Abstract
Background
Although maternal perinatal mental illnesses commonly present to and are primarily treated in general practice, few population-based estimates of this burden exist, and the most affected socioeconomic groups of pregnant women remain unclear.
Aim
To provide estimates of maternal depression, anxiety and serious mental illness (SMI) in UK general practice and quantify impacts of socioeconomic deprivation.
Design and setting
Cross-sectional analysis of prospectively recorded general practice records from a UK-wide database.
Method
A pregnancy ending in live birth was randomly selected for every woman of childbearing age, 1994–2009. Prevalence and diagnostic overlap of mental illnesses were calculated using a combination of medical diagnoses and psychotropic drug prescriptions. Socioeconomic deprivation was assessed using multivariate logistic regression, adjusting for calendar period and pregnancy history.
Results
Among 116 457 women, 5.1% presented with antenatal depression and 13.3% with postnatal depression. Equivalent figures for anxiety were 2.6% and 3.7% and for SMI 1/1000 and 2/1000 women. Socioeconomic deprivation increased the risk of all mental illnesses, although this was more marked in older women. Those age 35–45 years in the most deprived group had 2.63 times the odds of antenatal depression (95% confidence interval [CI] = 2.22 to 3.13) compared with the least deprived; in women aged 15–25 years the increased odds associated with deprivation was more modest (odds ratio = 1.35, 95% CI = 1.07 to 1.70). Similar patterns were found for anxiety and SMI.
Conclusion
Strong socioeconomic inequalities in perinatal mental illness persist with increasing maternal age. Targeting detection and effective interventions to high-risk women may reduce inequity and avoid substantial psychiatric morbidity.
Keywords: general practice, mental disorder, mothers, socioeconomic status
INTRODUCTION
Perinatal mental illness, especially depression, is a leading cause of maternal morbidity and mortality in high-income countries1,2 and has unfavourable impacts on short-term and long-term physical and mental health of offspring.3–5 Guidance from the UK’s National Institute for Health and Clinical Excellence (NICE) has emphasised that perinatal mental illness is one of the most important issues in women’s health6 yet up-to-date estimates of these conditions identified or treated at primary care level are not available. Although a recent systematic review7 reported 6.5–12.9% of women had depression during pregnancy and in the year postpartum, there are few estimates of maternal anxiety and serious mental illness (SMI; for example, bipolar disorder and psychosis) and hardly any estimates of concurrent diagnoses of different mental illnesses perinatally.
In addition, the latest UK national mental illness strategy8 highlighted the importance of reducing health inequalities as a key objective to promote mental wellbeing in the population, yet there remains a need for government to narrow the gap between policies and actions to effectively identify and treat those who are most vulnerable. While an association between mental illness and greater socioeconomic deprivation in the general population has been well documented,9 fewer studies have assessed this in the perinatal period and results are inconsistent.10–13 Furthermore, evidence that impacts of material deprivation may vary with age14 has not been assessed in pregnant women.
As most mental illness is detected and treated in general practice, and it provides the gateway to secondary care, a large population-based study was conducted to obtain current estimates of perinatal depression, anxiety, and SMI identified in UK general practice. Variation in illness was quantified by socioeconomic status and the extent of comorbidity or diagnostic overlap.
METHOD
Dataset and study population
Data from The Health Improvement Network (THIN) database of anonymised electronic patient records covering 446 general practices throughout the UK were used. THIN has a high standard of validated medical diagnoses, events, symptoms, and drug prescriptions.15 The study population comprised women aged 15–45 years with at least one recorded pregnancy ending in live birth between 1994 and 2009. To ensure no clustering effects, one pregnancy for each woman was randomly selected.
Measuring mental illness in and around pregnancy
In UK general practice it is common for patients to be prescribed psychotropic drugs without direct diagnostic indication for the prescription, likely reflecting the diagnostic pathway (for example, prescriptions of psychotropic drugs as part of diagnostic procedure) and routine clinical practice (for example, a doctor with knowledge of their patient’s clinical history will not need to record a new diagnosis of depression with each prescription for effective clinical care). Furthermore, individuals may receive more than one type of diagnosis, concurrently or at different times during their life. Therefore a comprehensive approach to define and distinguish between different types of mental illness in women’s records was adopted by using a combination of medical diagnoses and psychotropic drug prescriptions. The prevalence of clinically-diagnosed perinatal mental illness with or without treatment during the 9 months before pregnancy, during pregnancy (antenatal period) and during the 9 months after pregnancy (postnatal period) was estimated. Periods of 9-months were used as they were similar in length to the average pregnancy, minimising potential effects of different period lengths on prevalence estimates. Definitions of how clinically-recognised depression, anxiety, and SMI (bipolar disorder, schizophrenia, other psychotic disorders) were defined in and around pregnancy are in Box 1.
Box 1. Detailed definitions of mental illness within periods in and around pregnancy
Depression
Women were identified as having clinically-recognised depression in each period if they had records of depression and/or antidepressant prescriptions. Because antidepressants are prescribed for other mental illnesses (for example, anxiety), women who had been prescribed antidepressants but had no diagnosis of depression in their medical records were not included.
Anxiety
Women were identified as having clinically-recognised anxiety if they had records of anxiety and/or anxiolytic prescriptions during each period, excluding women who were prescribed anxiolytics with no diagnosis of anxiety throughout their medical record. Secondly, since anxiety is commonly treated using antidepressants, women with antidepressant prescriptions during the period and a diagnosis of anxiety at any time but without records of depression were identified.
Serious mental illness
Although serious mental illnesses are considered clinically to have life-long impact and NICE guidelines indicate the importance of knowing a woman’s history of psychotic illness,6 this study focused on evidence of currently recognised illness. Women were considered to have clinically-recognised bipolar disorder during each period if they had medical records of the illness and/or prescriptions of lithium or mood stabilisers during that time. Since mood stabilisers are also used for other conditions (for example, epilepsy), women prescribed mood stabilisers but with no diagnoses of bipolar disorder in their medical records were excluded. Women with schizophrenia and other psychotic disorders were identified in the same way and, as these are rare conditions, they were grouped together as ‘serious mental illness’.
How this fits in
Maternal perinatal mental illness has been highlighted as one of the most important issues in women’s health worldwide and there is considerable burden across the severity spectrum of maternal mental illnesses in UK general practice for women of all ages. Greater recognition that women in the most socioeconomically-deprived groups are at high risk is required at policy level; this should emphasise the need for trials of methods to effectively identify women and interventions to prevent and treat perinatal mental illness in high risk women in the primary care setting.
Statistical analyses
The prevalence of each mental illness was calculated as a proportion of all women during the periods before, during and after pregnancy. To provide estimates of overlapping illness, proportions of women with two or more different concurrent diagnoses were calculated. To estimate newly-diagnosed mental illness before, during and after pregnancy, data were restricted to women whose first mental illness recording fell in each 9-month period.
Logistic regression in Stata SE (version 11.1) was used to calculate odds ratios (ORs) for the association of each mental illness with socioeconomic deprivation, measured using household-level Townsend Index of Deprivation. To maintain anonymity in THIN, patients’ home postcodes are assigned a quintile of Townsend Index before data leave the general practice. As quintiles are based on census data distribution, they are representative of women’s relative socioeconomic position at national level. Given the effect of socioeconomic deprivation on mental illness could vary substantially across age,14 effect modification was assessed using the likelihood ratio test and presented prevalence estimates were used to show absolute risks of clinically-recognised mental illness in each quintile, stratified by maternal age (categorised as 15–24, 25–34, 35–45 years) alongside ORs adjusted for calendar period and women’s previously recorded live births. This was reassessed for women’s first clinically diagnosed mental illnesses during and after pregnancy.
RESULTS
A total of 116 457 women with at least one pregnancy ending in a live birth were identified. Median age at the end of pregnancy was 31 years (interquartile range 26–35 years) and the numbers (proportions) of pregnant women aged 15–24, 25–35, and 35–44 years were 21 341 (18.3%), 64 214 (55.1%) and 30 902 (26.5%) respectively. Of all women, 23.2% (26 984) were from the least socioeconomically-deprived group whereas 14.2% (16 524) were from the most socioeconomically-deprived group (5.3% (6172) had no socioeconomic group recorded).
Table 1 shows the clinical presentation of all mental illnesses in and around pregnancy. Compared with maternal depression in the period before pregnancy (9.3%), prevalence was lower during pregnancy (5.1%) and higher postpartum (13.3%). For anxiety and SMI, prevalence before pregnancy was similar to after, although clinical recording for both was lower during pregnancy (2.6% and 0.09% respectively). Compared with all clinical presentations, first presentations were less common but had similar prevalence patterns over the three periods, such that they were still lowest in the antenatal period (Table 1). Most women with SMI also had clinically-recognised depression or anxiety, yet most diagnostic overlap was between common mental illnesses with nearly 20% of women with antenatal depression also having anxiety (Figure 1).
Table 1.
Prevalence of maternal depression, anxiety and serious mental illnessa presenting to UK general practice in and around pregnancy (n = 116 457)
| Depression | Anxiety | SMIa | |
|---|---|---|---|
| n (%; 95% CI) | n (%; 95% CI) | n (%; 95% CI) | |
| Any presentation or treatment | |||
| During the 9 months before pregnancy | 10 802 (9.3; 9.1 to 9.4) | 4823 (4.1; 4.0 to 4.3) | 143 (0.12; 0.11 to 0.14) |
| During pregnancy | 5926 (5.1; 5.0 to 5.2) | 3084 (2.6; 2.6 to 2.7) | 110 (0.09; 0.08 to 0.11) |
| During the 9 months after pregnancy | 15 454 (13.3; 13.1 to 13.5) | 4325 (3.7; 3.6 to 3.8) | 176 (0.15; 0.13 to 0.18) |
| First presentation or treatmentb | |||
| During the 9 months before pregnancy | 3482 (3.0; 2.9 to 3.1) | 2253 (1.9; 1.9 to 2.0) | 30 (0.03; 0.02 to 0.04) |
| During pregnancy | 975 (0.8; 0.8 to 0.9) | 1196 (1.0; 1.0 to 1.1) | 14 (0.01; 0.01 to 0.02) |
| During the 9 months after pregnancy | 5814 (5.0; 4.9 to 5.1) | 1621 (1.4; 1.3 to 1.5) | 56 (0.05; 0.04 to 0.06) |
SMI includes bipolar disorder, schizophrenia or other related psychotic disorders.
Prevalence estimates are for presentation or treatment only when it first appeared in a woman’s record during the respective 9-month period, excluding any women with a history of the relevant mental illness. SMI = serious mental illness.
Figure 1.
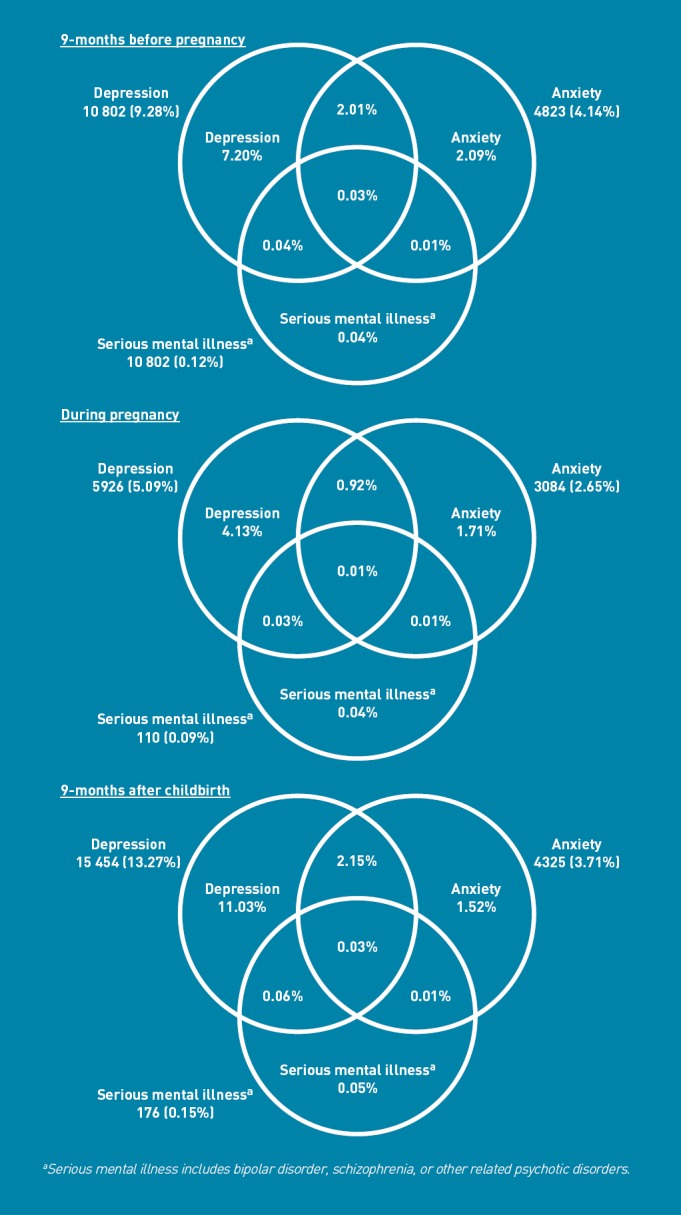
Venn diagrams of prevalence of maternal perinatal mental illnesses and overlap shown as proportions of all pregnant women (n = 116 457).
Tables 2–4 show absolute risks and adjusted ORs of clinically-recognised depression, anxiety, and SMI with socioeconomic deprivation, stratified by maternal age. The prevalence of maternal depression and anxiety was highest in the youngest women and lowest in the oldest women, although this pattern varied considerably by socioeconomic group. For women of all ages, the prevalence of all three mental illnesses during and after pregnancy increased with greater socioeconomic deprivation (Tables 2–4). After adjusting for calendar time and number of previously recorded live births, the odds of perinatal mental illness increased with each deprivation quintile, compared to women in the least socioeconomically-deprived quintile. In the youngest age group, several 95% confidence intervals (CIs) included unity, however, tests for trend with increasing socioeconomic deprivation were P<0.001 for depression (Table 2), P = 0.06 for anxiety (Table 3), and P = 0.30 and 0.13 for SMI during and after pregnancy (Table 4), which affected very few women. In older women, the degree of increase in odds of all three clinical mental illnesses with greater socioeconomic deprivation was more marked. For example, women aged 35–45 years from the most socioeconomically-deprived quintile had 2.6 times the odds of antenatal depression (OR = 2.63, 95% CI = 2.22 to 3.13), 2.5 times the odds of anxiety (OR = 2.48, 95% CI = 1.98 to 3.11) and 7.7 times the odds of SMI (OR = 7.68, 95% CI = 2.92 to 20.24) as those from the least socioeconomically-deprived quintile whereas in women aged 15–24 years, the equivalent ORs were 1.35 (95% CI = 1.07 to 1.70), 1.49 (95% CI = 1.06 to 2.09) and 1.59 (95% CI = 0.19 to 13.64) respectively (Tables 2–4). Similar patterns of risks were found postnatally. The P-values from the likelihood ratio tests for interaction between socioeconomic deprivation quintile and maternal age group were <0.001 for antenatal depression, postnatal depression and antenatal anxiety, and 0.09 for postnatal anxiety; however there was weak statistical evidence for such interaction in SMI (0.79 antenatal and 0.39 postnatal) with fairly small numbers of women in each age group.
Table 2.
Absolute risks and adjusted odds ratios for maternal depression associated with socioeconomic status, stratified by maternal age (n = 110 285a)
| Maternal age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–24 years | 25–34 years | 35–45 years | ||||||||||
| Socioeconomic status (deprivation quintile) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) |
| During pregnancye | 20 176 | 1223 | 6.1 | 60 722 | 2952 | 4.9 | 29 387 | 1416 | 4.8 | |||
| 1 (least deprivation) (n = 26 984) | 1843 | 94 | 5.1 | 1.00 | 15 732 | 492 | 3.1 | 1.00 | 9409 | 325 | 3.5 | 1.00 |
| 2 (n = 21 978) | 2479 | 109 | 4.4 | 0.85 (0.64 to 1.13) | 12 655 | 482 | 3.8 | 1.22 (1.07 to 1.38) | 6844 | 278 | 4.1 | 1.18 (1.00 to 1.39) |
| 3 (n = 23 028) | 4052 | 240 | 5.9 | 1.15 (0.90 to 1.47) | 13 069 | 661 | 5.1 | 1.61 (1.43 to 1.82) | 5907 | 287 | 4.9 | 1.41 (1.20 to 1.66) |
| 4 (n = 21 771) | 5838 | 364 | 6.2 | 1.21 (0.95 to 1.52) | 11 452 | 707 | 6.2 | 1.93 (1.72 to 2.17) | 4481 | 276 | 6.2 | 1.77 (1.50 to 2.09) |
| 5 (most deprivation) (n = 16 524) | 5964 | 416 | 7.0 | 1.35 (1.07 to 1.70) | 7814 | 610 | 7.8 | 2.39 (2.11 to 2.70) | 2746 | 250 | 9.1 | 2.63 (2.22 to 3.13) |
| P-value for trend | <0.001f | <0.001f | <0.001f | |||||||||
| During the 9 months after pregnancyc | 20 176 | 3550 | 17.6 | 60 722 | 7840 | 12.9 | 29 387 | 3235 | 11.0 | |||
| 1 (least deprivation) (n = 26 984) | 1843 | 236 | 12.8 | 1.00 | 15 732 | 1576 | 10.0 | 1.00 | 9409 | 836 | 8.9 | 1.00 |
| 2 (n = 21 978) | 2479 | 368 | 14.8 | 1.19 (1.00 to 1.41) | 12 655 | 1401 | 11.1 | 1.12 (1.04 to 1.21) | 6844 | 681 | 10.0 | 1.14 (1.02 to 1.27) |
| 3 (n = 23 028) | 4052 | 708 | 17.5 | 1.44 (1.23 to 1.69) | 13 069 | 1708 | 13.1 | 1.35 (1.25 to 1.45) | 5907 | 640 | 10.8 | 1.25 (1.12 to 1.39) |
| 4 (n = 1771) | 5838 | 1100 | 18.8 | 1.56 (1.34 to 1.82) | 11 452 | 1766 | 15.4 | 1.62 (1.50 to 1.74) | 4481 | 579 | 12.9 | 1.51 (1.35 to 1.69) |
| 5 (most deprivation) (n = 6524) | 5964 | 1138 | 19.1 | 1.58 (1.36 to 1.84) | 7814 | 1389 | 17.8 | 1.88 (1.74 to 2.03) | 2746 | 499 | 18.2 | 2.25 (1.99 to 2.53) |
| P-value for trend | <0.001f | <0.001f | <0.001f | |||||||||
5.3% (6,172) of the original population (116 457) had no socioeconomic status recorded.
Number of women in each deprivation quintile.
Number of women with depression.
Odds ratio and 95% CI adjusted for calendar time and number of previous known live births.
Likelihood ratio test for interaction between deprivation quintile and age group: during pregnancy P<0.001; after pregnancy P<0.001.
P-value for trend from least to greatest deprivation excluding women with missing information for socioeconomic status. AOR = adjusted odds ratio
Table 4.
Absolute risks and adjusted odds ratios for serious mental illness associated with socioeconomic status, stratified by maternal age (N = 110 285a)
| Maternal age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–24 years | 25–34 years | 35–45 years | ||||||||||
| Socioeconomic status (deprivation quintile) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) |
| During pregnancye | 20 176 | 13 | 0.06 | 60 722 | 48 | 0.08 | 29 387 | 44 | 0.15 | |||
| 1 (least deprivation) (N = 26 984) | 1843 | 1 | 0.05 | 1 | 15 732 | 7 | 0.04 | 1 | 9409 | 6 | 0.06 | 1 |
| 2 (N = 21 978) | 2479 | 0 | --- | --- | 12 655 | 7 | 0.06 | 1.24 (0.44 to 3.54) | 6844 | 5 | 0.07 | 1.14 (0.35 to 3.73) |
| 3 (N = 23 028) | 4052 | 3 | 0.07 | 1.37 (0.14 to 13.22) | 13 069 | 8 | 0.06 | 1.39 (0.50 to 3.82) | 5907 | 8 | 0.14 | 2.12 (0.73 to 6.11) |
| 4 (N = 21 771) | 5838 | 4 | 0.07 | 1.29 (0.14 to 11.53) | 11 452 | 14 | 0.12 | 2.84 (1.14 to 7.04) | 4481 | 12 | 0.27 | 4.28 (1.60 to 11.41) |
| 5 (most deprivation) (N = 16 524) | 5964 | 5 | 0.08 | 1.59 (0.19 to 13.64) | 7814 | 12 | 0.15 | 3.70 (1.45 to 9.42) | 2746 | 13 | 0.47 | 7.68 (2.92 to 20.24) |
| P-value for trend | 0.30f | 0.001f | <0.001f | |||||||||
| During the 9 months after pregnancye | 20 176 | 25 | 0.12 | 60 722 | 81 | 0.13 | 29 387 | 64 | 0.22 | |||
| 1 (least deprivation) (N = 26 984) | 1843 | 1 | 0.05 | 1 | 15 732 | 15 | 0.1 | 1 | 9409 | 13 | 0.14 | 1 |
| 2 (N = 21 978) | 2479 | 1 | 0.04 | 0.75 (0.05 to 11.98) | 12 655 | 17 | 0.13 | 1.42 (0.71 to 2.84) | 6844 | 9 | 0.13 | 0.95 (0.41 to 2.23) |
| 3 (N = 23 028) | 4052 | 3 | 0.07 | 1.38 (0.14 to 13.28) | 13 069 | 13 | 0.1 | 1.06 (0.50 to 2.22) | 5907 | 11 | 0.19 | 1.34 (0.60 to 3.00) |
| 4 (N = 21 771) | 5838 | 12 | 0.21 | 3.85 (0.50 to 29.66) | 11 452 | 25 | 0.22 | 2.35 (1.24 to 4.47) | 4481 | 15 | 0.33 | 2.42 (1.15 to 5.11) |
| 5 (most deprivation) (N = 16 524) | 5964 | 8 | 0.13 | 2.52 (0.31 to 20.16) | 7814 | 11 | 0.14 | 1.54 (0.70 to 3.36) | 2746 | 16 | 0.58 | 4.20 (2.01 to 8.78) |
| P-value for trend | 0.13f | 0.06f | <0.001f | |||||||||
Note: Serious mental illness includes bipolar disorder, schizophrenia or other related psychotic disorders.
5.3% (6172) of the original population (116 457) had no socioeconomic status recorded.
Number of women in each deprivation quintile.
Number of women with SMI.
Odds ratio and 95% CI adjusted for calendar time and number of previous known live births. SMI = serious mental illness.
Likelihood ratio test for interaction between deprivation quintile and age group: during pregnancy P = 0.79; after pregnancy P = 0.39.
P-value for trend from least to most deprivation excluding women with missing information for socioeconomic status. AOR = adjusted odds ratio.
Table 3.
Absolute risks and adjusted odds ratios for maternal anxiety associated with socioeconomic status, stratified by maternal age (N = 110 285a)
| Maternal age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–24 years | 25–34 years | 35–45 years | ||||||||||
| Socioeconomic status (deprivation quintile) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) | Nb | nc | % | AORd (95% CI) |
| During pregnancye | 20 176 | 559 | 2.8 | 60 722 | 1593 | 2.6 | 29 387 | 767 | 2.6 | |||
| 1 (least deprivation) (N = 26 984) | 1843 | 41 | 2.2 | 1.00 | 15 732 | 302 | 1.9 | 1.00 | 9409 | 189 | 2.0 | 1.00 |
| 2 (N = 21 978) | 2479 | 70 | 2.8 | 1.27 (0.86 to 1.88) | 12 655 | 293 | 2.3 | 1.21 (1.03 to 1.42) | 6844 | 135 | 2.0 | 0.98 (0.78 to 1.23) |
| 3 (N = 23 028) | 4052 | 116 | 2.9 | 1.29 (0.90 to 1.85) | 13 069 | 348 | 2.7 | 1.39 (1.19 to 1.62) | 5907 | 155 | 2.6 | 1.31 (1.06 to 1.62) |
| 4 (N = 1 771) | 5838 | 135 | 2.3 | 1.03 (0.72 to 1.47) | 11 452 | 345 | 3.0 | 1.56 (1.33 to 1.83) | 4481 | 153 | 3.4 | 1.71 (1.38 to 2.12) |
| 5 (most deprivation) (N = 16 524) | 5964 | 197 | 3.3 | 1.49 (1.06 to 2.09) | 7814 | 305 | 3.9 | 2.02 (1.72 to 2.38) | 2746 | 135 | 4.9 | 2.48 (1.98 to 3.11) |
| P-value for trend | 0.06f | <0.001f | <0.001f | |||||||||
| During the 9 months after pregnancye | 20 176 | 804 | 4.0 | 60 722 | 2330 | 3.8 | 29 387 | 974 | 3.3 | |||
| 1 (least deprivation) (N = 26 984) | 1843 | 65 | 3.5 | 1.00 | 15 732 | 477 | 3.0 | 1.00 | 9409 | 258 | 2.7 | 1.00 |
| 2 (N = 21 978) | 2479 | 82 | 3.3 | 0.93 (0.67 to 1.30) | 12 655 | 425 | 3.4 | 1.11 (0.97 to 1.27) | 6844 | 200 | 2.9 | 1.07 (0.88 to 1.29) |
| 3 (N = 23 028) | 4052 | 165 | 4.1 | 1.16 (0.86 to 1.55) | 13 069 | 502 | 3.8 | 1.27 (1.12 to 1.44) | 5907 | 184 | 3.1 | 1.14 (0.94 to 1.38) |
| 4 (N = 21 771) | 5838 | 230 | 3.9 | 1.11 (0.84 to 1.47) | 11 452 | 521 | 4.6 | 1.50 (1.32 to 1.70) | 4481 | 179 | 4.0 | 1.46 (1.20 to 1.77) |
| 5 (most deprivation) (N = 16 524) | 5964 | 262 | 4.4 | 1.24 (0.94 to 1.63) | 7814 | 405 | 5.2 | 1.69 (1.47 to 1.94) | 2746 | 153 | 5.6 | 2.06 (1.68 to 2.53) |
| P-value for trend | 0.06f | <0.001f | <0.001f | |||||||||
5.3% (6172) of the original population (116 457) had no socioeconomic status recorded.
Number of women in each deprivation quintile.
Number of women with anxiety.
Odds ratio and 95% CI adjusted for calendar time and number of previous known live births.
Likelihood ratio test for interaction between deprivation quintile and age group: during pregnancy P<0.001; after pregnancy P = 0.09.
P-value for trend from least to greatest deprivation excluding women with missing information for socioeconomic status. AOR = adjusted odds ratio.
After restricting to women’s first clinical recording of mental illness, prevalence estimates of all three mental illnesses presenting initially during or after pregnancy were substantially reduced across all age and socioeconomic group. The impact of socioeconomic deprivation also reduced, yet patterns of increasing ORs with greater deprivation generally persisted. The degree of increase with deprivation quintile was again greatest in women aged 35–45 years for antenatal depression, antenatal and postnatal anxiety, but not for postnatal depression which was instead greatest in women aged 15–24 years. Since very few women had a first clinical recording of SMI during or after pregnancy, it was not possible to stratify by age. Although adjusted ORs showed an association with deprivation, 95% CIs were extremely wide (data on first clinical recording are available on request from the authors).
DISCUSSION
Summary
A substantial burden of depression, anxiety, and SMI during the perinatal period presents and is managed in UK general practice among women with pregnancies ending in live births, although there are important socioeconomic inequalities in absolute risk. Higher risks of mental illness in mothers in more socioeconomically-deprived areas compared with those in less deprived areas persist with increasing maternal age. When women’s initial clinical presentation of mental illness was during or after pregnancy, the impact of socioeconomic deprivation remained yet reduced, indicating that this was partially due to a history of mental illness commonly recurring in the perinatal period.
Strengths and limitations
To the authors’ best knowledge, this is the largest study to examine the clinical prevalence and overlap of maternal depression, anxiety and SMI presenting to general practice in and around pregnancy. It is also the first to assess the joint effect of maternal age and socioeconomic status on the clinical burden of maternal perinatal mental illness in the UK. This study’s considerable sample size means that these findings are unlikely to be due to chance. Equally as data were obtained from a large general practice database with prospective recording, recall bias of mental illness was excluded.
This study’s definition of maternal mental illness relies on women presenting to and being correctly identified by practitioners. Such estimates quantify the primary care burden in pregnancy, thus excluding undiagnosed illness in women not disclosing feelings to their doctor or health visitor.16 A range of diagnostic tools (for example, the Edinburgh Postnatal Depression Scale) has been used in cohort studies of selected populations, yet there is no universal agreement that these are advantageous in a routine clinical setting (or for screening) and as such there are no widely applied cut-offs used in practice to diagnose mental illness.17,18 Case identification was based on diagnostic and prescribing records to reflect routine practice, similar to methods used by the Office for National Statistics and other published studies of mental illness in general practice databases.19–22 Since GPs occupy a gate-keeper role to health care, and are normally the first point of contact for non-emergency services, referred via midwives and health visitors, it is thought that these data are an ideal source for estimating the prevalence of mental illness presenting to general practice nationally. The similarity of this study’s prevalence estimates to studies restricting to diagnosed postnatal depression using standardised interviewing schedules23,24 and anxiety25 is reassuring. The decreased prevalence observed during pregnancy may reflect NICE guidelines to reduce psychotropic drug treatment during pregnancy which has been observed elsewhere,26 greater midwifery care antepartum, or diagnostic bias if GPs remain more likely to diagnose and treat mental illness postpartum.
Comparison with existing literature
Although numerous studies have estimated the prevalence of maternal depression, fewer have assessed anxiety and SMI and this study is the first to compare prospectively all clinically-recognised mental illnesses (depression, anxiety and SMI) and their concurrence before, during and after pregnancy in UK general practice. A systematic review of high-income countries found the prevalence of maternal postnatal depression was 13%,23 however individual study estimates varied widely. A systematic review of maternal anxiety concluded that there were too few studies to obtain adequate estimates around pregnancy; in three small studies generalised anxiety disorder ranged from 4.4–8.2% postpartum.25 Most previous studies assessed mental illness by study-specific screening with self-administered questionnaires,27 which means they are not directly comparable with this study.
Previous studies on SMI primarily rely on medical admissions and this study’s similar estimates indicate diagnoses are reasonably captured among women registered in general practice. Nager and colleagues12 examined Swedish first-time mothers (n = 502 767) and found about 0.07% had a first hospital admission for psychosis postpartum, very similar to this study’s estimate of first recorded SMI. This study’s estimates also concur with a 1987 Edinburgh registry study28 in which a higher proportion of psychiatric admissions presented after childbirth than during pregnancy. In Denmark, Munk-Olsen and colleagues29 examined over 1 million first-time mothers and found that compared with 6–11 months postpartum, medical contact for any mental disorder was more likely during the first month postpartum (relative risk = 3.49), and less likely during pregnancy (relative risk = 0.72), which is consistent with this study’s findings.
Sociodemographic factors have important impacts on maternal mental illness yet the joint effects of maternal age and socioeconomic deprivation among pregnant women and new mothers as predictors of important health burden in the population have not been adequately assessed. A recent US study30 of more than 75 000 non-pregnant women aged 18–44 years found that the prevalence of major depression was greater in women >35 years, unmarried, less educated, unable to work or unemployed or with low income than in women without such risk factors. Previous studies10,11,23,31 show that women with greater socioeconomic deprivation are more likely to have perinatal mental illness than those with less socioeconomic deprivation after adjusting for other sociodemographic factors.
Rich-Edwards and colleagues10 interviewed 1662 women from Boston in the US and found an increased odds of postnatal depression in those with financial hardship (OR = 3.6, 95% CI = 1.9 to 6.7 after adjusting for maternal age, race/ethnicity, immigration status, parity and income). As in this study’s UK population many women had a history of depression, which was by far the strongest risk factor for perinatal depression; however the effect of financial hardship remained when they excluded women with a history of depression. Women aged <23 years had more maternal depression during and after pregnancy than women aged 30–34 years (OR = 2.7, 95% CI = 1.4 to 5.2 mid-pregnancy and OR = 2.4, 95% CI = 1.1 to 5.4, 6 months postpartum after adjusting for race/ethnicity); however effects reduced and were not statistically significant after adjusting for household income, suggesting that effect of maternal age was largely driven by different financial circumstances.
Implications for practice and research
The considerable primary care burden of maternal perinatal mental illness found in this study highlights that women in more socioeconomically-deprived circumstances are at high risk, which needs greater recognition at policy level. As there is currently not enough evidence that perinatal screening tools are advantageous over clinical assessment in routine practice,17,18 this should emphasise the need for trials of methods to effectively identify women and interventions to prevent and treat perinatal mental illness among high-risk women in the primary care setting.
Funding
Joe West is supported by a University of Nottingham/National Institute for Health Research (NIHR) Senior Clinical Research Fellowship.
Ethical approval
Ethical approval for this study was obtained from the UK Medical Research Ethics Committee (reference 04/MRE01/9)
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Oates M. Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br Med Bull. 2003;67:219–229. doi: 10.1093/bmb/ldg011. [DOI] [PubMed] [Google Scholar]
- 2.Jablensky AV, Morgan V, Zubrick SR, et al. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry. 2005;162(1):79–91. doi: 10.1176/appi.ajp.162.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Howard LM, Goss C, Leese M, Thornicroft G. Medical outcome of pregnancy in women with psychotic disorders and their infants in the first year after birth. Br J Psychiatry. 2003;182(1):63–67. doi: 10.1192/bjp.182.1.63. [DOI] [PubMed] [Google Scholar]
- 4.Servili C, Medhin G, Hanlon C, et al. Maternal common mental disorders and infant development in Ethiopia: the P-MaMiE Birth Cohort. BMC Public Health. 2010;10(1):693. doi: 10.1186/1471-2458-10-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ban L, Gibson J, West J, Tata L. Association between perinatal depression in mothers and the risk of childhood infections in offspring: a population-based cohort study. BMC Public Health. 2010;10(1):799. doi: 10.1186/1471-2458-10-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. Antenatal and postnatal mental health. CG 45. 2007. http://guidance.nice.org.uk/CG45 (accessed 30 Aug 2012)
- 7.Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 8.HM Government. No health without mental health: a cross-government mental health outcomes strategy for people of all ages. London: DoH; 2011. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123766 (accessed 30 Aug 2012) [Google Scholar]
- 9.Lorant V, Deliège D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 10.Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60(3):221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobfoll SE, Ritter C, Lavin J, et al. Depression prevalence and incidence among inner-city pregnant and postpartum women. J Consult Clin Psychology. 1995;63(3):445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- 12.Nager A, Johansson L-M, Sundquist K. Are sociodemographic factors and year of delivery associated with hospital admission for postpartum psychosis? A study of 500 000 first-time mothers. Acta Psychiat Scand. 2005;112(1):47–53. doi: 10.1111/j.1600-0447.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 13.Valdimarsdóttir U, Hultman CM, Harlow B, et al. Psychotic illness in first-time mothers with no previous psychiatric hospitalizations: a population-based study. PLoS Med. 2009;6(2):e13. doi: 10.1371/journal.pmed.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreitman N, Carstairs V, Duffy J. Association of age and social class with suicide among men in Great Britain. J Epidemiol Community Health. 1991;45(3):195–202. doi: 10.1136/jech.45.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 16.Chew-Graham CA, Sharp D, Chamberlain E, et al. Disclosure of symptoms of postnatal depression, the perspectives of health professionals and women: a qualitative study. BMC Fam Pract. 2009;10:7. doi: 10.1186/1471-2296-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oates M. Postnatal depression and screening: too broad a sweep? Br J Gen Pract. 2003;53(493):596–597. [PMC free article] [PubMed] [Google Scholar]
- 18.Leverton TJ, Elliott SA. Is the EPDS a magic wand?: 1. A comparison of the Edinburgh Postnatal Depression Scale and health visitor report as predictors of diagnosis on the Present State Examination. J Reprod Infant Psychol. 2000;18(4):279–296. [Google Scholar]
- 19.Rait G, Walters K, Griffin M, et al. Recent trends in the incidence of recorded depression in primary care. Br J Psychiatry. 2009;195(6):520–524. doi: 10.1192/bjp.bp.108.058636. [DOI] [PubMed] [Google Scholar]
- 20.Dave S, Petersen I, Sherr L, Nazareth I. Incidence of maternal and paternal depression in primary care: a cohort study using a primary care database. Arch Pediatr Adolesc Med. 2010;164(11):1038–1044. doi: 10.1001/archpediatrics.2010.184. [DOI] [PubMed] [Google Scholar]
- 21.Martín-Merino E, Ruigómez A, Wallander M-A, et al. Prevalence, incidence, morbidity and treatment patterns in a cohort of patients diagnosed with anxiety in UK primary care. Fam Pract. 2010;27(1):9–16. doi: 10.1093/fampra/cmp071. [DOI] [PubMed] [Google Scholar]
- 22.Moser K, Majeed A. Prevalence of treated chronic diseases in general practice in England and Wales; trends over time and variations by the ONS area classification. London: Office for National Statistics; 1999. Office for National Statistics. [Google Scholar]
- 23.O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37–54. [Google Scholar]
- 24.Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry. 2006;67(8):1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- 26.Petersen I, Gilbert RE, Evans SJW, et al. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry. 2011;72(7):979–985. doi: 10.4088/JCP.10m06090blu. [DOI] [PubMed] [Google Scholar]
- 27.Evans J, Heron J, Francomb H, et al. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendell R, Chalmers J, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 29.Munk-Olsen T, Laursen TM, Pedersen CB, et al. Family and partner psychopathology and the risk of postpartum mental disorders. J Clin Psychiatry. 2007;68(12):1947–1953. doi: 10.4088/jcp.v68n1217. [DOI] [PubMed] [Google Scholar]
- 30.Farr SL, Bitsko RH, Hayes DK, Dietz PM. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542. doi: 10.1016/j.ajog.2010.07.007. e1–9. [DOI] [PubMed] [Google Scholar]
- 31.Warner R, Appleby L, Whitton A, Faragher B. Demographic and obstetric risk factors for postnatal psychiatric morbidity. Br J Psychiatry. 1996;168(5):607–611. doi: 10.1192/bjp.168.5.607. [DOI] [PubMed] [Google Scholar]


