Abstract
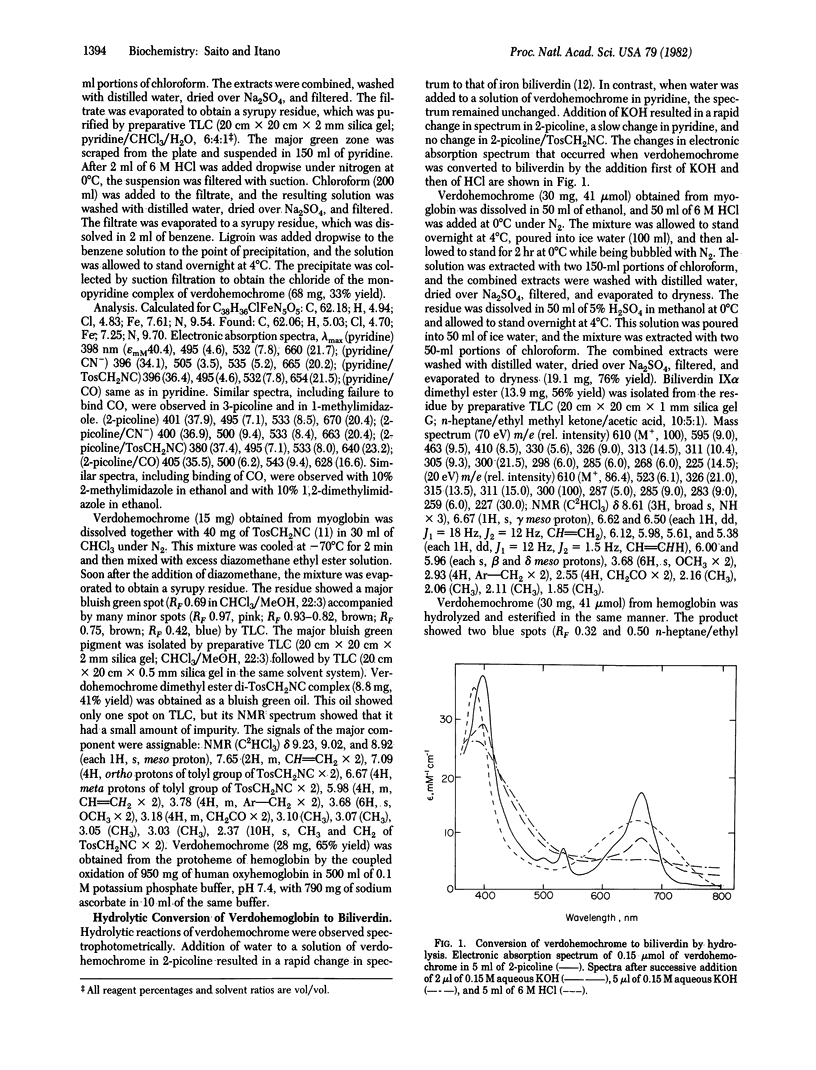
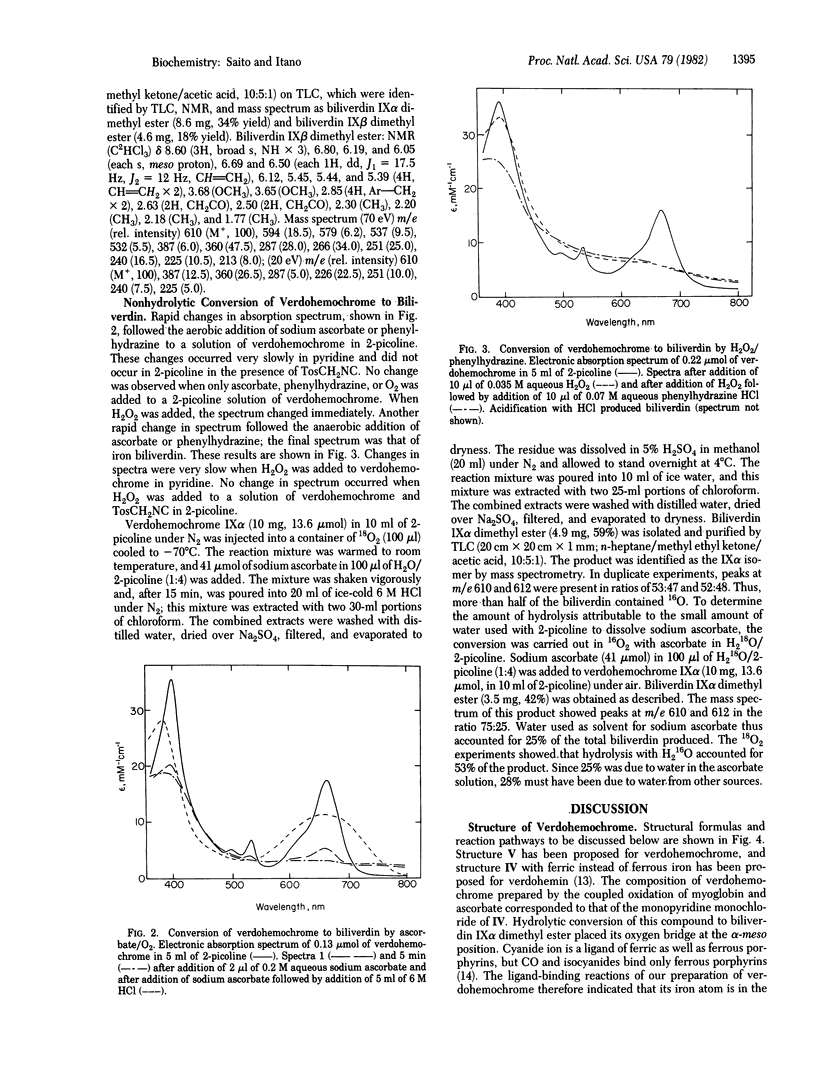
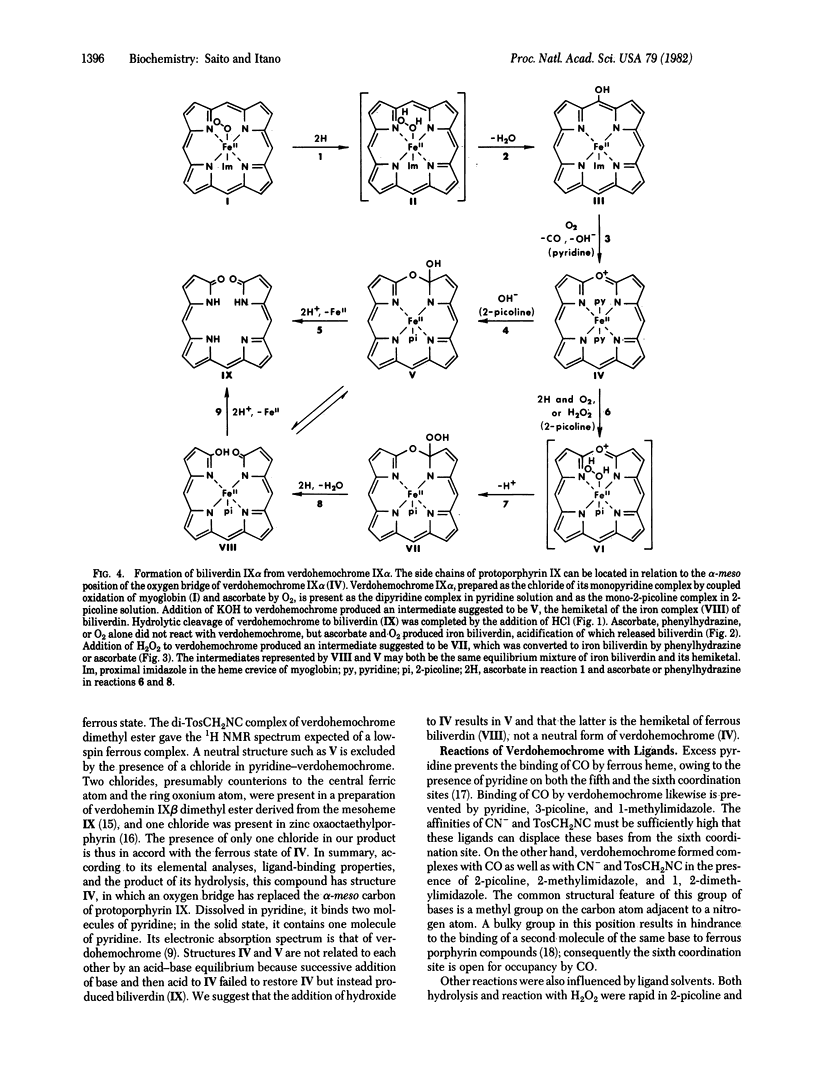
Several studies have shown that both terminal oxygen atoms of biliverdin are derived from molecular oxygen. Since the conversion of verdohemochrome to biliverdin has been assumed to be hydrolytic, these findings seemed to exclude verdohemochrome as an intermediate in the degradation of heme to biliverdin. Coupled oxidation of myoglobin and ascorbate yielded a pure preparation of verdohemochrome IX alpha. The structure and ferrous state of this product were determined from its composition, ligand reactions, 1H NMR spectrum, and conversion to biliverdin IX alpha dimethyl ester. Reaction with ascorbate and 18O2 converted this compound to biliverdin that contained an atom of 18O. Successive treatment of verdohemochrome, first oxidation with H2O2 and then reduction with phenylhydrazine, yielded the iron complex of biliverdin. These results showed that hydrolysis is not an obligatory step in the conversion of verdohemochrome to biliverdin and, moreover, indicated how heme can be converted, with verdohemochrome as an intermediate, into biliverdin in which the two terminal oxygen atoms are derived from different O2 molecules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- ANAN F. K., MASON H. S. An 018 study of the hemoglobin degradation to biliverdin in the model reaction. J Biochem. 1961 Jun;49:765–767. doi: 10.1093/oxfordjournals.jbchem.a127369. [DOI] [PubMed] [Google Scholar]
- Bonnett R., Dimsdale M. J. The meso-reactivity of porphyrins and related compounds. V. The meso-oxidation of metalloporphyrins. J Chem Soc Perkin 1. 1972;20:2540–2548. doi: 10.1039/p19720002540. [DOI] [PubMed] [Google Scholar]
- Bonnett R., McDonagh A. F. The meso-reactivity of porphyrins and related compounds. VI. Oxidative cleavage of the haem system. The four isomeric biliverdins of the IX series. J Chem Soc Perkin 1. 1973;9:881–888. doi: 10.1039/p19730000881. [DOI] [PubMed] [Google Scholar]
- Brown S. B., King R. F. An 18O double-labelling study of haemoglobin catabolism in the rat. Biochem J. 1975 Sep;150(3):565–567. doi: 10.1042/bj1500565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., King R. F. The mechanism of haem catabolism. Bilirubin formation in living rats by [18O]oxygen labelling. Biochem J. 1978 Feb 15;170(2):297–311. doi: 10.1042/bj1700297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Muljiani Z., Rousseau K., Borg D. C., Fajer J., Felton R. H. The chemistry of porphyrin pi-cations. Ann N Y Acad Sci. 1973;206:177–200. doi: 10.1111/j.1749-6632.1973.tb43211.x. [DOI] [PubMed] [Google Scholar]
- King R. F., Brown S. B. The mechanism of haem catabolism. A study of haem breakdown in spleen microsomal fraction and in a model system by 18O labelling and metal substitution. Biochem J. 1978 Jul 15;174(1):103–109. doi: 10.1042/bj1740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. Y. Studies on verdohemochrome. Biochemistry. 1966 Sep;5(9):2845–2852. doi: 10.1021/bi00873a010. [DOI] [PubMed] [Google Scholar]
- Saito S., Itano H. A. beta-meso-Phenylbiliverdin IX alpha and N-phenylprotoporphyrin IX, products of the reaction of phenylhydrazine with oxyhemoproteins. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5508–5512. doi: 10.1073/pnas.78.9.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H., Pimstone N. R., Trager W. F., Cooper D. Y., Schmid R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry. 1972 Apr 25;11(9):1716–1720. doi: 10.1021/bi00759a029. [DOI] [PubMed] [Google Scholar]
- Wang C. M., Brinigar W. S. A correlation of the visible and Soret spectra of dioxygen- and carbon monoxide-heme complexes and five-coordinate heme complexes with the spectra of oxy-, carboxy-, and deoxyhemoglobins. Biochemistry. 1979 Oct 30;18(22):4960–4977. doi: 10.1021/bi00589a026. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Features of the reaction of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1978 Jun 25;253(12):4230–4236. [PubMed] [Google Scholar]



