Abstract
Background
Better self management could improve quality of life (QoL) and reduce hospital admissions in chronic obstructive pulmonary disease (COPD), but the best way to promote it remains unclear.
Aim
To explore the feasibility, effectiveness and cost effectiveness of a novel, layperson-led, theoretically driven COPD self-management support programme.
Design and setting
Pilot randomised controlled trial in one UK primary care trust area.
Method
Patients with moderate to severe COPD were identified through primary care and randomised 2:1 to the 7-week-long, group intervention or usual care. Outcomes at baseline, 2, and 6 months included self-reported health, St George’s Respiratory Questionnaire (SGRQ), EuroQol, and exercise.
Results
Forty-four per cent responded to GP invitation, 116 were randomised: mean (standard deviation [SD]) age 69.5 (9.8) years, 46% male, 78% had unscheduled COPD care in the previous year. Forty per cent of intervention patients completed the course; 35% attended no sessions; and 78% participants completed the 6-month follow-up questionnaire. Results suggest that the intervention may increase both QoL (mean EQ-5D change 0.12 (95% confidence interval [CI] = –0.02 to 0.26) higher, intervention versus control) and exercise levels, but not SGRQ score. Economic analyses suggested that with thresholds of £20 000 per quality-adjusted life-year gained, the intervention is likely to be cost-effective.
Conclusion
This intervention has good potential to meet the UK National Institute for Health and Clinical Excellence criteria for cost effectiveness, and further research is warranted. However, to make a substantial impact on COPD self-management, it will also be necessary to explore other ways to enable patients to access self-management education.
Keywords: pilot projects; pulmonary disease, chronic obstructive; randomised controlled trial; self-care; self-management; patient education as topic; primary health care
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is associated with multiple problems for patients, including physical1 and psychological morbidity,2 socioeconomic deprivation,3 and a greatly reduced quality of life.4 Self-management has been defined as ‘the tasks that individuals must undertake to live with one or more chronic conditions. These tasks include having the confidence to deal with medical management, role management and emotional management of their conditions’.5 Thus, better self-management has the potential to reduce the impact of chronic conditions on patients.6 A meta-analysis concluded that educational interventions supporting self-management in COPD may reduce hospital admissions, but data were too sparse and heterogeneous to formulate recommendations about how this should best be delivered.7
Unlike the self-management interventions previously trialled in COPD,7 the Chronic Disease Self-Management Programme (CDSMP)8 has an explicit, theoretically driven behaviour-change basis: self-efficacy theory,9 (a major component of Bandura’s social cognitive theory).10 Self-efficacy can be understood as a person’s confidence in their ability to execute a particular behaviour; it has been shown to influence both psychological state9 and a variety of individual behaviours.11,12
It was hypothesised that a disease-specific version of the CDSMP targeted at those with moderate to severe disease might improve patients’ quality of life, reduce unplanned hospitalisations, and prove cost-effective. However, recruitment and retention may be a particular difficulty in COPD, where the uptake of pulmonary rehabilitation, a well-established, evidence-based intervention,13 could be as low as 30% to 39%.14 The Medical Research Council framework for complex interventions recommends a ‘carefully phased approach, starting with a series of pilot studies targeted at each of the key uncertainties in the design, and moving on to an exploratory and then a definitive evaluation’, when designing a new intervention.15 This article reports a pilot randomised controlled trial of a novel intervention based on the CDSMP in patients with moderate to severe COPD identified from primary care disease registers, designed to explore the feasibility, potential effect sizes, costs, and likely cost-effectiveness of such an intervention.
METHOD
Study design
Patients residing in a suburban borough with very high COPD prevalence16 were identified by 10 primary care teams from their disease registers, or from a community respiratory clinic, and invited by a letter to participate. Following baseline assessment, patients were randomised 2:1, intervention:control, maintaining allocation concealment. It was impossible to blind subjects to their allocation but primary care teams were unaware of patients’ allocated groups. All patients received GP care, and any other respiratory care, as usual. Questionnaires were self-completed by patients at home, in the presence of a researcher not associated with the intervention.
How this fits in
Patients with chronic obstructive pulmonary disease (COPD) may experience psychological as well as physical problems and a greatly reduced quality of life. Self-management education for patients with COPD may well reduce hospital admissions but how best to deliver such education, and its effect on quality of life, is unknown. This pilot study suggests that a lay-led, COPD-specific version of the Expert Patients Programme has good potential to be cost effective among patients with moderate to severe COPD and substantial morbidity. A larger trial of the intervention is warranted but there is also a need for alternative self-management support for patients who cannot, or will not, access such a group intervention.
Patients
Inclusion criteria were: aged >35 years, diagnosed COPD with a ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <0.7, plus either an exacerbation of COPD leading to unscheduled health care within the past year, or post-bronchodilator FEV1<80% predicted (moderate COPD17). Patients with life-threatening comorbidity, major psychological illness, inability to give informed consent, previous participation in another self-management programme, or lacking fluency in English18 were excluded. Previous involvement in pulmonary rehabilitation programmes did not influence eligibility.
COPD-specific self-management intervention
The intervention, Better Living with Long term Airways disease (BELLA), was a new disease-specific adaptation of the generic CDSMP19 developed by the Expert Patient Programme (EPP) Community Interest Company in the UK, and this group also developed a formal training programme for the tutors delivering the course. The course addressed five core self-management skills: defining the problem, decision making, finding and using resources, forming partnerships with healthcare providers, and taking action (making a short-term action plan and acting on it).8 Each course involved two trained lay (peer) tutors (at least one of whom had COPD), who delivered a structured, manualised, 3-hour session once a week for 7 weeks at a local community centre. During the sessions, the peer leaders modelled good self-management behaviours and responses.8 Each session covered six to eight different topics lasting 15–25 minutes (Appendix 1), and each week participants set themselves a personal goal for the next week and, at the subsequent meeting, discussed their success in achieving this goal. The course was highly participatory and content was designed to be particularly relevant for patients with COPD. It included an interactive session around COPD medications with a respiratory clinician. Other topics included: understanding the role of health beliefs, managing breathlessness, relaxation, energy conservation, managing fatigue, healthy eating, increasing physical activity, and addressing the emotional aspects of COPD. Participants were also given a copy of the generic EPP manual.20 At the final meeting, participants were introduced to members of a local COPD patients’ support group and encouraged to join. Six courses were run. Between nine and 18 participants were invited to each course and participants had the opportunity to defer starting a course. Patients who attended five or more sessions met the predetermined definition of a course ‘completer’. Intervention patients also received usual care, described below.
Patients in the control arm only received usual COPD care, which was not standardised in the area studied; some patients had regular outpatient follow-up in a community respiratory clinic or hospital, with a respiratory physician or specialist nurse; others were followed up on a regular or ad hoc basis in primary care. However, primary care doctors were reimbursed on an annual basis for having a register of patients with COPD, COPD diagnosis using post-bronchodilator spirometry, recording FEV1 and inhaler technique, and vaccinating patients against influenza.21 In the area studied, between 86.3% and 93.3% of general practices were meeting these targets at the time of study commencement.
Outcome measures
Outcomes were collected at baseline, 2, and 6 months after each course finished. Each patient in the control group was allocated the same follow-up dates as a patient in the intervention group randomly selected from those recruited around the same time. No primary outcome was specified as this was a pilot study.22 The following were collected: the St George’s Respiratory Questionnaire (SGRQ);23 the EQ-5D instrument, a generic measure of health-related quality of life (HRQoL);24 The Hospital Anxiety and Depression Scale (HADS);25 the Stanford self-efficacy scales around managing disease in general and communicating with physicians;26 and the Stanford self-management behaviour scales for exercise and communication with physicians.26 Participants were also asked to rate their current general health as very good, good, fair, poor, or very poor.
COPD-related healthcare use was extracted from primary care records from the start of the self-management course to 6 months after the course finished, or equivalent dates for control patients. This included telephone consultations, specialist nurse visits, GP surgery and home visits, out-of-hours consultations, rapid response team visits, emergency department attendance, hospital admissions, outpatient visits, and COPD-related medication. Primary care record data were also used to determine participants’ Cumulative Illness Rating Scale scores, a measure of the burden of comorbidity.27 To assess feasibility, data were collected on recruitment and participation, and a separate qualitative study on acceptability was conducted (reported elsewhere).28
Unit costs
Unit costs of resources used were obtained from national reference cost databases.28–30 Using the perspective of a healthcare provider, these unit costs were applied to individual healthcare records to estimate mean and median costs per patient for the intervention and control groups. The total cost for delivering BELLA was £30 000, including tutor training and the delivery of six courses with participant transportation if required.
Analysis
Since this was a pilot study, a sample size calculation was not performed.22 The researchers aimed for 120 participants because it was felt this would be a large enough sample to inform them about the practicalities of delivering several self-management courses led by patients with COPD, recruitment, uptake, and attrition.
Outcomes in each group were summarised using means, medians, and proportions as appropriate, and analysis of covariance (ANCOVA) linear regression models were fitted, using Stata (version 10.1), to obtain estimates of the difference in mean change scores between intervention and control groups.32 All patients were analysed in the group to which they were originally randomised, on an available-case basis.
The incremental cost-effectiveness ratio (ICER) is the measure of the mean additional cost per additional quality-adjusted life-year (QALY) gained by the intervention group. This was generated from EQ-5D scores using UK general population tariffs,24 and the total costs reported in the patient-level data from the intervention and control groups over 6 months.33 Patients who died or were lost to follow-up were excluded from the QALY calculations based on the study data. Bootstrapping with 1000 iterations and imputation was used for missing QALY data to explore variability in results and this was plotted on a cost-effectiveness plane. The plane showed the point where BELLA would be considered cost effective given alternative assumptions about society’s willingness to pay for improvements in the outcomes explored. A cost-effectiveness acceptability curve (CEAC) was also constructed. Such curves help illustrate the level of uncertainty surrounding cost-effectiveness thresholds and the probability that an intervention is considered cost effective.34
RESULTS
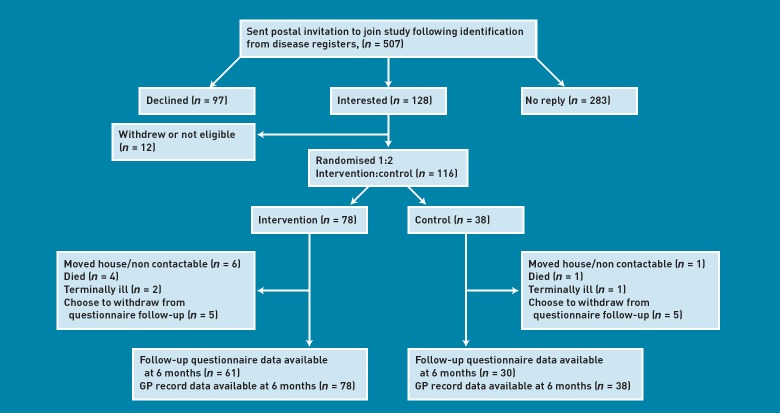
Invitation letters were sent to 507 patients; (44%) responded, 128 (25%) expressed an interest in participating, and 116 (23%) were recruited (Figure 1). Seventy-eight patients were randomised to the intervention and 38 to the control group. Ninety-one (78%) of the original participants completed the 6 month follow-up questionnaire. Attrition was similar in the intervention (n = 17, 22%) and control (n = 8, 21%) groups (reasons are shown in Figure 1). Among intervention patients, 64 (82%) agreed to be registered on a BELLA course, 27 (35%) did not attend any sessions, and 31 (40%) attended at least five sessions.
Figure 1.
Flow chart for the study.
Some differences in baseline characteristics arose because of the relatively small numbers in this study (Table 1). Very little difference in change in outcomes was seen between baseline and 2 month follow-up in either group (data not shown). Table 2 compares the mean changes between baseline and 6 months after completion of the course (or equivalent date) in the two groups. As this was a pilot study, P-values are not shown.22 Although there was a suggestion of a difference in changes in the symptoms and activities components of the SGRQ, favouring control and intervention groups respectively, the mean change in total SGRQ scores was similar in both groups (mean difference –0.4, 95% confidence interval [CI] = –5.1 to 4.4, favouring the intervention). Nevertheless, the 95% CI does not rule out a clinically important difference (4 points) in a larger study, although this looks unlikely. Similarly, there was little difference in mean change in HADS anxiety or depression scores between the two groups. Changes in scores for self-efficacy to manage the disease and to communicate with doctors tended to be very small: less than one-third or one-tenth of a standard deviation (SD), favouring control and intervention respectively.
Table 1.
Baseline characteristics of study participants
| Characteristic | Intervention (n = 78) | Control (n = 38) |
|---|---|---|
| Sex, male:female | 40:38 | 13:25 |
| Age, years | 69.0 ± 9.8 | 70.5 ± 10.0 |
| Marital and employment status, n (%) | ||
| Married | 47 (60) | 16 (42) |
| Single | 4 (5) | 1 (3) |
| Widowed | 13 (17) | 13 (34) |
| Divorced | 14 (18) | 8 (22) |
| Lives alone | 28 (36) | 13 (34) |
| Currently employed | 12 (15) | 2 (5) |
| Age completed full-time education, yearsa | 15.1 ± 2.0 | 16.1 ± 7.4 |
| Smoking and COPD status | ||
| Current smoker, n (%) | 24 (31) | 8 (21) |
| Ever smoker, n (%) | 68 (87) | 33 (87) |
| Mean pack-years | 47.6 ± 30.6 | 50.2 ± 35.8 |
| Had pulmonary rehab, n (%) | 10 (13) | 10 (26) |
| Cumulative Illness Rating Scale Scoreb | 12.1 ± 4.5 | 12.9 ± 5.1 |
| COPD exacerbation in past 12 months, n (%) | 60 (77) | 26 (68) |
| Unscheduled COPD care in past year, n (%) | 62 (80) | 29 (76) |
| Years since COPD diagnosed | 7.6 ± 9.7 | 6.0 ± 7.8 |
| BMI,c kg/m2 | 26.4 ± 5.4 | 27.4 ± 6.4 |
| Using oxygen, n (%) | 13 (17) | 7 (18) |
| Lung function | ||
| FEV1, litres | 1.32 ± 0.54 | 1.27 ± 0.58 |
| FEV1, % predicted | 53.9 ± 22.6 | 54.6 ± 23.4 |
| FVC, litres | 2.40 ± 0.78 | 2.04 ± 0.54 |
| FVC, % predicted | 77.6 ± 22.3 | 74.4 ± 20.1 |
| FEV1:FVC | 0.55 ± 0.15 | 0.59 ± 0.17 |
Data shown are mean ± standard deviation unless otherwise indicated.
Three participants had never been in full-time education.
See text, higher scores represent greater comorbidity.
BMI, based on self-report height and weight. BMI = body mass index. COPD = chronic obstructive pulmonary disease. FEV1 = forced expiratory volume in 1 second. FVC = forced vital capacity.
Table 2.
Mean of outcomes at baseline and 6 months and differences between intervention and control groups at 6 months (available case analysis)
| Control, n = 30 | Intervention, n = 61 | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Estimated differencea (95% CI) | Direction of effect favours | |
| SGRQ, mean ± SE | ||||||
| Symptoms | 58.0 ± 4.85 | 50.4 ± 4.70 | 54.9 ± 3.62 | 52.5 ± 3.27 | 4.7 (–3.0 to 12.4) | Control |
| Activities | 57.3 ± 3.98 | 58.7 ± 2.98 | 55.5 ± 2.74 | 53.0 ± 2.55 | –4.8 (–11.3 to 1.8) | Intervention |
| Impacts | 34.4 ± 3.66 | 31.8 ± 3.26 | 36.6 ± 2.45 | 33.9 ± 2.62 | 0.3 (–5.1 to 5.7) | Control |
| Total score | 45.3 ± 3.49 | 43.1 ± 3.18 | 45.4 ± 2.34 | 42.8 ± 2.47 | –0.4 (–5.1 to 4.4) | Intervention |
| Exercise, minutes/week | ||||||
| Strengthening | 22.0 ± 9.7 | 9.0 ± 4.4 | 22.6 ± 6.1 | 21.6 ± 5.8 | 12.4 (–3.8 to 28.6) | Intervention |
| Aerobic | 56.0 ± 12.3 | 43.5 ± 10.9 | 65.7 ± 10.5 | 59.1 ± 10.4 | 10.1 (–16.3 to 35.5) | Intervention |
| Self-efficacy to: | ||||||
| Communicate with doctors | 8.0 ± 0.5 | 7.7 ± 0.5 | 8.5 ± 0.3 | 8.2 ± 0.3 | 0.2 (–0.6 to 1.1) | Intervention |
| Manage disease | 7.4 ± 0.3 | 7.8 ± 0.2 | 7.7 ± 0.2 | 7.4 ± 0.2 | –0.5 (–1.0 to 0.1) | Control |
| HADS scores | ||||||
| Anxiety | 6.7 ± 0.8 | 6.7 ± 0.8 | 6.1 ± 0.5 | 5.7 ± 0.6 | –0.5 (–1.8 to 0.9) | Intervention |
| Depression | 4.8 ± 0.5 | 5.1 ± 0.4 | 5.4 ± 0.4 | 5.7 ± 0.4 | 0.2 (–0.8 to 1.3) | Control |
| EQ-5D | ||||||
| EQ-5D index scores | 0.76 ± 0.04 | 0.57 ± 0.07 | 0.73 ± 0.04 | 0.68 ± 0.04 | 0.12 (–0.02 to 0.26) | Intervention |
| Current health, n | ||||||
| Very good | 0 | 2 | 2 | 4 | ||
| Good | 7 | 4 | 13 | 12 | ||
| Fair | 17 | 15 | 26 | 31 | ||
| Poor | 5 | 9 | 17 | 13 | ||
| Very poor | 1 | 0 | 3 | 1 | ||
HADS = Hospital Anxiety and Depression Scale. SE = standard error. SGRQ = St George’s Respiratory Questionnaire.
From the ANCOVA, regression coefficient for the intervention versus the control groups.
At 6 months, there was a tendency for the intervention group to be doing more exercise than the control group (Table 2). Although the effect estimates were modest, 12.4 minutes more exercise per week (95% CI = –3.8 to 28.6) for strengthening exercises and 10.1 more minutes per week (95% CI = –16.3 to 35.5) for aerobic exercise, they were relatively large when compared to the total weekly exercise reported at baseline (Table 2). This difference arose because the level of self-reported exercise declined more in the control group than in the intervention group. Similarly, EQ-5D utility scores deteriorated from baseline to follow-up in both groups, but the mean decline tended to be considerably smaller in the intervention group (difference in mean change 0.12, 95% CI = –0.02 to 0.26). Over the 6 month follow-up period, two members of the control group became regular smokers, while in the intervention group, two quit smoking. Overall, there was a suggestion that self-reported heath improved in the intervention group and declined in the control group, but the numbers are small and these data are only descriptive.
While the cost of the intervention per person in the intervention arm of the trial was £385, the cost per participant attending was much higher at £588, as 27 patients in the intervention group failed to attend. Including healthcare resource use, at 6 months the mean cost per patient in the treatment arm of the trial (SD) was £877 (£1218), and the median cost was £551. In the control arm, these costs were £395 (£822) and £109, respectively (Table 3).
Table 3.
Parameters used in the economic evaluation and mean values for the cost-effectiveness evaluation
| Item | Value | Notes |
|---|---|---|
| Intervention prices | ||
| Total price for the intervention | £30 000 | Total amount charged by EPP CIC: £24 000 for seven courses and £6000 for staff training |
| Price per person in the intervention arm | £385 | Price per person in the intervention group (78 patients) |
| Healthcare service costs | ||
| A&E visit | £111 | A&E treatment national mean29 |
| Community matron home visit | £9 | Clinical support worker nursing (community)29 |
| Community respiratory clinic | £138 | Same cost as an outpatient visit (expert opinion: Dr S Taylor) |
| Course session attendance | £129 | £30 000 paid for all courses divided among the 232 patient sessions attended over the duration of the six courses. Cost of each course £4000; lay person total training costs £6000 |
| GP practice | £36 | GP surgery consultation29 |
| GP home visit | £58 | GP home visit29 |
| Home rapid response | £190 | Rapid-response low-cost episode29 |
| Hospital admission (1 day) | £319 | National reference costs COPD non-elective short stay (1 day or less)30 |
| Hospital admission (>1 day) | £267 | COPD non-elective long stay without NIV and with complications30 |
| Out of hours, per day | £200 | Expert opinion: Dr S Taylor |
| Outpatients hospital | £138 | Cost weighted by the most frequent COPD visits (consultant-led follow-up attendance non-admitted face to face £118 weighted by 71% and consultant-led first attendance non-admitted face to face £186 weighted by 29%)30 |
| Telephone | £22 | Telephone consultation30 |
| Antibiotic prescription | £2 | Doxycycline 200 mg for 7 days31 |
| Steroid prescription | £7 | Prednisolone 40 mg for 7 days31 |
| Rescue pack | £9 | Doxycycline 200 mg for 7 days + prednisolone 40 mg for 7 days31 |
| Mean values for cost-effectiveness analysis | ||
| Cost per person in intervention arm | £877 | Includes the intervention and healthcare services costs |
| Cost per person in control arm | £395 | Includes healthcare services costs |
| QALY per person in intervention arm | 0.682 | Calculated using EQ-5D scores from baseline, 2, and 6 months |
| QALY per person in control arm | 0.569 | Calculated using EQ-5D scores from baseline, 2, and 6 months |
A&E = accident and emergency. EPP CIC = Expert Patients Programme Community Interest Company. NIV = non-invasive ventilation. QALY = quality-adjusted life-year.
The ICER was £11 710 per QALY gained over 6 months. In order to adjust for any differences due to outliers, a bootstrap was conducted with 1000 replications. This suggested a moderate increase in both outcome and costs for patients in the intervention group (Figure 2). A CEAC was then constructed, suggesting that if society was only willing to pay £20 000 (€22 533, $37 000) per additional QALY gained, then the BELLA intervention would have a 75% probability of being cost effective (Figure 3). If a willingness-to-pay threshold of £30 000 (€33 800, $55 500) per QALY gained were used, then the intervention would have an 86% probability of being cost effective.
Figure 2.
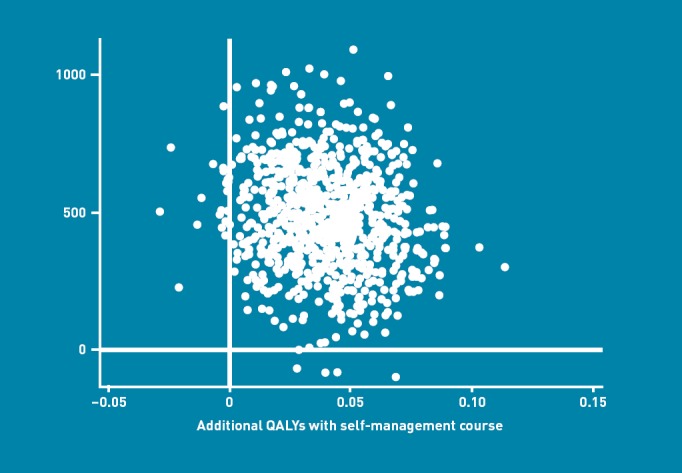
Incremental cost-effectiveness ratios (ICERs) predicted from a bootstrap calculation with 1000 replications, showing the relationship between the mean difference in costs and mean difference in benefits for the BELLA intervention.
Figure 3.
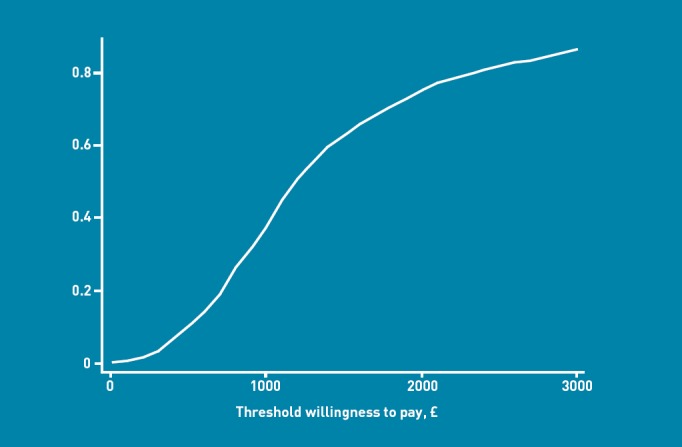
Cost-effectiveness acceptability curve (CEAC) for the BELLA intervention showing the probability that the intervention is cost effective across different willingness-to-pay thresholds.
DISCUSSION
Summary
The results of this pilot study suggest that a COPD-specific version of the self-management course led by lay tutors with COPD, and including a small health professional component, is feasible, may improve health outcomes, and is potentially cost effective. At 2 months’ follow-up, little difference could be seen between those in the intervention and control arms of the study, but by 6 months greater differences had emerged in favour of the intervention group in both self-reported exercise levels and HRQoL. This finding supports the theory that self-management skills gained on the course, such as goal setting and action planning, develop with practice over time. The costs of the intervention did not appear to be offset by a decrease in the utilisation of healthcare services by 6 months. However, if the moderate benefit in HRQoL demonstrated here is replicated in a larger, definitive study, the intervention is highly likely to be cost effective using the threshold range of £20 000–30 000 per QALY adopted by the National Institute for Health and Clinical Excellence (NICE) in England and Wales. It is worth noting that the potential cost per QALY seen in this study is similar to the lowest estimates for cost per QALY for tiotropium compared to placebo in patients with moderate to severe COPD.35
Strengths and limitations
The study findings vindicate the strategy of conducting a pilot study before a definitive effectiveness trial. Only one-quarter of patients with moderate to severe COPD identified from primary care disease registers expressed an interest in participating in the study, and 35% of those offered the self-management courses failed to attend any sessions. To detect a clinically significant four-point difference in SGRQ with 80% power and 5% significance and the 22% attrition seen in this study, 402 patients per group would be needed.36 Similarly, to detect the 0.14 benefit in EQ-5D Index score seen in this study, 119 patients per group would be needed. It would be valuable to consider ways in which the uptake of the intervention might be facilitated and encouraged and to develop versions of the intervention for participants who remain unwilling or unable to attend group self-management courses.
This study was conducted with the agreement of primary care physicians, and patients were invited to join the study via a letter sent from their primary care practices; however, the intervention was not otherwise integrated into primary or secondary care and participation in the course was not specifically recommended or endorsed by healthcare professionals. Face-to-face recruitment during routine consultations, and specific endorsement of the course by members of the primary care team, might have increased participation rates, a suggestion supported by some qualitative literature on attendance at pulmonary rehabilitation.37,38 Others have found that health professionals may want to be more directly engaged in community-run self-management groups,39 or that health professionals might be more confident in referring patients to self-management courses if they were more aware of course content.40 Thus, the intervention might benefit from complementary education to members of the primary care team on self-management in COPD.
The results of the economic analyses come with several caveats. Recently, Ringbaek and colleagues have suggested that the EQ-5D may not be the most appropriate utility-based indicator for COPD patients when assessing the effects of pulmonary rehabilitation, as it may not fully capture improvements in health status due to ceiling effects;41 this was not evident in the present study (data not shown). Secondly, the price of the course delivery was fixed before recruitment. If recruiting efforts could increase session attendance, and the new attendees have better outcomes than the non-attendees, then the cost effectiveness of the BELLA course would increase. As the 6-month follow-up period restricts the ability to look at any long-term health benefits that may occur as a result of the course, further modelling is needed to extrapolate beyond the trial time period. Finally, the economic analysis is conservative and does not include potential effects on social care service utilisation or the impacts on informal caregiver time, which can be substantial for this group of patients.
Comparison with existing literature
This study is unique in examining a theoretically driven, group self-management education intervention for COPD delivered by trained lay people.7 Only four other published studies have reported randomised controlled trials of group self-management education in COPD and none of these attempted to recruit patients from disease registers.41–45 Unlike BELLA, all of these studies relied on education from healthcare professionals, along with exercise or other components delivered by healthcare professionals in three studies.43–45 More recently, Rice and colleagues have described large reductions in emergency department attendance and all-cause hospitalisation in patients with COPD at high risk of hospitalisation, with a disease-management intervention consisting of a single 1–1.5 hour group education session and monthly follow-up phone calls by a case manager.46 Their study was not able to demonstrate any improvement in HRQoL using the SGRQ, but the response rate for returning questionnaires was low.
Implications for future research
This self-management intervention has good potential to be cost effective and improve HRQoL, exercise levels, and self-rated health in patients with moderate to severe COPD, and warrants further research. However, it is also important to explore ways to enable and encourage patients to access self-management education, which may include working more closely with primary care teams.
Acknowledgments
We would like to thank: all study participants; participating primary care practices; Church Elm Lane Respiratory Clinic (now Porters Avenue); the lay tutors; our study steering group patient and carer representatives; Barking and Dagenham, Havering and Hertfordshire Breath Easy groups and members of The Lung Club, Leyton; London EPP CIC; Mr Jim Phillips; Ms Julie Atkins; Social Action for Health; and The British Lung Foundation.
Appendix 1. Structured BELLA course overview
| Week | Session content |
|---|---|
| 1 | Welcome, introduction, ground rules, what is self-management? Balancing life with a long-standing health problem What is COPD? Why do exercise? Goal setting and action planning |
| 2 | Follow-up and feedback Doing too much/overtiring oneself, breathing, symptom scanning Managing breathlessness, role of health beliefs in COPD, positive thinking Goal setting and action planning |
| 3 | Follow-up and feedback Positive self-talk, being more active, healthy eating and COPD Muscle relaxation, pacing yourself, sleep Goal setting and action planning |
| 4 | Follow-up and feedback Managing medication, COPD and socialising, managing fatigue Interactive session on COPD medications with a local respiratory consultant Goal setting and action planning |
| 5 | Follow-up and feedback Depression, managing the emotional impact of COPD, using distraction Breathing, recognising setbacks, physical activity, solving problems Goal setting and action planning |
| 6 | Follow-up and feedback Managing setbacks, managing COPD, better communication with doctors and nurses physical activity and relaxation Goal setting and action planning |
| 7 | Follow-up and feedback Making choices deals and decisions, guided imagery, revisiting the programme, sharing successes and goals Planning to stay well, introduction to members of a local COPD patient support group and encouragement to join, celebration tea party |
Funding
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit programme (Grant no. PB-PG-0906-11172). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
Ethics committee
The study was approved by the UK National Research Ethics Service (Joint UCL/UCLH Committee on the Ethics of Human Research Committee Alpha, REC reference number: 07/H0715/110).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 2.Hynninen KM, Breitve MH, Wiborg AB, et al. Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res. 2005;59(6):429–443. doi: 10.1016/j.jpsychores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Anto JM, Vermeire P, Vestbo J, Sunyer J. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982–994. doi: 10.1183/09031936.01.17509820. [DOI] [PubMed] [Google Scholar]
- 4.Schlenk EA, Erlen JA, Dunbar-Jacob J, et al. Health-related quality of life in chronic disorders: a comparison across studies using the MOS SF-36. Qual Life Res. 1998;7(1):57–65. doi: 10.1023/a:1008836922089. [DOI] [PubMed] [Google Scholar]
- 5.Adams K, Greiner AC, Corrigan JM. The 1st annual crossing the quality chasm summit — a focus on communities. Washington DC: The National Academic Press; 2004. [PubMed] [Google Scholar]
- 6.World Health Organization. 2008–2013 Action plan for the global strategy for the prevention and control of noncommunicable diseases. Geneva: World Health Organization; 2009. [Google Scholar]
- 7.Effing T, Monninkhof EEM, van der Valk PP, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(4):CD002990. doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 9.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1985. [Google Scholar]
- 11.Bandura A. Self-efficacy: the exercise of control. New York, NY: Freeman; 1997. [Google Scholar]
- 12.Stajkovic AD, Luthans F. Self-efficacy and work-related performance: a meta-analysis. Psychol Bull. 1998;124(2):240–261. [Google Scholar]
- 13.Lacasse Y, Brosseau L, Milne S, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Harris D, Hayter M, Allender S. Improving the uptake of pulmonary rehabilitation in patients with COPD: qualitative study of experiences and attitudes. Br J Gen Pract. 2008;58(555):703–710. doi: 10.3399/bjgp08X342363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical Research Council. Complex interventions guide. London: Medical Research Council; 2008. www.mrc.ac.uk/complexinterventionsguidance (accessed 28 Aug 2012) [Google Scholar]
- 16.British Lung Foundation. Invisible lives: chronic obstructive pulmonary disease (COPD) — finding the missing millions. London: British Lung Foundation; 2008. [Google Scholar]
- 17.Calverley PMA, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053–1061. doi: 10.1016/s0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- 18.Lindh M, Lurie M, Sanne H. A randomized prospective study of vocational outcome in rehabilitation of patients with non-specific musculoskeletal pain: a multidisciplinary approach to patients identified after 90 days of sick-leave. Scand J Rehabil Med. 1997;29(2):103–112. [PubMed] [Google Scholar]
- 19.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing utilization and costs: a randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 20.NHS Expert Patients Programme. Self management of long term health conditions — a handbook for people with chronic disease. Boulder, Colorado: Bull Publishing Company; 2002. [Google Scholar]
- 21.QOF indicators 2008/09. http://www.dhsspsni.gov.uk/qof_indicators_2008_09.pdf (accessed 28 Aug 2012)
- 22.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones P, Spencer S, Adie S. The St George’s Respiratory Questionnaire. Manual version 2.1. London: St George’s, University of London; 2003. [Google Scholar]
- 24.Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. York: Centre for Health Economics, University of York; 1995. [Google Scholar]
- 25.Snaith RP, Zigmond AS. The Hospital Anxiety and Depression Scale with the Irritability-Depression-Anxiety Scale and the Leeds Situational Anxiety Scale: Manual. Windsor: The NFER-Nelson Publishing Company; 1994. [Google Scholar]
- 26.Lorig K, Stewart A, Ritter P, et al. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage Publications, Inc; 1996. [Google Scholar]
- 27.Miller MD, Towers A. A manual of guidelines for scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) Pittsburgh, PA: University of Pittsburgh; 1991. [Google Scholar]
- 28.Sohanpal R, Seale C, Taylor SJC. Learning to manage COPD: a qualitative study of reasons for attending and not attending a COPD-specific self-management programme. Chronic Respiratory Disease. 2012;9:163–174. doi: 10.1177/1479972312444630. [DOI] [PubMed] [Google Scholar]
- 29.Curtis LA. Unit costs of health and social care 2008. Canterbury: PSSRU, University of Kent at Canterbury; 2008. [Google Scholar]
- 30.Department of Health. NHS reference costs 2007–08. London: Department of Health; 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945 (accessed 28 Aug 2012) [Google Scholar]
- 31.Royal Pharmaceutical Society of Great Britain. British National Formulary: 56 2008. London: RPS Publishing and BMJ Publishing Group; 2008. [Google Scholar]
- 32.Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MF, Schulpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 34.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106. [DOI] [PubMed] [Google Scholar]
- 35.Oba Y. Cost-effectiveness of long-acting bronchodilators for chronic obstructive pulmonary disease. Mayo Clin Proc. 2007;82(5):575–582. doi: 10.4065/82.5.575. [DOI] [PubMed] [Google Scholar]
- 36.Machin D, Campbell MH, Tan SB, Tan SH. Sample size tables for clinical studies. 3rd edn. Chichester: Wiley-Blackwell; 2009. [Google Scholar]
- 37.Fischer MJ, Scharloo M, Abbink JJ, et al. Participation and drop-out in pulmonary rehabilitation: a qualitative analysis of the patient’s perspective. Clin Rehabil. 2007;21(3):212–221. doi: 10.1177/0269215506070783. [DOI] [PubMed] [Google Scholar]
- 38.Arnold E. Adherence to pulmonary rehabilitation: a qualitative study. Resp Med. 2005;100(10):1716–1723. doi: 10.1016/j.rmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Gately C, Rogers A, Sanders C. Re-thinking the relationship between long-term condition self-management education and the utilisation of health services. Soc Sci Med. 2007;65(5):934–945. doi: 10.1016/j.socscimed.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Blakeman T, Macdonald W, Bower P, et al. A qualitative study of GPs’ attitudes to self-management of chronic disease. Br J Gen Pract. 2006;56(527):407–414. [PMC free article] [PubMed] [Google Scholar]
- 41.Ringbaek T, Brondum E, Martinez G, Lange P. EuroQoL in assessment of the effect of pulmonary rehabilitation COPD patients. Respir Med. 2008;102(11):1563–1567. doi: 10.1016/j.rmed.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Howland J, Nelson EC, Barlow PB, et al. Chronic obstructive airway disease. Impact of health education. Chest. 1986;90(2):233–238. doi: 10.1378/chest.90.2.233. [DOI] [PubMed] [Google Scholar]
- 43.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17(3):232–240. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- 44.Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Educ Couns. 2004;52(3):259–266. doi: 10.1016/S0738-3991(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 45.Monninkhof E, van der Valk P, van der Palen J, et al. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;22(5):815–820. doi: 10.1183/09031936.03.00047003. [DOI] [PubMed] [Google Scholar]
- 46.Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(7):890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]