Abstract
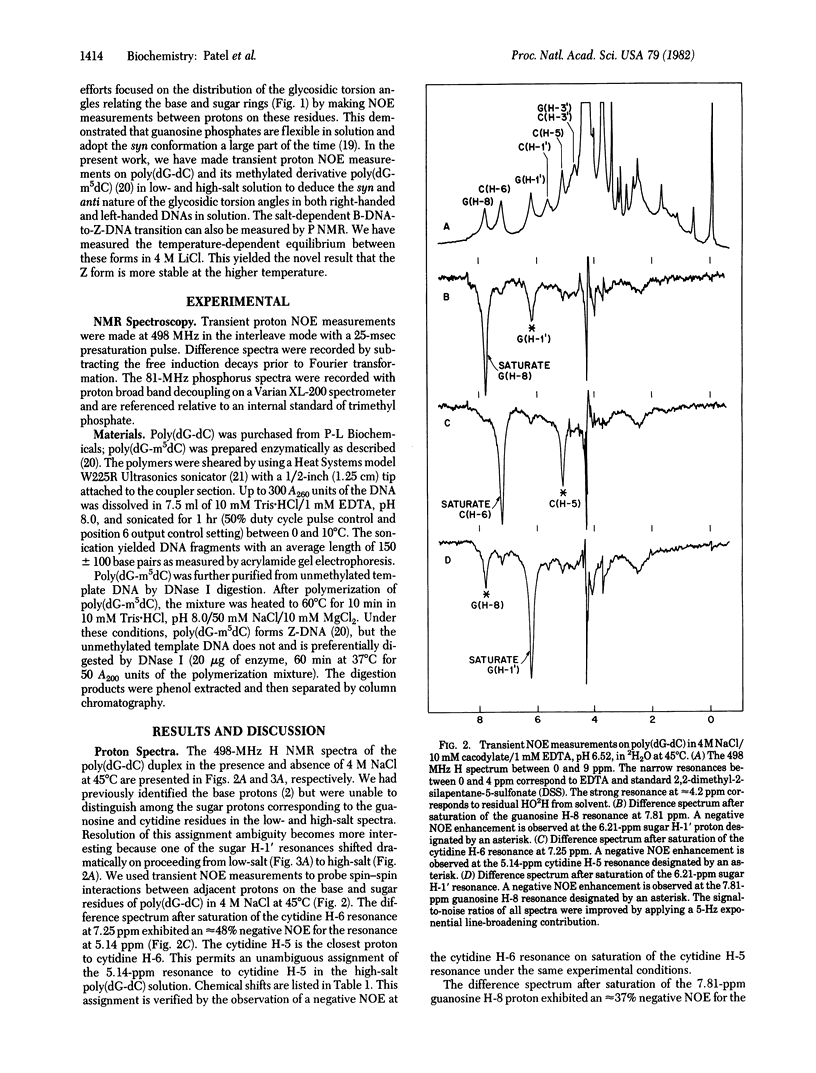
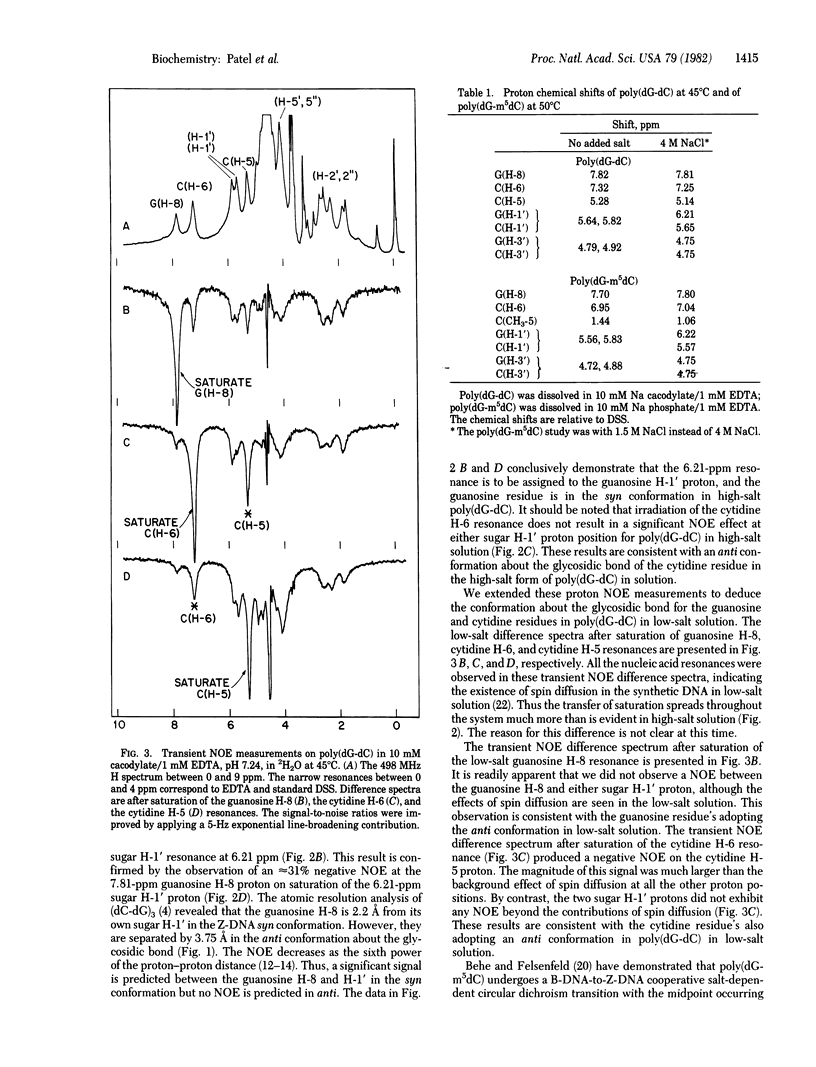
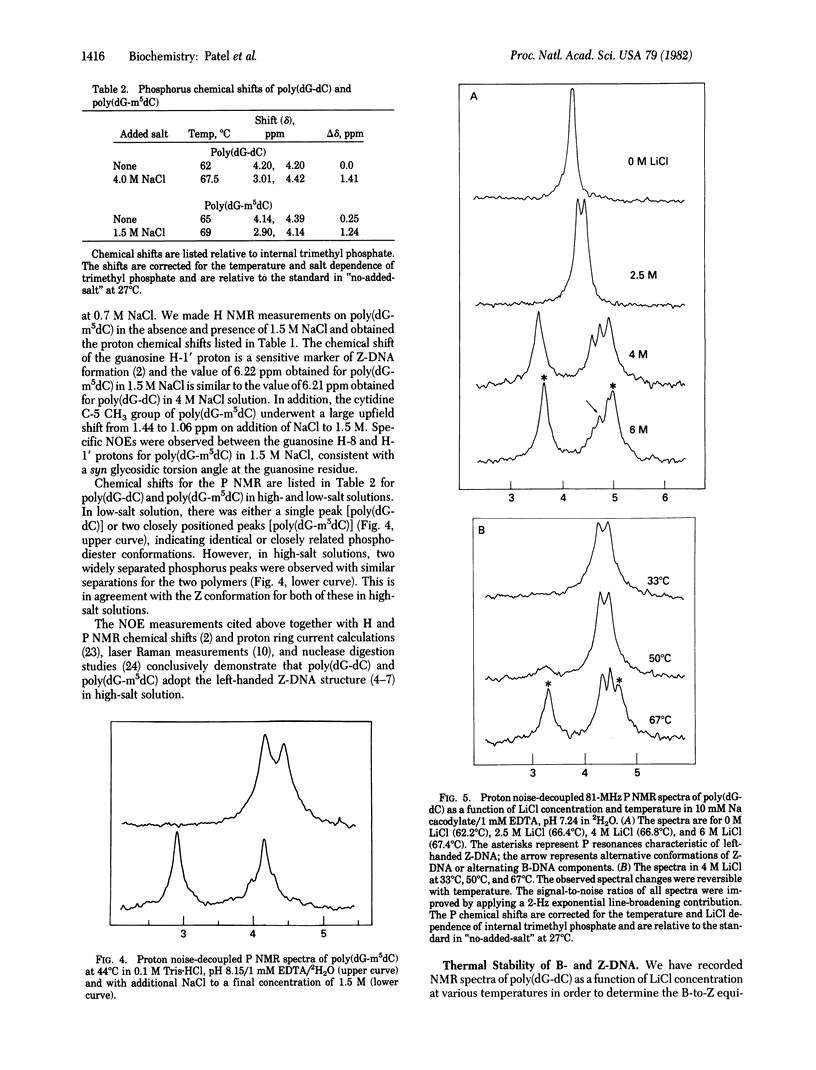
We have differentiated between syn and anti glycosidic torsion angles in nucleic acid duplexes by measuring the transient nuclear Overhauser effect (NOE) between the sugar H-1' protons and the purine H-8 and pyrimidine H-6 base protons. The transient NOE measurements demonstrate a syn glycosidic torsion angle at guanosine and an anti glycosidic torsion angle at cytidine in poly(dG-dC) in 4 M NaCl and in poly(dG-m5dC) in 1.5 M NaCl solution. These features have been observed previously in the left-handed Z-DNA conformation of (dC-dG)3 in the crystalline state. By contrast, transient NOE studies demonstrate that both guanosine and cytidine residues adopt the anti conformation about the glycosidic bond for the right-handed poly(dG-dC) and poly(dG-m5dC) conformation in a low-salt solution. We have used P NMR to monitor the equilibrium between B- and Z-DNA forms of poly(dG-dC) in LiCl solutions; at high temperatures, the equilibrium shifts from B- to Z-DNA.
Full text
PDF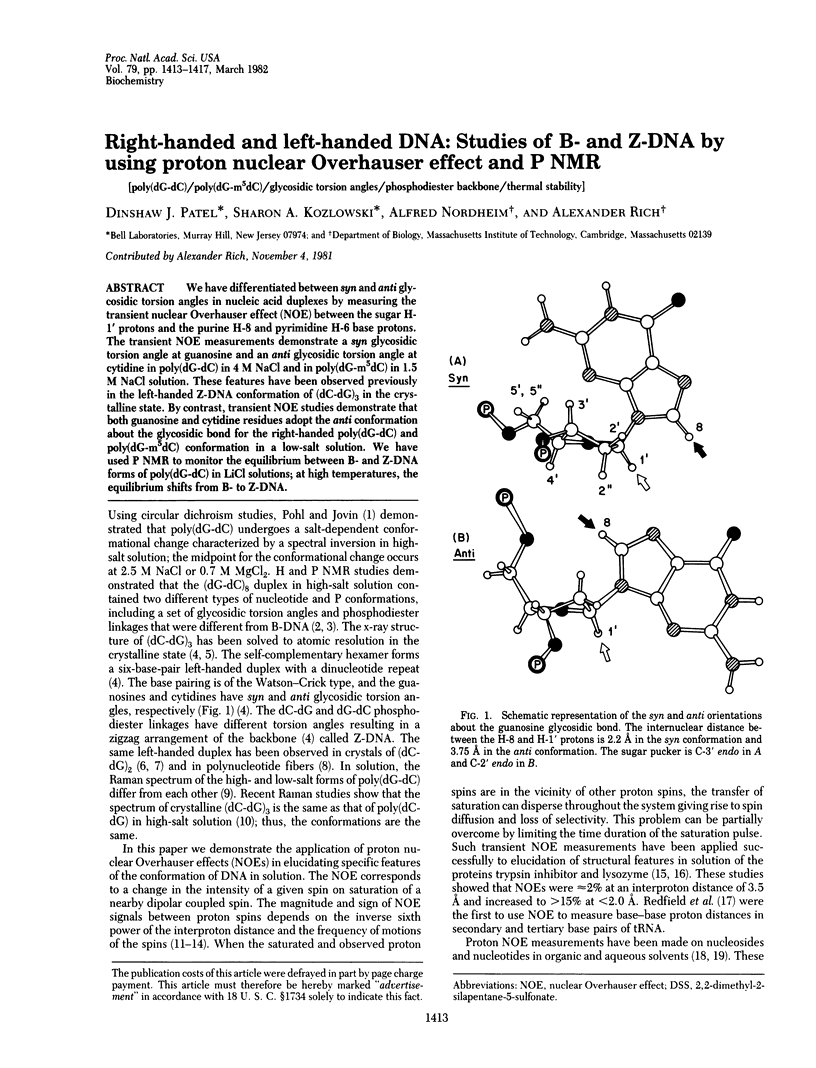

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Zimmerman S., Felsenfeld G. Changes in the helical repeat of poly(dG-m5dC) . poly(dG-m5dC) and poly(dG-dC) . poly(dG-dC) associated with the B-Z transition. Nature. 1981 Sep 17;293(5829):233–235. doi: 10.1038/293233a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Kolpak F. J., Wang A. H., Quigley G. J., van Boom J. H., van der Marel G., Rich A. The tetramer d(CpGpCpG) crystallizes as a left-handed double helix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4016–4020. doi: 10.1073/pnas.77.7.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Hart P. A., Davis J. P. Pyrimidine nucleoside conformational analysis. Nuclear Overhauser effect and circular dichroism correlations. J Am Chem Soc. 1971 Feb 10;93(3):753–760. doi: 10.1021/ja00732a033. [DOI] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Mitra C. K., Sarma M. H., Sarma R. H. Left-handed deoxyribonucleic acid double helix in solution. Biochemistry. 1981 Mar 31;20(7):2036–2041. doi: 10.1021/bi00510a046. [DOI] [PubMed] [Google Scholar]
- Narasimhan V., Bryan A. M. Temperature-induced perturbations in the circular dichroic spectrum of the synthetic polymer poly[d(G-C)]. Biochim Biophys Acta. 1976 Jul 16;435(4):433–437. doi: 10.1016/0005-2787(76)90209-4. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Suggs J. W., Cox S. D. Right-handed alternating DNA conformation: poly(dA-dT) adopts the same dinucleotide repeat with cesium, tetraalkylammonium, and 3 alpha, 5 beta, 17 beta-dipyrrolidinium steroid dimethiodide cations in aqueous solution. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4063–4067. doi: 10.1073/pnas.78.7.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R. P., Krishna N. R., Sakai T. T., Glickson J. D. Proton NMR studies of ternary complexes of bleomycin with metal ions and nucleic acid. Biochem Biophys Res Commun. 1980 Nov 17;97(1):270–278. doi: 10.1016/s0006-291x(80)80164-1. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Poulsen F. M., Hoch J. C., Dobson C. M. A structural study of the hydrophobic box region of lysozyme in solution using nuclear Overhauser effects. Biochemistry. 1980 Jun 10;19(12):2597–2607. doi: 10.1021/bi00553a011. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Redfield A. G., Gupta R. K. Pulsed NMR study of the structure of cytochrome c. Cold Spring Harb Symp Quant Biol. 1972;36:405–411. doi: 10.1101/sqb.1972.036.01.052. [DOI] [PubMed] [Google Scholar]
- Son T. D., Guschlbauer W., Guéron M. Flexibility and conformations of guanosine monophosphates by the Overhauser effect. J Am Chem Soc. 1972 Nov 1;94(22):7903–7911. doi: 10.1021/ja00777a038. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G., Richarz R., Perkins S. J. Individual assignments of the methyl resonances in the 1H nuclear magnetic resonance spectrum of the basic pancreatic trypsin inhibitor. Biochemistry. 1978 Jun 13;17(12):2253–2263. doi: 10.1021/bi00605a001. [DOI] [PubMed] [Google Scholar]



