Abstract
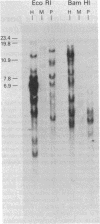
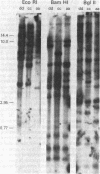
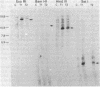
A porcine genomic clone encoding a major histocompatibility, (MHC) antigen was isolated by direct screening of a swine genomic library with a heterologous human MHC cDNA probe. Mouse L cells transformed with DNA from the clone stably express swine MHC antigen. Pig alloantisera specifically lyse transformant but not control cell lines in a complement-mediated cytotoxicity assay. Direct immunoprecipitation of radiolabeled cellular protein from transformed lines by pig alloantiserum results in the coprecipitation of swine MHC heavy chain and mouse beta 2-microglobulin, demonstrating the association of heterologous subunits of MHC antigens.
Full text
PDF



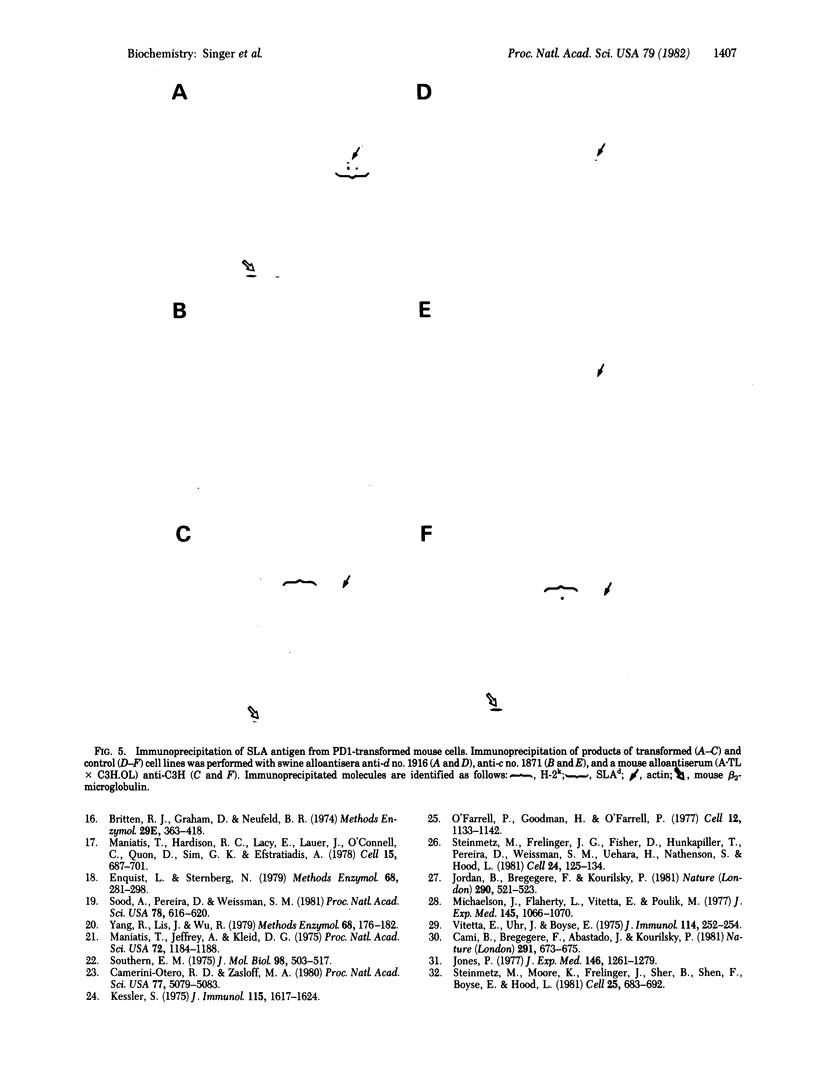
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos D. B., Kostyu D. D. HLA--a central immunological agency of man. Adv Hum Genet. 1980;10:137-208, 385-6. doi: 10.1007/978-1-4615-8288-5_2. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Zasloff M. A. Nucleosomal packaging of the thymidine kinase gene of herpes simplex virus transferred into mouse cells: an actively expressed single-copy gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5079–5083. doi: 10.1073/pnas.77.9.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami B., Brégégère F., Abastado J. P., Kourilsky P. Multiple sequences related to classical histocompatibility antigens in the mouse genome. Nature. 1981 Jun 25;291(5817):673–675. doi: 10.1038/291673a0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Bregegere F., Kourilsky P. Human HLA gene segment isolated by hybridization with mouse H-2 cDNA probes. Nature. 1981 Apr 9;290(5806):521–523. doi: 10.1038/290521a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Leight G. S., Sachs D. H., Rosenberg S. A. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977 Mar;23(3):271–276. [PubMed] [Google Scholar]
- Lunney J. K., Sachs D. H. Transplantation in minature swine. IV. Chemical characterization of MSLA and Ia-like antigens. J Immunol. 1978 Feb;120(2):607–612. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. J., Gilliland G. L., Lunney J. K., Osborne B. A., Rudikoff S., Sachs D. H. Transplantation in miniature swine. IX. Swine histocompatibility antigens: isolation and purification of papain-solubilized SLA antigens. J Immunol. 1981 Aug;127(2):769–775. [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Vitetta E., Poulik M. D. Molecular similarities between the Qa-2 alloantigen and other gene products of the 17th chromosome of the mouse. J Exp Med. 1977 Apr 1;145(4):1066–1070. doi: 10.1084/jem.145.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Orr H. T., López de Castro J. A., Lancet D., Strominger J. L. Complete amino acid sequence of a papain-solubilized human histocompatibility antigen, HLA-B7. 2. Sequence determination and search for homologies. Biochemistry. 1979 Dec 11;18(25):5711–5720. doi: 10.1021/bi00592a030. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976 Dec;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Winn H. J., Russell P. S. The immunologic response to xenografts. Recognition of mouse H-2 histocompatibility antigens by the rat. J Immunol. 1971 Aug;107(2):481–492. [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Trägärdh L., Rask L., Wiman K., Fohlman J., Peterson P. A. Complete amino acid sequence of pooled papain-solubilized HLA-A, -B, and -C antigens: relatedness to immunoglobulins and internal homologies. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1129–1133. doi: 10.1073/pnas.77.2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Capra J. D. The protein products of the murine 17th chromosome: genetics and structure. Adv Immunol. 1978;26:147–193. doi: 10.1016/s0065-2776(08)60230-8. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Association of a beta2-microglobulin-like subunit with H-2 and TL alloantigens on murine thymocytes. J Immunol. 1975 Jan;114(1 Pt 1):252–254. [PubMed] [Google Scholar]
- Watanabe B., Ishimaru T., Usa T., Nagataki S. [Sarcoidosis associated with diabetes insipidus and hypothalamo-pituitary dysfunction--a case report]. Horumon To Rinsho. 1982 Dec;30(12):1407–1413. [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]