paucity of data relating differences in afferent physiological responsiveness to the striking differences in fiber innervation patterns. We previously reported that utricular afferents exhibit high sensitivities to linear acceleration with advanced phase values relative to linear acceleration, more than any species examined to date (Si et al., 1997). No work in birds has provided correlative evidence of afferent innervation structure and physiological responsiveness for the vestibular receptor organs. However, in chinchillas, calyx afferents in the central macula were shown to have the most irregular discharge rates, the highest sensitivities, and the most advanced phase values to linear acceleration as compared to extrastriola innervating afferents (Goldberg et al., 1990). In addition to serving as linear accelerometers, recent evidence suggests that the lagena may also function as a magnetic sense detector in birds (Wu and Dickman, 2011). If the lagena does function in a dual role of accelerometer and magnetoreceptor, it has yet to be determined if lagenar afferents respond to both modalities or maintain separate functional qualities.
Highlights.
The lagena lies in a parasagittal plane with lateral directed edges.
Lagena hair cell planar polarities were oriented away from a central reversal line.
Calyx and dimorph afferents innervated the striola region of the lagena.
Bouton afferents innervated the extrastriola.
Calyx, dimorph, and bouton fibers had increasing complexities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Baird RA, Lewis ER. Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena. Brain Res. 1986;369:48–64. doi: 10.1016/0006-8993(86)90512-3. [DOI] [PubMed] [Google Scholar]
- Baird RA, Schuff NR. Peripheral innervation patterns of vestibular nerve afferents in the bullfrog utriculus. J Comp Neurol. 1994;342:279–298. doi: 10.1002/cne.903420210. [DOI] [PubMed] [Google Scholar]
- Blanks RHI, Precht W. Functional characterization of primary vestibular afferents in the frog. Exp Brain Res. 1976;25:369–390. doi: 10.1007/BF00241728. [DOI] [PubMed] [Google Scholar]
- Brandt HM, Apkarian AV. Biotin-dextran: a sensitive anterograde tracer for neuroanatomic studies in rat and monkey. J Neurosci Methods. 1992;45:35–40. doi: 10.1016/0165-0270(92)90041-b. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Morphological identification of physiologically characterized afferents innervating the turtle posterior crista. J Neurophysiol. 2000;83:1124–1242. doi: 10.1152/jn.2000.83.3.1202. [DOI] [PubMed] [Google Scholar]
- Britcha AM, Peterson EH. Functional architecture of vestibular primary afferents from the posterior semicircular canal of a turtle, Pseudemys Trachemyus scripta elegans. J Comp Neurol. 1994;344:481–492. doi: 10.1002/cne.903440402. [DOI] [PubMed] [Google Scholar]
- Caston J, Precht W, Blanks RHI. Response characteristics of frog’s lagena afferents to natural stimulation. J Comp Physiol. 1977;118:273–289. [Google Scholar]
- Chang JSY, Popper AN, Saidel WM. Heterogeneity of sensory hair cells in a fish ear. J Comp Neurol. 1992;324:621–640. doi: 10.1002/cne.903240413. [DOI] [PubMed] [Google Scholar]
- Cortopassi KA, Lewis ER. High-frequency tuning properties of bullfrog lagenar vestibular afferent fibers. J Vestib Res. 1996;6:105–119. [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophys. 2005;93:251–266. doi: 10.1152/jn.00746.2003. [DOI] [PubMed] [Google Scholar]
- Dickman JD. Spatial orientation of semicircular canals and afferent sensitivity vectors in pigeons. Exp Brain Res. 1996;111:8–20. doi: 10.1007/BF00229550. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Fang Q. Differential central projections of vestibular afferents in pigeons. J Comp Neurol. 1996;367:110–131. doi: 10.1002/(SICI)1096-9861(19960325)367:1<110::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Huss D, Lowe M. Morphometry of otoconia in the utricle and saccule of developing Japanese quail. Hear Res. 2004;188:89–103. doi: 10.1016/S0378-5955(03)00377-0. [DOI] [PubMed] [Google Scholar]
- Edds-Walton PL, Popper AN. Dendritic arbors on the saccule and lagena in the ear of the goldfish, Carassius auratus. Hear Res. 2000;141:229–242. doi: 10.1016/s0378-5955(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Fay RR, Edds-Walton PL. Directional response properties of saccular afferents of the toadfish (Opsanus tau) Hear Res. 1997;111:1–21. doi: 10.1016/s0378-5955(97)00083-x. [DOI] [PubMed] [Google Scholar]
- Fay RR, Olsho LW. Discharge patterns of lagenar and saccular neurons of the goldfish eighth nerve: displacement sensitivity and directional characteristics. Comp Biochem Physiol. 1979;62:377–386. [Google Scholar]
- Fernandez C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervations patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–181. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervations patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Fernández C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervations patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol. 1995;73:1253–1269. doi: 10.1152/jn.1995.73.3.1253. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:791–804. doi: 10.1152/jn.1990.63.4.791. [DOI] [PubMed] [Google Scholar]
- Haque A, Huss D, Dickman JD. Afferent innervations patterns of the pigeon’s horizontal crista ampullaris. J Neurophysiol. 2006;96:3293–3304. doi: 10.1152/jn.00930.2005. [DOI] [PubMed] [Google Scholar]
- Harada Y, Taniguchi M, Namatame H, Iida A. Magnetic materials in otoliths of bird and fish lagena and their function. Acta Otolaryngol. 2001;121:590–595. [PubMed] [Google Scholar]
- Harada Y. Experimental analysis of behavior of homing pigeons as a result of functional disorders of their lagena. Acta Otolaryngol. 2002;122:132–137. doi: 10.1080/00016480252814126. [DOI] [PubMed] [Google Scholar]
- Huss D, Navaluri R, Faulkner K, Dickman JD. Development of otolith receptors in Japanese quail. Dev Neurobiol. 2010;70:436–455. doi: 10.1002/dneu.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM. On the structure of the macula lagenae in birds with some notes on the avian maculae utriculi and sacculi. Vidensk Meddr dansk naturh Foren. 1970;133:121–147. [Google Scholar]
- Jorgensen JM, Andersen T. On the structure of the avian maculae. Acta Zoologica. 1973;54:121–130. [Google Scholar]
- Kushida H. A study of cellular swelling and shrinkage during fixation, dehydration and embedding in various standard media. J Electron Microscopy. 1962;11:135–138. [Google Scholar]
- Ladhams A, Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol. 1996;366:335–347. doi: 10.1002/(SICI)1096-9861(19960304)366:2<335::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Leonard RB, Kevetter GA. Molecular probes of the vestibular nerve I. Peripheral termination patterns of calretinin, calbindin and peripherin containing fibers. Brain Res. 2002;928:8–17. doi: 10.1016/s0006-8993(01)03268-1. [DOI] [PubMed] [Google Scholar]
- Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol. 2008;99:718–733. doi: 10.1152/jn.00831.2007. [DOI] [PubMed] [Google Scholar]
- Li CX, Gong M, Huang YN, Tang ZQ, Chen L. Morphometry of otoliths in chicken macula lagena. Neurosci Lett. 2006;404:83–86. doi: 10.1016/j.neulet.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Loe PR, Tomko DL, Werner G. The neural signal of angular head position in primary afferent vestibular nerve axons. J Physiol (Lond) 1973;219:29–50. doi: 10.1113/jphysiol.1973.sp010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Popper AN. Morphological polarizations of sensory hair cells in the three otolithic organs of a teleost fish: fluorescent imaging of ciliary bundles. Hear Res. 1998;126:47–57. doi: 10.1016/s0378-5955(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Lu Z, Popper N. Neural response directory correlates of hair cell orientation in a teleost fish. Journal of Comparative Physiology A. 2001;187:453–465. doi: 10.1007/s003590100218. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Acoustic response properties of lagenar nerve fibers in the sleeper goby, Dormitator latifrons. J Comp Physiol. 2003;189:889–905. doi: 10.1007/s00359-003-0462-7. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus) J Comp Neurol. 2008;511:47–64. doi: 10.1002/cne.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernandez C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol. 1995;73:1270–1281. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- MacNaughtan IPJ, McNally WJ. Some experiments which indicate that the frog’s lagena has an equilibrial function. J Laryngol Otol. 1946;61:204–214. doi: 10.1017/s0022215100007842. [DOI] [PubMed] [Google Scholar]
- Manley GA, Haeseler C, Brix J. Innervation patterns and spontaneous activity of afferent fibres to the lagenar macula and apical basilar papilla of the chick’s cochlea. Hear Res. 1991;6:211–226. doi: 10.1016/0378-5955(91)90172-6. [DOI] [PubMed] [Google Scholar]
- Moravec WJ, Peterson EH. Differences between stereocilia numbers on type I and type II vestibular hair cells. J Neurophysiol. 2004;92:3153–3160. doi: 10.1152/jn.00428.2004. [DOI] [PubMed] [Google Scholar]
- Platt C, Jørgensen JM, Popper AM. The inner ear of the lungfish Protopterus. J Comp Neurol. 2004;471:277–288. doi: 10.1002/cne.20038. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehororgan der Wirbelthiere. Vol. 2. Stockholm: Samson and Wallin; 1884. Das gehororgan der reptilian, der vogel, und der saugethiere; pp. 1–368. [Google Scholar]
- Richardson KC, Jarett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Biotechnic & Histochemistry. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Rennie KJ, Cochran SL, Kevetter GA, Correia MJ. Vestibular type I and type II hair cells: I. Morphological identification in pigeon and gerbil. J Vestib Res. 1997;7:393–406. [PubMed] [Google Scholar]
- Rosenhall U. Some morphological principles of the vestibular maculae in birds. Arch Klin Exp Ohr. 1970;197:154–182. doi: 10.1007/BF00306164. [DOI] [PubMed] [Google Scholar]
- Rowe MH, Peterson EH. Quantitative analysis of stereociliary arrays on vestibular hair cells. Hearing Res. 2004;190:10–24. doi: 10.1016/S0378-5955(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Schessel DA, Ginzberg R, Highstein SM. Morphophysiology of synthetic transmission between type I hair cells and vestibular primary afferents. An intracellular study employing horseradish peroxidase in the lizardCalotes versicolor. Brain Res. 1991;544:1–16. doi: 10.1016/0006-8993(91)90879-z. [DOI] [PubMed] [Google Scholar]
- Si X, Angelaki DE, Dickman JD. Response dynamics and orientation vectors of otolith afferents in pigeons. Exp Brain Res. 1997;117:242–250. doi: 10.1007/s002210050219. [DOI] [PubMed] [Google Scholar]
- Si X, Zakir M, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol. 2003;89:1660–1677. doi: 10.1152/jn.00690.2002. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Speck JD. Expression of GATA3 and Tenascin in the avian vestibular maculae: Normative patterns and changes during sensory regeneration. J Comp Neurol. 2007;500:646–657. doi: 10.1002/cne.21153. [DOI] [PubMed] [Google Scholar]
- Werner CF. The differentiation of the maculae in the labyrinth, in particular in mammals. Z Anat Entwicklungesch. 1933;99:696–709. [Google Scholar]
- Wu LQ, Dickman JD. Magnetoreception in an avian brain in part mediated by inner ear lagena. Current Biology. 2011;21:418–423. doi: 10.1016/j.cub.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Peterson EH. Hair bundle heights in the utricle: differences between macular location and hair cell types. J Neurophysiol. 2006;95:171–186. doi: 10.1152/jn.00800.2005. [DOI] [PubMed] [Google Scholar]
- Zakir M, Dickman JD. Regeneration of vestibular otolith afferents following ototoxic damage. J Neuroscience. 2006;26:2881–2893. doi: 10.1523/JNEUROSCI.3903-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakir M, Huss D, Dickman JD. Afferent innervation patterns of the saccule in pigeons. J Neurophys. 2003;89:534–550. doi: 10.1152/jn.00817.2001. [DOI] [PubMed] [Google Scholar]